Abstract
The expression of sucrose-phosphate synthase II (SPSII) and sucrose transporters ShSUT1A and ShSUT4 were determined by RT-PCR and qRT-PCR in the sink and source leaves and in rind and pith of mature internodes of four high-yielding Hawaiian sugarcane cultivars. Expression of SPSII, ShSUT1A, and ShSUT4 was lower in pith than in rind, except in one cultivar, but else quite similar in the cultivars. The strong expression of transporter ShSUT4 in the rind of the internodes may hint to a special role of ShSUT4 in the rind. ShSUT4-expression in the sink and source leaves was similar in all four cultivars, whereas large differences were found for the expression of ShSUT1A and SPSII between the source and sink leaves and between the cultivars. The levels of sucrose precursors were doubled in source leaves compared to sink leaves, whereas they were higher in immature internode compared to mature internode. The role of sucrose transporters and SPSII in leaves and internodes is discussed, but the large differences, which were observed in the transcript levels of SPSII and sucrose transporters between some cultivars, although all the cultivars were similarly high-yielding cultivars, show that SPSII and SUT transcript levels cannot be used as indicators of high-yield cultivars.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Sugarcane is grown commercially because of its ability to store large amounts of sucrose in its stem parenchyma. The increase of sucrose yield has been always a goal and in recent years also for the increasing demand of biofuel and biomass production (Cheavegatti-Gianotto et al. 2011). Therefore, the mechanism of sucrose accumulation in sugarcane stem is also nowadays a top priority in sugarcane research. Sucrose is the common transport sugar in the phloem of nearly all plants, but also nearly all plants including grasses synthesize macromolecules such as starch or fructans as storage compounds to avoid osmotic problems which would be created by the accumulation of small molecules (Zhu et al. 1997). Breeding for high sugar yield in sugarcane achieved values of 20% sucrose on fresh weight (FW) basis, which corresponds to 700 mM sucrose in the stem (Moore 1995). This high concentration of sucrose is probably evenly distributed between vacuole, symplast, and apoplast of the mature stem parenchyma (Hawker 1965; Welbaum and Meinzer 1990) and it is the mechanically strong rind tissue which prevents stem cracking, which sometimes indeed occurs at high rainfall. The sucrose content in the phloem of sugarcane has not been measured so far; however, in maize and barley it has been estimated at 1 M and probably the same concentration may be present in sugarcane (Ohshima et al. 1990; Winter et al. 1992). Thus, there is a possibility to have small concentration gradient between phloem and storage parenchyma which could drive sucrose exit into the storage parenchyma cells even through symplastic connections which bridge the permeability barrier around the bundle sheath cells (Jacobsen et al. 1992). Uphill sucrose transport may not be required for sucrose storage in the stem, but transporters may be useful to enhance the sucrose flow from cell to cell over the apoplast or for retrieval of sucrose. The structure of sugarcane stem plays an essential role in sucrose transport from phloem to the storage parenchyma tissue. The vascular bundles of sugarcane stem are surrounded by a layer of fiber cells that become progressively lignified with development (Rae et al. 2005a). It has been suggested that these layers can prevent and/or impede apoplastic movement of solutes during the period of sucrose accumulation (Casu et al. 2003). A sucrose transporter belonging to the SUT1 group has been isolated from sugarcane (Casu et al. 2003; ElSayed et al. 2010; Rae et al. 2005a), which is preferentially localized in the mature leaves and in the bundle sheath of maturing internodes. Also, an SUT4-group transporter has been identified in the internode (ElSayed et al. 2013). Furthermore, previous studies proposed that SUT1 was greatly discriminating for sucrose, but the gene family of SUTs is not fully understood in sugarcane due to the difficult challenge caused by its high degree of polyploidy and heterozygosity genome (Rae et al. 2005b; Reinders et al. 2006).
Sucrose phosphate synthase II (SPSII) is one of the key enzymes that regulate the sucrose synthesis pathway, which has been widely studied (Doehlret and Huber 1993). Several studies provided strong evidence that SPSII is involved in the regulation of carbohydrate partitioning and in sugar metabolism in general (Galtier et al. 1993; Worrell et al. 1991). Additionally, the parenchyma-based synthesis of sucrose by sucrose-phosphate synthase II (SPSII) will recycle sucrose hydrolysis products from invertase action and indeed SPSII can be considered as a marker for high sucrose storage (Grof et al. 2007).
Sugarcane stalk bagasse consists of two major components; pith and rind. Pith is the inner part of sugarcane stalk bagasse while rind is the outer part of it. Sugar transport in sugarcane from phloem to pith is assumed to involve movement via the apoplast (Glasziou and Gayler 1972). Therefore, the capacity for sugar uptake from free space by pith parenchyma is a vital step. Pith and the pith–rind boundary of the internodes are less intractable, especially in cultivars that contain low lignin. Even though the pith and pith–rind boundary both represent only 24–26% of the internode sugarcane dry mass, they are motivating elements appropriate for direct enzymatic hydrolysis, yielding moderate to high glucose yield (Várnai et al. 2014). Therefore, this study was aimed to analyze the expression of sucrose transporter genes (ShSUT1A and ShSUT4) and SPSII in pith and rind of the sugarcane stem of some commercial cultivars and, for comparison in their leaves. The purpose was to gain insight into physiological conditions in sugarcane that are responsible for the high level of sucrose storage. Four high-yielding commercial cultivars from Hawaii with different demands for soils and climate were selected for these studies to prove whether their high-yielding property can be traced back to a common feature of sucrose synthesis and sucrose transport genetic activity.
Materials and methods
Plant material
Saccharum spp. hybrids (H65-7052, H73-6110, H87-4319, and H87-4094) were grown in test plots within plantation fields in Oahu and Maui, Hawaii, for 12 months. The plants were harvested by hand, the green leaf tops and the stems were separated, weighed and chopped for sugar extraction and determination as described previously (Lehrer et al. 2007). The same cultivars were grown in the greenhouse in pots containing garden soil at Bayreuth University, Germany, at 24 ± 1 °C with a 12 h photoperiod. The uppermost leaf with fully developed dewlap (the top visible dewlap TVD) and the attached internode beneath was numbered as TVD#1. The leaves with TVD#5–10 are fully mature and considered as full source leaves, the leaves with lower numbers (i.e. to the top of the shoot) are considered as sink leaves.
Expression of sucrose transporters and sucrose phosphate synthase studied by RT-PCR
Total RNA from source and sink leaves and from mature internode (TVD#9) of four sugarcane cultivars was isolated and subsequently, cDNA synthesis was performed according to ElSayed et al. (2010). RT-PCR with primers listed in Table 1 was used to determine the expression of ShSUT1A, ShSUTA4, and SPSII in rind and pith tissues of sugarcane internode #9 and in sink and source leaves as well. The PCR program, including the design of primer sets, was optimized using semi-qPCR with different numbers of PCR-cycles.
Real time PCR assay
The quantitative real-time PCR (qRT-PCR) analysis was conducted using the iCycler™ Thermal Cycler (Bio-Rad, USA) by utilizing the iQ™ SYBR Green Supermix (Bio-Rad). The RT-PCR reaction was performed in a final volume of 20 µL according to the manufacturer’s instructions. The iCycler™ program was as follows: 95 °C for 1 min, then 45 cycles at 95 °C for 30 s, 58 °C for 40 s, and 72 °C for 45 s, and then a final extension at 72 °C for 10 min followed by a melting curve program (55–95 °C in increasing steps of 0.5 °C). GAPDH and 25S rRNA genes were used as controls to normalize the qRT-PCR. LinRegPCR software was used to investigate the efficiency of each reaction according to Ruijter et al. (2009). Signal values were subsequently derived from the threshold cycles after subtracting the average signal of the background using the equation of Pfaffl (2001).
In situ hybridization of ShSUT1A transcript in internode section
The in situ hybridization was performed according to Woo et al. (1999) with some modifications as described by ElSayed et al. (2010).
Analysis of sugar and metabolites
Sugar yield of cultivars H65-7052, H73-6110, H87-4319, and H87-4094 was determined from plants in the Hawaiian fields as described by Lehrer et al. (2007). The harvest of these plants was made by hand, the green leaf tops were chopped off and the remaining stalks were collected as stems.
Sucrose analysis
A 20 μL of the extract was heated at 90 °C for 10 min with 1.38 mL KOH (7.5%). From the resulted reaction, 140 μL was incubated with 1 mL anthrone reagent (150 mg anthrone, 76 mL sulfuric acid and 30 mL water) for 20 min at 37 °C. After that 200 μL of the mixture were measured in a microplate reader at 630 nm.
Metabolites were measured in the sink and source leaves of sugarcane cultivar H73-6110, using gas chromatography–mass spectrometry (Supelco, Bellefonte, CA, USA). The collected leaf samples were frozen in liquid nitrogen. Extraction and measurement of the metabolites in the leaf tissue were performed as described by Roessner et al. (2000). Normalized data was calculated as detailed by Roessner et al. (2001).
Statistical analysis
The data were analyzed by analysis of variance (ANOVA) and Student–Newman–Keuls (SNK) test (p < 0.05) using SAS statistical software (SAS Institute, Cary, NC).
Results
Sugar yield and sucrose and starch precursors
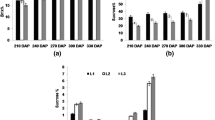
Under the experimental field conditions (which were irrigated dryland plantation fields) the four cultivars had very similar sugar yields per field area (Fig. 1A). Sugar yield is the product of biomass yield and the sugar content in the stem. The sucrose concentration in the harvested stems was also very similar in all four cultivars, although small differences existed, for example, cultivar H87-4094 had the lowest sugar yield despite the highest concentration of sucrose in the stem (Fig. 1A, B). Large differences existed in the sugar concentration in the green leaf top (Fig. 1C). Cultivar H87-4319 showed the highest sucrose concentration in the green leaf top (72.4 µmol/g FW) and H65-7052 and H87-4094 the lowest (Fig. 1C). As expected, the green leaf top had in average only one-third of the sucrose concentration in the stem.
The green leaf top consists of mature source leaves (more downward on the stem) and younger, immature sink leaves (towards the apex of the shoot). Sugar and sugar metabolite concentrations were measured in one cultivar (H73-6110) as an example (Table 2). Sucrose was high in source and sink leaf. However, the precursors of sucrose, namely sucrose-phosphate, hexose-phosphates and UDP-glucose were usually double in the source leaf compared to the sink leaf. Also, the precursor of starch, ADP-glucose, and ATP was higher in source leaves, only inorganic phosphate, which serves among others to drive out triose-phosphates from the chloroplast, was higher in sink leaves (Table 2).
The sugar and metabolite concentrations were also measured in the young, immature stem and in the mature, ripe stem of the sugarcane cultivar H73-6110 as an example (Table 3). Sucrose concentration was six-fold higher in the mature internode (#12) than in the immature internode (#5). In contrast, the glucose and fructose concentrations were 40-fold lower in the mature internode compared to immature internode, where they were at the same level as sucrose. The precursors of sucrose, glucose-6-P, UDP-glucose, glucose-1-P and fructose-6-P concentrations were higher, in average double, in immature internode (#5) compared with the mature internode (#12) (Table 3).
Expression of sucrose transporters and sucrose-phosphate synthase in rind and pith of mature internode
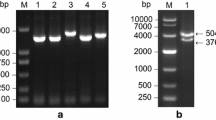
The cross section of the sugarcane stem shows a central pith area with wide parenchyma cells and dispersed bundles and a densely fibrous rind with sometimes incomplete bundles and few thin-walled parenchyma cells (Fig. 2A left). The increasing size of sclerenchyma sheets of the bundles towards and in the rind and the densely packed bundles are very obvious under UV-light, which excites the lignified cell walls (Fig. 2A right). The pith parenchyma is the main place of sugar storage, whereas the rind serves for mechanical stabilization and firmness of the stem. Two sucrose transporter genes had been identified in Hawaiian sugarcane cultivars, ShSUT1A and ShSUT4. ShSUT1A can be localized in cells of the internode bundles (Fig. 2B), no signal with the sense probe (Fig. 2C).
A Cross sections of sugarcane stalk showing the fibrous rind and the soft pith in a fresh overall section (left) and magnified under UV light (right), which elicits the lignified cell walls pictures were taken from latimesblogs.latimes.com (left) and shutterstock.com (right) and modified. B In situ localization of ShSUT1A in sugarcane internode (#9) in cv. H87-4094 (red arrows) using antisense probe. Strong transcript signals were present in parenchymatic cells of phloem (p) and vascular bundles (vb) of internode (mx metaxylem), scale bar = 100 µm. C For comparison in situ localization using sense probe of ShSUT1A in sugarcane internode (#9) of cv. H87-4094 (color figure online)
Transcripts of both transporters ShSUT1A and ShSUT4 were expressed in rind and pith tissue (Fig. 3A), however, there were differences between the four Hawaiian high-yield sugarcane cultivars. Quantitative RT-PCR was performed to measure the transcript amount for ShSUT1A, ShSUT4 and SPSII genes in the cultivars for quantitative comparison (Fig. 3B). ShSUT1A was approximately one quarter less expressed in pith than in rind in three cultivars, whereas in H65-7052, the ShSUT1A amount in the pith was double of that in the rind (Fig. 3B). The amount of ShSUT4 transcripts was approximately the same in all four cultivars, but was clearly much lower in pith than in rind, within average a three-fold higher amount in rind than in pith tissue. The expression of SPSII was different in the four cultivars and in the case of three cultivars higher in rind than in pith (Fig. 3B), whereas it was the same in rind and pith in cultivar H65-7052, which had the lowest SPSII amount of the cultivars anyway.
A Expression patterns of ShSUT1A, ShSUT4 and sucrose phosphate synthase (SPSII) in rind and pith of Hawaiian sugarcane hybrids. 25S rRNA was amplified from the same samples to serve as loading control. M: DNA molecular size markers 50 bp and 1 kb (Fermentas, St. Leon Rot, Germany). B Transcript levels of ShSUT1A, ShSUT4 and SPSII genes in rind and pith tissues for different Hawaiian sugarcane cultivars. Transcript amounts were quantified by qPCR and related to 25S rRNA contents. Data are the means of nine values corresponding to three biological (=RNA extractions) replicates and three technical (=PCR amplifications) replicates per sugarcane cultivar. The bar on top of each column represents the standard error of the mean. The small letters indicate which results are significantly different from each other according to the Duncan´s Multiple Range Tests (p < 0.05). Straight letters compare rind values, italic letters the pith values
Expression of sucrose transporters and sucrose-phosphate synthase in sink and source leaves
Large differences up to four-fold were observed in the ShSUT1A transcript amounts in the four cultivars (Fig. 4A). Two cultivars (H65-7052 and H73-6110) had similar levels in source and sink leaves, although double in H73-6110, whereas H87-4094 had a four-fold higher amount in sink leaves than source leaves and H87-4319 just the inverse (Fig. 4B).
A RT-PCR for sucrose transporters ShSUT1A, ShSUT4 and sucrose phosphate synthase (SPSII) in the sink and source sugarcane leaves. The lower panel shows the transcription of ribosomal RNA (25S rRNA 108 bp) as transcription and amplification control. M: DNA molecular size markers 50 bp and 1 kb (Fermentas, St. Leon Rot, Germany). B Transcript levels of ShSUT1A, ShSUT4 and SPSII genes in sink and source leaves for different Hawaiian sugarcane cultivars. Transcript amounts were quantified by qPCR and related to 25S rRNA contents. Data are the means of nine values corresponding to three biological (=RNA extractions) replicates and three technical (=PCR amplifications) replicates per sugarcane cultivar. The bar on top of each column represents the standard error of the mean. The small letters indicate which results are significantly different from each other according to the Duncan´s Multiple Range Tests (p < 0.05). Straight letters compare sink leaf values, italic letters the source leaf values
Small variances were found between the sugarcane cultivars for the ShSUT4 transcripts in the sink and source leaves (Fig. 4A). In general, the amount in source leaves was about 20% lower than in sink leaves. This was true for all four tested cultivars, although H65-7052 was a bit lower in source leaves than in sink leaves (Fig. 4B).
Relatively large differences were found for the transcript of SPSII between the source and sink leaves and between the cultivars (Fig. 4A). H87-4319 had a high and approximately similar transcript level of SPSII in source and sink leaves, whereas the other three cultivars had an up to three-fold higher level in the source leaves than in sink leaves (Fig. 4B). The SPSII-transcript level in source leaves of two of these three cultivars (H73-6110 and H87-4094) was approximately as high as in source and sink leaves of cultivar H87-4319, whereas it was only half of that in source leaves of cultivar H65-7052 and less in sink leaves of that cultivar (Fig. 4B).
Discussion
The importance of sucrose synthesis and sucrose transport is obvious in sugarcane, where sucrose is not only the transported sugar in the phloem but also the storage compound in the stem. The key enzyme for sucrose synthesis is sucrose-phosphate synthase, whose activity correlates with the sucrose content in sugarcane leaves of different cultivars (Grof et al. 1998) and is well-thought-out to be a major target for cumulative sucrose biosynthesis in the leaves of crop plants (Hoffmann-Thoma et al. 1996). Sucrose synthesis is rate limited depends on SPSII activity and is strongly regulated by light and metabolites (Huber and Huber 1996), also in some C4 species (Ohsugi and Huber 1987) including sugarcane (Köhler et al. 1988). Since SPSII-activity is highly regulated downstream of the transcript level by several factors including metabolites (reviewed in Huber and Huber 1996), the small expression differences of SPSII between the sink and source leaves cannot be further interpreted. Besides SPSII vacuolar (acid) invertases have been described to show an important role in regulating sucrose fluxes in sink tissues and their activities have been linked to sucrose accumulation in the sugarcane stem (Zhu et al. 1997). The difference between SPSII-activity and soluble acid invertase activity correlates best with sugar yield of cultivars, not SPSII alone (Lingle and Tew 2008; Zhu et al. 1997). The metabolites that act as an ancestors for sucrose and starch biosynthesis are lower in mature internode (#12) and young leaves than in the mature leaves and immature internode (#5) (Tables 2, 3), which may be of significance for a higher sucrose and/or starch biosynthesis rate. In mature leaves, where assimilates are backed up, these metabolites improved, possibly by reversal of sucrose synthase activity in direction of UDP-glucose. In addition, the hexose-phosphate level is higher in source leaves than in sink leaves and the inorganic phosphate level is lower (Table 2), a situation which favors sucrose synthesis by SPSII in source leaves, because hexose-phosphate activates, SPSII-activity and inorganic phosphate inhibit SPSII-activity. In addition, the higher sucrose-phosphate-precursor levels (UDP-glucose and hexose-phosphates) are higher in source leaves than in sink leaves. SPSII is also crucial for sucrose synthesis and sucrose cycling in the maturing stem, although no qualitative difference for SPSII expression in internodes was found between the four Hawaiian cultivars (Fig. 3).
The role of sucrose transporters in phloem loading is well understood, with the recovery of sucrose along the transport path in the stem. The localization of ShSUT1A in phloem is, consequently, no exception; however, its role in storing sucrose in the parenchyma is less understood (ElSayed et al. 2010). Sucrose transporters transcripts were found in all tested tissues, source leaves, sink leaves and internode rind and pith tissue. Sucrose transporters exhibited high expression levels in the sink leaves and the source leaves, suggesting SUTs may play an important role in leading sucrose in sink and source as well before sucrose accumulation (Zhang et al. 2016). So far two sucrose transporters were found in sugarcane ShSUT1A and ShSUT4 (Casu et al. 2003; ElSayed et al. 2013). ShSUT1A was localized mostly in the bundle sheath and a possible role for this transporter in the sucrose partitioning between bundle and storage parenchyma was postulated (Rae et al. 2005b), but ShSUT1A is also present in cells within the bundles in the phloem of internodes (Fig. 2B), thus a role in phloem loading and sucrose recovery also appears possible. SUT4 plays a role for transporting sucrose from mesophyll vacuoles to their cytoplasm in rice (Aoki et al. 2003) and Arabidopsis (Schulz et al. 2011) and it has been shown to be more important at early developmental stage than in mature plants (Zhang et al. 2016). In the current study, SUT4 was relatively equal in source and sink in all four tested cultivars and might be characteristic for sucrose accumulation in sugarcane, in contrast to ShSUT1A.
Sucrose storage in maturing internodes becomes increasingly symplastic in parallel with the maturation development, in which a lignified barrier is established around the bundle sheath, averting the apoplastic transfer from phloem to storage parenchyma (Jacobsen et al. 1992; Rae et al. 2005b). ShSUT4 was more expressed in the rind part of the stem than in the pith in all four cultivars, whereas ShSUT1A was more expressed in the rind in two sugarcane cultivars (H87-4319 and H73-6110). The expression of SPSII and of sucrose transporters in the rind is interesting because microarray studies and QTL-analysis have shown that high sucrose yield did not correlate with sucrose metabolism enzymes but with enzymes of lignin and cell wall synthesis (Casu et al. 2004; Mcintyre et al. 2006; Papini-Terzi et al. 2009), which are especially strong in the rind of the sugarcane stem. It is already known that the mechanical stability of the rind is crucial to prevent stem cracking, which may occur because of the very high sucrose concentration in the apoplast (Hawker 1965; Welbaum and Meinzer 1990). In addition, the rind serves as a protection against insect pests (White et al. 2006). Therefore, properties of the rind will make the difference between low-yield and high-yield cultivars, in case that all cultivars are relatively similar in their synthesis and storage capacity of sucrose.
Previous studies have compared low and high-yield cultivars and no significant correlation was found between yield and sucrose synthesis enzymes (Casu et al. 2004; Mcintyre et al. 2006; Papini-Terzi et al. 2009). Our studies took the opposite approach and compared four high-yield cultivars for differences in transcripts for sucrose synthesis enzyme and transporters. Here large differences were indeed found, although the yields were the same (Fig. 1). Thus the sugar yield may be more determined by fluxes of sucrose in the phloem and in the storage parenchyma (Komor et al. 1996; Rohwer and Botha 2001) and by properties of the rind, which is well equipped with SPSII and sucrose transporters.
Previous studies have compared low and high-yield cultivars and no significant correlation was found between yield and sucrose synthesis enzymes (Casu et al. 2004; Mcintyre et al. 2006; Papini-Terzi et al. 2009). Our studies took the opposite approach and compared four high-yield cultivars for differences in transcripts for sucrose synthesis enzyme and transporters. Here large differences were indeed found, although the yields were the same (Fig. 1), only shSUT4, which is supposed to be important in early development, expressed the similar transcripts levels in all cultivars. Thus the sugar yield may be more determined by fluxes of sucrose in the phloem and in the storage parenchyma (Komor et al. 1996; Rohwer and Botha 2001). The emphasis should be given to the properties of the rind, which is well equipped with SPSII and sucrose transporters and which may be very significant for high yield cultivars.
References
Aoki N, Hirose T, Scofield GN, Whitfeld PR, Furbank RT (2003) The sucrose transporter gene family in rice. Plant Cell Physiol 44:223–232
Casu RE, Grof CP, Rae AL, Mcintyre CL, Dimmock CM, Manners JM (2003) Identification of a novel sugar transporter homologue strongly expressed in maturing stem vascular tissues of sugarcane by expressed sequence tag and microarray analysis. Plant Mol Biol 52:371–386
Casu RE, Dimmock CM, Chapman SC, Grof CP, Mcintyre CL, Bonnett GD, Manners JM (2004) Identification of differentially expressed transcripts from maturing stem of sugarcane by in silico analysis of stem expressed sequence tags and gene expression profiling. Plant Mol Biol 54:503–517
Cheavegatti-Gianotto A et al (2011) Sugarcane (Saccharum X officinarum): a reference study for the regulation of genetically modified cultivars in Brazil. Trop Plant Biol 4:62–89
Doehlret DC, Huber SC (1993) Regulation of spinach leaf sucrose phosphate synthase by glucose-6 phosphate, inorganic phosphate and pH. Plant Physiol 73:989–994
ElSayed AI, Ramadan MF, Komor E (2010) Expression of sucrose transporter (ShSUT1) in a Hawaiian sugarcane cultivar infected with Sugarcane yellow leaf virus (SCYLV). Physiol Mol Plant Pathol 75:56–63
ElSayed AI, Weig A, Sariyeva G, Hummel E, Shih-Long Y, Bertolini A, Komor E (2013) Assimilate export inhibition in Sugarcane yellow leaf virus-infected sugarcane is not due to less transcripts for sucrose transporters and sucrose-phosphate synthase or to callose deposition in sieve plates. Physiol Mol Plant Pathol 81:64–73
Galtier N, Foyer CH, Huber J, Volker TA, Huber SC (1993) Effects of elevated sucrose phosphate synthase activity on photosynthesis, assimilate partitioning and growth in tomato (Lycopersicum esculentum var. UC82B). Plant Physiol 101:535–543
Glasziou KT, Gayler KR (1972) Storage of sugars in stalks of sugar cane. Bot Rev 38:471–490
Grof CP, Knight DP, Mcneil SD, Lunn JE, Campbell JA (1998) A modified assay method shows leaf sucrose-phosphate synthase activity is correlated with leaf sucrose content across a range of sugarcane varieties. Aust J Plant Physiol 25:499–502
Grof CP, Albertson PL, Bursle J, Perroux JM, Bonnett GD, Manners JM (2007) Sucrose-phosphate synthase, a biochemical marker of high sucrose accumulation in sugarcane. Crop Sci 47:1530–1539
Hawker JS (1965) The sugar content of cell walls and intercellular spaces in sugarcane stems and its relation to sugar transport. Aust J Biol Sci 18:959–969
Hoffmann-Thoma G, Hinkel K, Nicolay P, Willenbrink J (1996) Sucrose accumulation in sweet sorghum stem internodes in relation to growth. Physiol Plant 97:277–284
Huber SC, Huber JL (1996) Role and regulation of sucrose-phosphate synthase in higher plants. Annu Rev Plant Physiol Plant Mol Biol 47:431–444
Jacobsen KR, Fisher DG, Maretzki A, Moore PH (1992) Developmental changes in the anatomy of the sugarcane stem in relation to phloem unloading and sucrose storage. Bot Acta 105:70–80
Köhler J, Komor E, Thom M, Maretzki A (1988) Activity of sucrose-phosphate synthase in sugar cane leaves. Phytochemistry 27:1605–1608
Komor E, Zingsheim O, Sprügel H (1996) Cycles of sugar transport and sucrose metabolism in sugarcane tissue: quantitative determination. In: Wilson JRHD, Campbell JA, Garride AL (eds) Sugarcane: research towards efficient and sustainable production. CSIRO, Brisbane, pp 89–91
Lehrer AT, Moore PH, Komor E (2007) Impact of sugarcane yellow leaf virus (ScYLV) on the carbohydrate status of sugarcane: comparison of virus-free plants with symptomatic and asymptomatic virus-infected plants. Physiol Mol Plant Pathol 70:180–188
Lingle SE, Tew TL (2008) A comparison of growth and sucrose metabolism in sugarcane germplasm from Louisiana and Hawaii. Crop Sci 48:1155–1163
Mcintyre CL et al (2006) The identification and characterisation of alleles of sucrosephosphate synthase gene family III in sugarcane. Mol Breed 18:39–50
Moore PH (1995) Temporal and spatial regulation of sucrose accumulation in the sugarcane stem. Aust J Plant Physiol 22:661–679
Ohshima T, Hayashi H, Chino M (1990) Collection and chemical composition of pure phloem sap from Zea mays L. Plant Cell Physiol 31:735–737
Ohsugi R, Huber SC (1987) Light modulation and localization of sucrose phosphate synthase activity between mesophyll cells and bundle sheath cells in C4 species. Plant Physiol 84:1096–1101
Papini-Terzi FS et al (2009) Sugarcane genes associated with sucrose content. BMC Genom 10:1–21
Pfaffl MW (2001) A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res 29:e45
Rae AL, Grof CP, Casu RE, Bonnett GD (2005a) Sucrose accumulation in the sugarcane stem: pathways and control points for transport and compartmentation. Field Crops Res 92:159–168
Rae AL, Perroux JM, Grof CP (2005b) Sucrose partitioning between vascular bundles and storage parenchyma in the sugarcane stem: a potential role for the ShSUT1 sucrose transporter. Planta 220:817–825. doi:10.1007/s00425-004-1399-y
Reinders A, Sivitz AB, Hsi A, Grof CPL, Perroux JM, Ward JM (2006) Sugarcane ShSUT1: analysis of sucrose transport activity and inhibition by sucralose. Plant Cell Environ 29:1871–1880
Roessner U, Wagner C, Kopka J, Trethewey R, Willmitzer L (2000) Simultaneous analysis of metabolites in potato tuber by gas chromatography–mass spectrometry. Plant J 23:131–142
Roessner U, Luedemann A, Brust D, Fiehn O, Linke T, Willmitzer L, Fernie A (2001) Metabolic profiling and phenotyping of genetically and environmentally modified plant systems. Plant Cell Environ 13:11–29
Rohwer JM, Botha FC (2001) Analysis of sucrose accumulation in the sugarcane culm on the basis of in vitro kinetic data. Biochem J 358:437–445
Ruijter JM, Ramakers C, Hoogaars W, Bakker O, van den Hoff MJB, Karlen Y, Moorman AFM (2009) Amplification efficiency: linking baseline and bias in the analysis of quantitative PCR data. Nucleic Acids Res 37:e45
Schulz A et al (2011) Proton-driven sucrose symport and antiport are provided by the vacuolar transporters SUC4 and TMT1/2. Plant J 68:129–136
Várnai A, Costa TH, Faulds CB, Milagres AM, Siika-Aho M, Ferraz A (2014) Effects of enzymatic removal of plant cell wall acylation (acetylation, p-coumaroylation, and feruloylation) on accessibility of cellulose and xylan in natural (non-pretreated) sugar cane fractions. Biotechnol Biofuels 7:153
Welbaum GE, Meinzer FC (1990) Compartmentation of solutes and water in developing sugarcane stalk tissue. Plant Physiol Biochem 93:1147–1153
White WH, Tew TL, Richard EP (2006) Association of sugarcane pith, rind hardness, and fiber with resistance to the sugarcane borer. J Am Soc Sugar Cane Technol 26:87–100
Winter H, Lohaus G, Heldt HW (1992) Phloem transport of amino acids in relation to their cytosolic levels in barley leaves. Plant Physiol 99:996–1004
Woo H-H, Orbach M, Hirsch A-M, Hawes MC (1999) Meristem-localized inducible expression of a UDP-glycosyltransferase gene is essential for growth and development in pea and alfalfa. Plant Cell 11:2303–2316
Worrell AC, Bruneau JM, Summerfelt K, Boersig M, Voelker TA (1991) Expression of a maize sucrose-phosphate synthase in tomatoes alters leaf carbohydrate partitioning. Plant Cell 3:1121–1130
Zhang Q, Hu W, Zhu F, Wang L, Yu Q, Ming R, Zhang J (2016) Structure, phylogeny, allelic haplotypes and expression of sucrose transporter gene families in Saccharum. BMC Genom 17:88. doi:10.1186/s12864-016-2419-6
Zhu YJ, Komor E, Moore PH (1997) Sucrose accumulation in the sugarcane stem is regulated by the difference between the activities of soluble acid invertase and sucrose phosphate synthase. Plant Physiol 115:609–616
Acknowledgements
Dr. ElSayed and Dr. Ebrahim thank the DAAD for a visiting scholarship, the research by Dr. Lehrer was supported by DFG.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
ElSayed, A.I., Lehrer, A., Ebrahim, M. et al. Assessment of sucrose transporters, metabolites and sucrose phosphate synthase in different sugarcane tissues. Physiol Mol Biol Plants 23, 703–712 (2017). https://doi.org/10.1007/s12298-017-0454-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12298-017-0454-7