Abstract
24-Epibrassinolide (EBL) and/or spermine (Spm) applications regulate photosynthetic process and hormonal balance in drought-stressed plants. A pot experiment was conducted to investigate the potential effects of 25 mg l−1 EBL and/or 0.1 mg l−1 Spm applied to maize (Zea mays L.; hybrid Giza 129) exposed to water deficiency (50 and 75% field capacity). Plastic pots were planted with maize plants and designed in a complete randomized design with four replications. Drought significantly impaired photosynthetic pigments content, photochemical reactions of photosynthesis, net photosynthetic rate, transpiration rate, stomatal conductance, maximum quantum efficiency of PSII photochemistry, electron transport rate, actual photochemical efficiency of PSII, photochemical quenching coefficient, effective quantum yield of PSII photochemistry, activities of Rubisco, Rubisco activase, and carbonic anhydrase, seeds carbohydrate content as well as concentrations of auxins, cytokinins, and gibberellins. These changes were significantly modulated in drought-affected plants after EBL and Spm combined application. Moreover, this combined treatment under water shortage conditions inhibited the increased concentrations of intercellular CO2, non-photochemical quenching coefficients, and abscisic acid as well as diminished the enhanced activity of glycolate oxidase. These results reinforce the utility of this combined treatment not only in improving the photosynthetic capability but also in regulating the hormonal homeostasis as a powerful strategy to enhance the plant drought tolerance. Indeed, exogenous application of 25 mg l−1 Spm + 0.1 mg l−1 EBL can preserve the photosynthetic apparatus activity under water deficiency.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Drought is a major environmental stress factor negatively affecting the global agricultural productivity. It is a major bottleneck that restrains sustainable development of agriculture worldwide. About one third of the world’s arable land suffers from this harsh condition (Ribaut et al. 2012). It alters a series of physiological, biochemical, and molecular responses (Farooq et al. 2009). Photosynthesis is among the most severely affected processes during water stress. The photosynthetic impairment during water deficiency is mainly caused by decreases in total chlorophyll contents, distortion in chlorophyll ultra structures, inhibition in photosystem II (PSII) activity, changes in chlorophyll fluorescence and gas exchange traits, as well as inhibition in Rubisco activation (Zhang et al. 2015; Anjum et al. 2016; Gleason et al. 2017). Under drought conditions, reactive oxygen species (ROS) production was induced by inhibition in CO2 fixation that is accompanied by over-reduction in the electron transport chain. Generation of ROS can cause photo-oxidation, induce membrane structure degradation, and disrupt thylakoid membrane organization (Miller et al. 2010; Talaat 2019a).
Plants respond to water deficiency by activating different signaling pathways, which assist plants in adjusting their metabolism to maintain a defensive regime. The synthesis and accumulation of key osmolytes such as soluble sugars in plant tissues can provide a symptom of the degree of tolerance to water stress (Talaat et al. 2015; Talaat and Shawky 2016). Soluble sugars regulate gene expression as well as provide carbon skeletons, membrane stability, and osmotic adjustment (Sun et al. 2016) and their accumulation are often regarded as basic strategy for the protection and survival of plants under drought conditions (Talaat 2019b). Furthermore, major endogenous phytohormones, such as auxin, abscisic acid, cytokinin, and gibberellic acid, also play crucial roles in plant adaptation to water deficiency conditions (Shakirova et al. 2016). In this respect, abscisic acid protects photosynthesis under water stress by regulating stress-response genes expression (Fan et al. 2016) and by promoting stomatal closure via multiple cascades of cellular-biochemical events (Divi et al. 2010). Cytokinins also protect the photosynthetic machinery via inhibiting the negative impact of stress condition on both the chlorophyll concentration and the photochemical efficiency (Chernyadev 2009).
Brassinosteroids (BRs) as plant-specific steroidal hormones can promote plant growth and induce plant stress tolerance (Divi and Krishna 2009; Talaat and Shawky 2012, 2013; Todorova et al. 2016; Dong et al. 2017) by involving in a complex signaling network via modulating other hormones levels and sensitivity (Divi et al. 2010). The exogenous application of 24-epibrassinolide, an active form of BRs, ameliorated a drought-induced inhibition in the photosynthetic efficiency, mainly owing to an alter in the photosynthetic activity of chloroplast, photochemical reactions of photosynthesis, photosynthetic pigments content, chlorophyll fluorescence attributes, gas exchange parameters, Rubisco and Rubisco activase activities, osmoprotectants accumulation, and endogenous phytohormones production (Hu et al. 2013; Shakirova et al. 2016; Gill et al. 2017; Zhao et al. 2017). Indeed, the mechanism underlying BR-enhanced photosynthesis under drought conditions is still poorly understood. Accordingly, studying this mechanism is a critical issue to improve plant drought tolerance.
Polyamines (PAs) are nitrogenous growth regulators that play critical roles in a range of developmental and physiological processes and can enhance plant stress tolerance (Todorova et al. 2016) mainly by protecting LHC-II proteins in photosynthetic apparatus (Hamdani et al. 2011), by scavenging free radical and modulating certain ion channels activity (Todorova et al. 2016), as well as by inducing cross-talk with other phytohormones (Li et al. 2016). All PAs forms can ameliorate the detrimental effects of water stress; however, spermine (Spm), a tetramine form of PAs, is more effective (Farooq et al. 2009). Spm enhanced plant water stress tolerance and regulated photosynthetic ability through retention of chlorophyll, enhancing gas exchange characteristic, scavenging free radicals, and altering endogenous phytohormones balance (Huang et al. 2014; Li et al. 2016; Todorova et al. 2016). However, few reports are focused on the role played by Spm on the photosynthetic activity in response to water deficit.
Maize is one of the most important cereal crops worldwide (Zhang et al. 2018). It is a highly sensitive species to water deficit, especially when plants exposed to water deprivation at the flowering time. Moreover, water shortage at the early stage of plant growth may disturb the photosynthetic efficiency (Ribaut et al. 2012). This investigation interests in studying the negative effects of water deficiency on the plant photosynthetic capability. Furthermore, considering the importance of BRs and PAs, this approach is an attempt to study the impact of 24-epibrassinolide (EBL) and/or Spm on photosynthetic efficiency and phytohormonal status under water-stressed conditions. Indeed, no studies have been undertaken to unravel the potential of EBL and Spm combined application in sustaining photosynthesis under drought conditions. Therefore, the present study, as a first approach, was designed to investigate the effect of this combined application on maize performance in water-limited environments. It was hypothesized that this application may alleviate drought-induced irreversible harm to plant photosynthetic system. To investigate this, changes in photosynthetic pigments concentration, gas exchange parameters, photochemical reactions activity, chlorophyll fluorescence system, Rubisco, Rubisco activase, carbonic anhydrase and glycolate oxidase activities, total soluble sugars concentration, seeds carbohydrate content, as well as endogenous phytohormones concentration were examined under the influence of EBL and/or Spm exogenous treatments in maize plants subjected or not to water deficit conditions.
2 Materials and Methods
2.1 Experimental Design, Plant Material, Treatments, and Growth Conditions
Pot experiment was conducted on June 3 of 2013 and 2014 in the greenhouse of the Department of Plant Physiology, Faculty of Agriculture, Cairo University, Egypt. Twelve experimental treatments were used (three soil water levels × four spraying treatments) and they were arranged in a complete randomized design with four replicates.
One month after sowing, the plants that were exposed to three soil water conditions [well-watered (100% field capacity; WW) and drought stress (75 and 50% field capacity; WD1 and WD2)] were used. Soil water contents (SWC) were 15.5, 11.6, and 7.7%, respectively, which were calculated as: SWC % = [(FW − DW)/DW] × 100 (Coombs et al. 1987).
Plants at 60 days old from each water stress treatment were sprayed with 0.00 (double-distilled water; DDW), 0.1 mg l−1 EBL, 25 mg l−1 Spm, and 25 mg l−1 Spm + 0.1 mg l−1 EBL. EBL (C28H48O6, MW = 480.7) and Spm (C10H26N4, MW = 202.3) were purchased from Sigma (USA). Tween 20 (0.05%) was added as surfactant at the time of treatment. Preliminary screening was performed for various concentrations of EBL and Spm to obtain the optimum responses and the concentrations of 0.1 mg l−1 EBL and 25 mg l−1 Spm were selected (Talaat et al. 2015; Talaat and Shawky 2016). The spraying was done when the maize plants had 6–8 leaves fully developed (during pre-female inflorescence emerging stage).
Maize (Zea mays L.; hybrid Giza 129) seeds were obtained from the Agriculture Research Center, Ministry of Agriculture, Egypt. Hybrid Giza 129 was selected based on its high yield productivity and I tried to increase its drought tolerance by using EBL and/or Spm foliar application. Thirty-centimeter diameter plastic pots that were 35 cm deep were filled with 15 kg clay loamy soil (sand 37%, silt 28%, clay 35%) and were supplemented with ammonium nitrate (33.5% N), calcium superphosphate (15.5% P2O5), and potassium sulfate (48% K2O) at the rate of 2.0, 2.0, and 0.5 g pot−1, respectively. In addition, 2.0 g pot−1 ammonium nitrate was added 30 days after planting. Soil chemical analysis was carried out following the procedures of Cottenie et al. (1982) and presented in Table 1. Four seeds were sown in each pot, and two uniform seedlings were kept after the seedlings reached the first true leaf stage. Plants were regularly watered to maintain optimum soil moisture until stress imposition.
The plants were sampled after 25 days of EBL and Spm foliar application to assess the following physiological and biochemical parameters. At maturity, seeds were collected, and extraction and determination of total carbohydrate content in maize seeds were carried out.
2.2 Photosynthetic Pigments Measurement
Concentrations of chlorophyll a, chlorophyll b, and carotenoids in fresh leaves were determined after extraction with 80% (v/v) acetone and the absorbance was measured at 663, 645, and 470 nm, respectively, using a UV–vis spectrophotometer. The amount of chlorophylls and carotenoids was estimated by the equations of Lichtenthaler (1987).
2.3 Chloroplast Isolation and Measurement of Photosynthetic Photochemical Reactions Activity
The leaves’ chloroplast was isolated with the method described by Cerovic and Plesnicar (1984). PSII-mediated electron transport from H2O to p-benzoquinone (pBQ) was determined according to the method described by (Tiwari et al. 1997). PSI-mediated electron transport was measured in terms of oxygen consumption using 2,6-dichlorophenol indophenols (DCPIP) as electron donor and methyl viologen (MV) as final acceptor.
2.4 Gas Exchange Measurements
Leaf gas exchange parameters were analyzed at 8:30–11:30 am in the greenhouse using a LI-COR-6400 Portable Photosynthesis System (LI-COR Inc., Lincoln, NE, USA) on three fully expanded intermediate leaves in four plants of each treatment. Irradiance level was set at 800 μmol photons m−2 s−1. Air temperature, air relative humidity, and CO2 concentration were set at ambient conditions in the greenhouse. The net photosynthetic rate (Pn, μmol CO2 m−2 s−1), stomatal conductance (Gs, mol H2O m−2 s−1), transpiration rate (Tr, mmol H2O m−2 s−1), and intercellular CO2 concentration (Ci, μmol CO2 mol air−1) of maize (Zea mays L.) leaves were measured.
2.5 Chlorophyll Fluorescence Analysis
Chlorophyll fluorescence measurements were determined following the procedure described by Lu et al. (2003) on the same leaves as those used for the gas exchange attributes. Chlorophyll fluorescence in dark- and light-adapted leaves was excited and measured. Minimal (F0), maximal (Fm) fluorescence yields and maximum quantum efficiency of PSII photochemistry (Fv/Fm) was determined after a 30-min dark acclimation of selected leaves, and Fv/Fm was calculated as (Fm − F0)/Fm. The steady-state fluorescence yield (Fs) in light-adapted leaves, the maximum chlorophyll fluorescence level (Fm′), and the minimal fluorescence level in light-adapted state (F0′) were determined and the actual photochemical efficiency of PSII (ΦPSII) was calculated as (Fm′ − Fs)/Fm′. Photochemical quenching coefficient (qP) was calculated as (Fm′ − Fs)/(Fm′ − F0′). Electron transport rate was calculated as ETR = (Fm′ − Fs)/Fm′ × PPFD × 0.5 × 0.84, where PPFD is photosynthetic photon flux density incident on the leaf, 0.5 as the factor that assumes equal distribution of energy between the two photosystems, and 0.84 as the factor for leaf absorbance. Effective quantum yield of PSII photochemistry was calculated as (Fv′/Fm') = (Fm′ − F0′)/Fm′. Using the fluorescence data obtained with the same dark-adapted and steady-state-illuminated leaves, non-photochemical quenching coefficients (qN) were calculated as qN = 1 − (Fm′ − F0′)/(Fm − F0).
2.6 Assay of Rubisco and Rubisco Activase Activity
Estimation of Rubisco activity was done as described by Jiang et al. (2012). The Rubisco activation state (%) was calculated using the ratio initial activity/total activity. The Rubisco activase (RCA) activity was estimated using a Rubisco Activase Assay Kit (Genmed Scientifics Inc., Wilmington, DE, USA).
2.7 Assay of Carbonic Anhydrase and Glycolate Oxidase Activities
Carbonic anhydrase (CA; EC 4.2.1.1) activity was assayed using the method of Dwivedi and Randhawa (1974). To fresh leaf tissues, cysteine hydrochloride solution was added and samples were incubated at 4 °C for 20 min. Then phosphate buffer (pH 6.8), alkaline bicarbonate solution, and bromothymol blue were added to the leaf tissues and they were incubated at 5 °C for 20 min. Finally, titration was done against 0.05 N HCl.
Glycolate oxidase (GO; EC 1.1.3.15) activity was determined by following glyoxylate phenylhydrazone formation at 324 nm for 3 min after an initial lag phase of 1 min as described by Blasco et al. (2010). For this, fresh leaf tissues were extracted by PVPP and 1 ml of 50 mM Tris-HCl buffer (pH 7.8) with 0.01% Triton X-100 and 5 mM dithiothreitol (DTT) were added and then centrifuged at 30,000×g for 20 min. The GO assay was performed in a reaction medium containing 50 μl plant extract, 50 mM Tris-HCl buffer (pH 7.8), 3.3 mM phenylhydrazine HCl (pH 6.8), 0.009% Triton X-100, and 5 mM glycolic acid. Protein concentration was quantified according to Lowry et al. (1951).
2.8 Determination of Total Soluble Sugars Concentration
Total soluble sugars were estimated by the anthrone reagents method (Irigoyen et al. 1992). Five milliliters anthrone sulfuric acid solution (75% v/v) was added to 0.1 ml supernatant. The mixture was boiled in 90 °C for 15 min, after it was refrigerated in a cool water bath (0 °C). The absorbance was read at 620 nm, using a spectrophotometer and compared with the calibration curve, using pure glucose (Sigma).
2.9 Estimation of Seeds Carbohydrate Content
Extraction and determination of total carbohydrate content in dried ground maize seeds were carried out according to Yih and Clark (1965) and Dubois et al. (1956). Dried ground seeds was extracted with 1.5 N H2SO4 and then centrifuged at 4000×g for 10 min. For 1 ml of the extract, 1 ml of 5% distilled phenol was added and absorbance was taken at 490 nm using spectrophotometer.
2.10 Extraction and GC-MS Analysis of Hormones
Method for auxins, cytokinins, gibberellins, and abscisic acid determination according to Nehela et al. (2016) was applied with minor modifications. One hundred milligrams of dried ground leaves was extracted with 2 ml of ice-cold extraction solvent (methanol/water/HCl (6 N); 80/19.9/0.1; v/v/v). The extract was centrifuged at 25,000×g for 5 min at 4 °C and then the supernatant was collected and used in phytohormones determination.
2.11 Statistical Analysis
Experimental data were statistically analyzed according to complete randomized design, with a two-way factorial arrangement (Snedecor and Cochran 1980). Combined analysis was made for the two growing seasons, since their results followed a similar trend. All values are expressed as mean ± standard error of mean (SE) of four replicates. The analysis of variance (ANOVA) was performed using SAS software (SAS Institute, Cary NC). Differences between treatments were separated by the least significant difference (LSD) test at a 0.05 probability level.
3 Results
Photosynthetic pigments were significantly (p < 0.05) impaired under water-limited environments. However, EBL and/or Spm exogenous treatments ameliorated the decomposition of chlorophyll a, chlorophyll b, and carotenoids under this harsh condition (Fig. 1). EBL and Spm combined application significantly (p < 0.05) elevated the content of chlorophyll a by 30.6, 40.6, and 50.0%, that of chlorophyll b by 38.0, 50.8, and 94.4%, that of carotenoids by 41.2, 61.5, and 125.0%, and that of total pigments by 34.2, 45.8, and 68.3% under normal (WW) and drought conditions (WD1 and WD2), respectively, compared with control (double-distilled water) treatment.
Changes of chlorophyll a, chlorophyll b, carotenoids, and total pigments content in leaves of maize plants treated with [control (DDW), 25 mg l−1 Spm, 0.1 mg l−1 EBL, and 25 mg l−1 Spm + 0.1 mg l−1 EBL] under normal (WW) and drought stress conditions (WD1 and WD2). Values are the mean ± SE (n = 4), and asterisks indicate significant differences (p < 0.05) compared with the control (sprayed with DDW) plants
Water deficiency as well as EBL and/or Spm treatments had strong impacts on the photochemical reactions of photosynthesis (Fig. 2). PSI and PSII activities were significantly (p < 0.05) decreased by water stress treatments. Indeed, they showed 30.6 and 59.7% inhibition, respectively, under drought condition at 50% of field capacity (WD2). However, EBL and/or Spm applications considerably restored photosynthetic electron transport under water-stressed conditions. EBL and Spm combined application significantly (p < 0.05) increased PSI activity by 9.4, 18.9, and 39.0%, and that of PSII activity by 17.5, 52.5, and 126.1% under normal (WW) and drought conditions (WD1 and WD2), respectively, compared to control (double-distilled water) treatment.
Changes of PSI and PSII electron transport activities (μmol O2 mg−1 Chl h−1) in leaves of maize plants treated with [control (DDW), 25 mg l−1 Spm, 0.1 mg l−1 EBL, and 25 mg l−1 Spm + 0.1 mg l−1 EBL] under normal (WW) and drought stress conditions (WD1 and WD2). Values are the mean ± SE (n = 4), and asterisks indicate significant differences (p < 0.05) compared with the control (sprayed with DDW) plants
Water deficit conditions considerably hampered the gas exchange attributes. It significantly (p < 0.05) decreased the net photosynthetic rate, transpiration rate, and stomatal conductance, while it increased the intercellular CO2 concentration (Fig. 3). EBL and/or Spm applications overcame the decline in the net photosynthetic rate, transpiration rate, and stomatal conductance traits and enhanced their values in water-stressed plants. EBL and Spm combined application significantly (p < 0.05) improved the net photosynthetic rate by 37.7, 45.0, and 86.1%, that of stomatal conductance by 57.1, 86.4, and 121.7%, and that of the transpiration rate by 45.7, 62.0, and 104.2% compared to values of control plants under normal (WW) and drought conditions (WD1 and WD2), respectively. However, combined application significantly (p < 0.05) decreased the intercellular CO2 concentration value by 28.2, 34.8, and 40.9% under normal (WW) and drought conditions (WD1 and WD2), respectively, in comparison to control treatment.
Changes of gas exchange parameters [net photosynthetic rate (Pn, μmol CO2 m−2 s−1), stomatal conductance (Gs, mol H2O m−2 s−1), transpiration rate (Tr, mmol H2O m−2 s−1), and intercellular CO2 concentration (Ci, μmol CO2 mol−1)] in leaves of maize plants treated with [control (DDW), 25 mg l−1 Spm, 0.1 mg l−1 EBL, and 25 mg l−1 Spm + 0.1 mg l−1 EBL] under normal (WW) and drought stress conditions (WD1 and WD2). Values are the mean ± SE (n = 4), and asterisks indicate significant differences (p < 0.05) compared with the control (sprayed with DDW) plants
Upon water stress, chlorophyll fluorescence parameters were altered. The maximum quantum efficiency of PSII photochemistry, the actual photochemical efficiency of PSII, the effective quantum yield of PSII photochemistry, the electron transport rate, and the photochemical quenching coefficient values of water-stressed plants were significantly (p < 0.05) decreased compared to that of un-stressed ones (Fig. 4). In contrast, EBL and/or Spm exogenous treatments mitigated the deleterious effect of drought and induced increases in their values. The non-photochemical quenching coefficients value was significantly (p < 0.05) raised by drought, while it decreased by these exogenous treatments. EBL and Spm combined application alleviated the detrimental impact of water deficiency and significantly (p < 0.05) enhanced the maximum quantum efficiency of PSII photochemistry by 21.0, 43.1, and 113.9%, that of the actual photochemical efficiency of PSII by 20.0, 57.1, and 111.8%, that of the effective quantum yield of PSII photochemistry by 18.9, 42.5, and 92.0%, that of the electron transport rate by 19.0, 40.8, and 92.3%, and that of the photochemical quenching coefficient by 16.1, 32.0, and 103.3% compared to values of control plants under normal (WW) and drought conditions (WD1 and WD2), respectively.
Changes of chlorophyll fluorescence attributes [maximum quantum efficiency of PSII photochemistry (Fv/Fm), actual photochemical efficiency of PSII (ΦPSII), effective quantum yield of PSII photochemistry (Fv′/Fm′), electron transport rate (ETR), photochemical quenching coefficient (qP), and non-photochemical quenching coefficients (qN)] in leaves of maize plants treated with [control (DDW), 25 mg l−1 Spm, 0.1 mg l−1 EBL, and 25 mg l−1 Spm + 0.1 mg l−1 EBL] under normal (WW) and drought stress conditions (WD1 and WD2). Values are the mean ± SE (n = 4), and asterisks indicate significant differences (p < 0.05) compared with the control (sprayed with DDW) plants
The total and initial Rubisco activity and the RCA activity were assayed to investigate the mechanism by which EBL and/or Spm exogenous applications regulates CO2 fixation under drought conditions. They were significantly (p < 0.05) decreased in response to water deficiency. However, treated plants by EBL and/or Spm had higher Rubisco and RCA activity than that in control plants under drought conditions (Fig. 5). EBL and Spm combined application improved the drought tolerance and significantly (p < 0.05) increased the initial Rubisco activity by 15.4, 32.9, and 103.4%, that of total Rubisco activity by 9.0, 20.0, and 65.8%, that of Rubisco activation state by 5.9, 10.6, and 22.9%, and that of RCA activity by 17.6, 23.1, and 115.4% compared to values of control plants under normal (WW) and drought conditions (WD1 and WD2), respectively.
Changes in the activities of initial and total Rubisco (μmol m−2 s−1), Rubisco activase (μmol ECM min−1), and the Rubisco activation state (%) in leaves of maize plants treated with [control (DDW), 25 mg l−1 Spm, 0.1 mg l−1 EBL, and 25 mg l−1 Spm + 0.1 mg l−1 EBL] under normal (WW) and drought stress conditions (WD1 and WD2). Values are the mean ± SE (n = 4), and asterisks indicate significant differences (p < 0.05) compared with the control (sprayed with DDW) plants
Carbonic anhydrase activity was significantly (p < 0.05) decreased with decreasing soil water content (Fig. 6). EBL and/or Spm applications alleviated the impact of water deficiency and improved its activity. EBL and Spm combined application significantly (p < 0.05) enhanced its activity by 15.0, 46.7, and 72.7% under normal (WW) and drought conditions (WD1 and WD2), respectively, compared with control treatment. Glycolate oxidase activity was significantly (p < 0.05) increased in plants grown under drought conditions (Fig. 6). However, combined application decreased its value by 12.5, 15.8, and 33.3% compared to value of control plants under normal (WW) and drought conditions (WD1 and WD2), respectively.
Changes of carbonic anhydrase (CA, mol CO2 kg−1 leaf FM s−1) and glycolate oxidase (GO, nmol min−1 mg−1 protein) activity in leaves of maize plants treated with [control (DDW), 25 mg l−1 Spm, 0.1 mg l−1 EBL, and 25 mg l−1 Spm + 0.1 mg l−1 EBL] under normal (WW) and drought stress conditions (WD1 and WD2). Values are the mean ± SE (n = 4), and asterisks indicate significant differences (p < 0.05) compared with the control (sprayed with DDW) plants
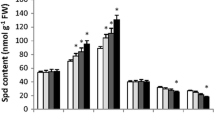
Plants grown under water-limited soils showed significant (p < 0.05) enhancement in the total soluble sugars concentration (Fig. 7). EBL and/or Spm treatments under normal (WW) condition did not change its value. However, when these treatments were accompanied with water deficit conditions, they elevated its accumulation. EBL and Spm combined application enhanced total soluble sugars concentration by 2.8, 22.3, and 63.2% under normal (WW) and drought conditions (WD1 and WD2), respectively, in comparison to control treatment.
Changes of total soluble sugars (mg g−1 DW) concentration in leaves of maize plants treated with [control (DDW), 25 mg l−1 Spm, 0.1 mg l−1 EBL, and 25 mg l−1 Spm + 0.1 mg l−1 EBL] under normal (WW) and drought stress conditions (WD1 and WD2). Values are the mean ± SE (n = 4), and asterisks indicate significant differences (p < 0.05) compared with the control (sprayed with DDW) plants
Seeds carbohydrate content was significantly (p < 0.05) decreased under water shortage conditions (Fig. 8). EBL and/or Spm treatments alleviated the deleterious impact of drought and enhanced its content. EBL and Spm combined application enhanced seeds carbohydrate content by 6.4, 26.6, and 70.1% under normal (WW) and drought conditions (WD1 and WD2), respectively, compared with control treatment.
Changes in the seeds carbohydrate content (mg g−1 DW) in maize plants treated with [control (DDW), 25 mg l−1 Spm, 0.1 mg l−1 EBL, and 25 mg l−1 Spm + 0.1 mg l−1 EBL] under normal (WW) and drought stress conditions (WD1 and WD2). Values are the mean ± SE (n = 4), and asterisks indicate significant differences (p < 0.05) compared with the control (sprayed with DDW) plants
Drought significantly altered the endogenous phytohormones production. It increased abscisic acid (ABA) accumulation and decreased indole-3-acetic acid (IAA), gibberellins (GAs), and cytokinins (CKs) concentrations (Fig. 9). EBL and/or Spm treatments alleviated the detrimental effect of water stress on the hormonal balance and caused dramatic changes in it. EBL and Spm combined application significantly (p < 0.05) increased IAA concentration by 19.5, 70.5, and 180.0%, that of GAs concentration by 16.4, 67.9, and 132.2%, and that of CKs concentration by 15.7, 55.4, and 120.6%, compared to values of control plants under normal (WW) and drought conditions (WD1 and WD2), respectively. However, combined application significantly (p < 0.05) decreased ABA concentration by 44.3 and 61.5% under drought conditions (WD1 and WD2), respectively, compared to control treatment.
Changes of indole-3-acetic acid (IAA, μg g−1 DW), gibberellins (GAs, μg g−1 DW), cytokinins (CKs, μg g−1 DW), and abscisic acid (ABA, ng g−1 DW) concentration in leaves of maize plants treated with [control (DDW), 25 mg l−1 Spm, 0.1 mg l−1 EBL, and 25 mg l−1 Spm + 0.1 mg l−1 EBL] under normal (WW) and drought stress conditions (WD1 and WD2). Values are the mean ± SE (n = 4), and asterisks indicate significant differences (p < 0.05) compared with the control (sprayed with DDW) plants
4 Discussion
Water deficiency is a major abiotic factor that severely reduces plant productivity; therefore, minimizing this loss is a critical issue to ensure the global agricultural food security. Indeed, over 20% of the annual maize yield losses worldwide are attributed to drought (Ribaut et al. 2012). Plant growth regulators such as 24-epibrassinolide and spermine have major roles in enhancing tolerance to environmental stresses. Hence, using their exogenous applications can be a potential strategy to improve maize performance under water stress conditions. My previous studies have demonstrated that EBL and/or Spm exogenous treatments enhanced maize drought tolerance by improving antioxidant enzymes activity, increasing ascorbate and glutathione content, inducing organic solutes production, altering polyamines pool, enhancing protein synthesis, as well as affecting ethylene and ROS production (Talaat et al. 2015; Talaat and Shawky 2016). The current study is an attempt to investigate if EBL and/or Spm treatments have significant impacts on other physiological and biochemical criteria. It reveals a novel role of EBL and/or Spm treatments on maize drought tolerance based on their fundamental role on photosynthetic process and endogenous phytohormonal status.
Water stress significantly (p < 0.05) hampered photosynthetic performance (Table 2) by damaging both gas exchange capacity (Fig. 3) and chlorophyll fluorescence kinetics (Fig. 4). It also significantly disrupted photosynthetic activities mainly via altering the photosynthetic pigments content (Fig. 1), photochemical reactions of photosynthesis (Fig. 2), photosynthetic enzymes activity (Figs. 5 and 6), and carbohydrates accumulation (Figs. 7 and 8). It also altered the endogenous phytohormones production (Fig. 9). However, EBL and/or Spm foliar applications mitigated water deficiency effect and maintained leaf photosynthetic ability (Table 2) by improving chlorophylls and carotenoids content, PSI and PSII activities, photosynthesis-related enzymes activity, stomatal conductance and by reducing photorespiration. Furthermore, these exogenous treatments sustained maize drought tolerance by maintaining hormonal balance (Fig. 9). Notably, EBL and Spm enhanced drought tolerance by improving photosynthesis as well as by maintaining endogenous phytohormones profile.
Chlorophylls and carotenoids are considered as fundamental components in the photosynthetic complex (Pastenes et al. 2005; Talaat 2013). Data in Fig. 1 demonstrates that EBL and/or Spm applications significantly (p < 0.05) ameliorated the deleterious effect of drought and induced increases in the photosynthetic pigments content, indicating that these exogenous treatments could ameliorate the negative effect of stress on the chloroplast structure. Indeed, the damaging effect on pigments content may directly be linked with water deficiency-induced chloroplast disruption, chlorophyll oxidation by free radicals, chlorophyll degradation by chlorophyllase, and/or inhibitory effect on pigment biosynthesis enzymes (Ahmed et al. 2009) that may prompt structural changes in light harvesting complex, alter light fixation capacity, and consequently decrease photosynthetic efficiency. In contrast, EBL and/or Spm foliar treatments mitigated water deficiency effect and increased pigments concentration, which could possibly be attributed to their positive effect on pigments biosynthesis as was also reported earlier by Divi and Krishna (2009). Spm can stabilize the molecular composition of the thylakoid membranes (Popovic et al. 1979), and thus prevent pigments’ losses, delay senescence, and increase efficiency of light capture under stressful conditions. Additionally, carotenoids can act as efficient quenchers of singlet oxygen (Qin et al. 2007). Thus, the higher carotenoids amount in treated stressed plants compared to that in non-treated ones may limit the chlorophyll damage induced by ROS. Hence, it is worth to mention that sprayed water-stressed plants by EBL and/or Spm counteracted the decrease in the photosynthetic pigments production to sustain the photosynthetic activity.
Core consequence of drought conditions is the diminishing in PSI and PSII activities; particularly PSII (Fig. 2). This decline in the photochemical reactions of photosynthesis could be due to the harmful effect of water stress on photosynthetic pigments content (Fig. 1). A decrease in chlorophyll concentration may lower the consumption of photons for light-driven electron transport in drought-exposed plants leading to photoinhibition. Another potentially reason for this decline in the PSII activity is the destruction of the chloroplasts resulting from thylakoids envelope breakdown and destabilization of pigment protein complexes (Miller et al. 2010). Water stress also induces damage in PSII major proteins D1 and D2 and in PSII oxygen evolving complex (Sapeta et al. 2013). However, EBL and/or Spm treatments significantly (p < 0.05) alleviated drought-mediated reduction in the photochemical reactions of photosynthesis (Fig. 2). This ameliorative effect could be attributed to EBL and Spm favorable impact on (a) protecting PSII against over-excitation (Hamdani et al. 2011; Hu et al. 2013) and (b) enhancing antioxidant enzymes activity and antioxidant molecules content (Talaat et al. 2015; Talaat and Shawky 2016) that protect PSII against ROS damaging effect. Furthermore, PAs by binding to portions of PSII intrinsic polypeptides can provide stability to their conformation against stressful conditions (Hamdani et al. 2011).
Parallel decreases in gas exchange attributes (net photosynthetic rate, transpiration rate, and stomatal conductance) were detected in drought-exposed plants (Fig. 3). This is achieved mainly by the decrease in leaf area (Talaat et al. 2015), reduction in photosynthetic pigments content (Fig. 1), and impairment in photosynthetic photochemical reactions (Fig. 2). Decreased the stomatal conductance under water deficit conditions allows plants to limit the transpiration rate; moreover, it also reduces CO2 absorption, which ultimately results in a reduction in the net photosynthetic rate (Gleason et al. 2017). Additionally, data in the same figure implied that drought stress increased the intercellular CO2 concentration, as was also reported by Zhang et al. (2015). This result proves that drought can induce CO2 to accumulate in intercellular areas, which indirectly limits carbon assimilation and induces poor performance of the photosynthetic apparatus. However, results in Fig. 3 also illustrated that treated stressed plants by EBL and/or Spm significantly (p < 0.05) increased the levels of the net photosynthetic rate, stomatal conductance, and transpiration rate with a reduction in the intercellular CO2 concentration than the corresponding untreated ones. Increased stomatal conductance in treated plants, particularly under the stress conditions, led to higher CO2 diffusion into leaf thereby favoring higher net photosynthetic rate. EBL improved the net photosynthetic rate under water stress condition by increasing absorbed light energy utilization (Hu et al. 2013) and by enhancing stomatal opening and thus increase the chance to allow more CO2 into the leaves (Gill et al. 2017). Hence, it is interesting to underline that EBL and/or Spm might enhance photosynthetic capability under drought through ameliorating both stomatal and non-stomatal limitations.
Chlorophyll fluorescence can be used as a rapid technique to detect the changes in the photosynthetic activity in stressed plants (Osório et al. 2013). Drought significantly (p < 0.05) decreased the maximum quantum efficiency of PSII photochemistry (Fv/Fm), electron transport rate (ETR), effective quantum yield of PSII photochemistry (Fv′/Fm'), actual photochemical efficiency of PSII (ΦPSII), and photochemical quenching coefficient (qP) values, while it significantly increased the non-photochemical quenching coefficients (qN) (Fig. 4) that could be due to severe damage in the PSII reaction center. The high non-photochemical quenching coefficients could imply high thermal energy dissipation ability (Hu et al. 2013). The maximum quantum efficiency of PSII photochemistry is a reliable indicator for photo-damage and its lower value in stressed leaves indicates that the rate of breakdown of the D1-protein of PSII exceeds the ability to repair the photo-damage in these plants (Gururani et al. 2015). Stress conditions also induced electron transfer blockage at the PSII acceptor side (Mehta et al. 2010). Apparently, PSII is generally considered to be more susceptible to the effects of drought than PSI (Fig. 2). This effect is apparent as reductions of both the maximum (Fv/Fm) and actual (ΦPSII) quantum yields of PSII and concomitant increases in the non-photochemical quenching coefficients. However, EBL and/or Spm treatments restored the deleterious impact of water stress on these parameters (Fig. 4), indicating alleviation of the photoinhibition, improvement of the photochemical efficiency, increment of the capacity of CO2 assimilation, and improvement of the efficiency of light utilization in stressed-maize plants and thus acclimation to this harsh condition. EBL under water stress conditions might protect PSII against over-excitation (Zhao et al. 2017), enhance thylakoid membranes flexibility, and improve energy redistribution between PSI and PSII (Dobrikova et al. 2014). From this point of view, it is worth mentioning that treated stressed plants can remain potential PSII efficiency and exhibit greater PSII function than that of stressed untreated ones.
Ribulose-bisphosphate carboxylase (Rubisco) catalyzes the incorporation of CO2 into ribulose 1,5-bisphosphate and the photosynthetic performance is largely determined by its activation state. RCA maintain Rubisco in an active conformation (Chen et al. 2015). As shown in Fig. 5, drought conditions significantly (p < 0.05) reduced Rubisco and RCA activities as well as Rubisco activation state, and consequently the carboxylation capacity was impaired in these plants. This decline in Rubisco activation state is thought to be directly correlated to the impairing in Rubisco activase activity, as was also reported by Zhang et al. (2015). However, EBL and/or Spm treatments increased the Rubisco activation state (Fig. 5) that could be attributed to their favorable role on enhancing the Rubisco activase activity (Fig. 5). In this concern, Zhao et al. (2017) found that EBL improved the photosynthetic activity under water deficiency by enhancing the 38–39-kDa RCA subunit that was associated with modification in the values of initial Rubisco activity and net photosynthetic rate. BRs could also regulate Rubisco activity by upregulating rbcL and rbcS genes expression, which encode subunits of this enzyme (Xia et al. 2009) and/or by protecting enzymes involved in ribulose 1,5-bisphosphate regeneration (Divi and Krishna 2009). Furthermore, PAs could incorporate with the large subunit of Rubisco (Hamdani et al. 2011) and thus enhance CO2 assimilation. Accordingly, recovery of the activity of Calvin cycle enzyme could enhance the photosynthetic capacity.
Water shortage conditions significantly (p < 0.05) inhibited carbonic anhydrase activity (Fig. 6). This inhibition could be attributed to (a) decrease CO2 uptake by inducing stomatal closure (Fig. 3) and/or (b) excessive ROS production that may inhibit enzyme synthesis or alter enzyme activity. Contrarily, treated water-stressed plants by EBL and/or Spm enhanced carbonic anhydrase activity and that corresponded well with the enhanced photosynthetic efficiency. This enhancement in the enzyme activity can be explained by the positive impact of these exogenous treatments on (a) stomatal conductance (Fig. 3) that facilitates the uptake and availability of CO2 and/or (b) inducing the synthesis of carbonic anhydrase by involving the specific gene expression (Divi and Krishna 2009).
Stomatal closure induced by water-stressed environments decreases the ratio of CO2/O2 and increases photorespiration and glycolate production. In peroxisomes, H2O2 oxidized glycolate to glycolate oxidase (Miller et al. 2010). In the present study, it is worth noting that activity of glycolate oxidase was enhanced in response to water deficiency. However, treated stressed plants by EBL and/or Spm decreased its activity (Fig. 6) that may be due to their possible involvement in the enhancement of stomatal conductance (Fig. 3).
As shown in Fig. 7, drought stress resulted in increasing the total soluble sugars concentration, which was further increased by EBL and/or Spm treatments, indicating stress ameliorative properties of EBL and Spm. Sugars biosynthesis enhancement by EBL could be attributed to the crucial role played by EBL on upregulating the related gene expression (Divi and Krishna 2009). Indeed, plants respond to drought stress using different physiological and biochemical strategies. One of them is the accumulation of osmoregulation substances in plant cells, such as soluble sugars that can regulate osmotic potential, maintain cell turgor pressure, scavenge radical, serve as a sink for energy and carbon as well as confer protection to the stability of membrane structures and metabolism-related enzymes (Sun et al. 2016). Maintaining higher turgor pressure in treated stressed plants may contribute to keeping the stomata open and the photosynthetic rate relatively high. Furthermore, sugars also activate specific ROS scavenging systems via interplaying with redox and hormone signals (Ramel et al. 2009).
Drought-caused decreases in seeds carbohydrate content, which was significantly (p < 0.05) alleviated by EBL and/or Spm exogenous treatments (Fig. 8). This ameliorative effect could have been at least in part due to the role played by EBL and Spm on pigments content, PSI and PSII performance, gas exchange, chlorophyll fluorescence, and Rubisco activity. Concerning to Divi and Krishna (2009) and Talaat and Shawky (2012), BR applications can induce photosynthetic efficiency and enhance sink strength and phloem uploading that stimulate assimilate flow from source to sink organs and resulted in higher seeds carbohydrate content.
Hormonal profile has a critical fundamental role in controlling plant drought resistance. As shown in Fig. 9, significantly (p < 0.05) high level of ABA was detected in plants exposed to drought conditions, whereas concentrations of IAA, GAs, and CKs were significantly decreased. Apparently, endogenous hormones were significantly influenced by drought and closely associated with photosynthetic activity. Abscisic acid induces stomatal closure to prevent water losses under drought environments that disturb gas exchange and consequently inhibit photosynthesis process. Abscisic acid can also regulate stress-response genes expression (Fan et al. 2016). However, a converse trend was detected when water-stressed plants were sprayed by EBL and/or Spm as they counteracted the negative effects of water stress by diminishing the level of stress-induced hormonal imbalance. Indeed, endogenous phytohormones are essential pre-requisites for improving plant stress tolerance and maintaining high photosynthetic capacity. In this respect, higher concentration of cytokinins in treated stressed plants may protect the photosynthetic machinery by inhibiting the negative impact of stress condition on chlorophyll concentration and on photochemical efficiency (Chernyadev 2009).
Brassinosteroids exert anti-stress effects both independently as well as through cross-talk with other stress-related hormones pathways (Divi et al. 2010; Shakirova et al. 2016). In this connection, Divi et al. (2010) reported that there was a cross-talk between abscisic acid and brassinosteroids that can regulate stomatal development under water-stressed condition. Brassinosteroids also modulate cytokinins levels by regulating the expression of genes participating in cytokinin signaling (Divi and Krishna 2009). Furthermore, it is worth noting that the positive effect of Spm in inducing hormonal balance in water-stressed plants is based on its capacity to interact with other phytohormones, as was also reported by Li et al. (2016). In this respect, Radhakrishnan and Lee (2013) found that Spm alters abscisic acid metabolic pathway and thus induce stomatal regulation under water-stressed conditions.
The novel aspect of the current study is that exogenous EBL and/or Spm applications alter the concentrations of abscisic acid, indole-3-acetic acid, gibberellins, and cytokinins in maize plants subjected to drought, which suggests that a general interaction existed between endogenous phytohormones and both EBL and Spm and that might be involved in the simultaneous improvement in photosynthetic carbon fixation efficiency and thereby enhance plant stress tolerance.
Taking into account the physiological responses of maize plants to EBL and/or Spm treatments under drought conditions, it is evident that these foliar applications can alleviate drought-induced injury to the photosynthetic system by counteracting the decrease of photosynthetic pigments concentration (Fig. 1), improving the performance of photosynthetic photochemical reactions (Fig. 2), maintaining the integrity of gas exchange parameters (Fig. 3), alleviating the disturbance of chlorophyll fluorescence system (Fig. 4), altering the activities of Rubisco, Rubisco activase, carbonic anhydrase and glycolate oxidase (Figs. 5 and 6), inducing the accumulation of compatible solutes (Fig. 7), sustaining the seeds carbohydrate content (Fig. 8), and regulating the plant hormonal system (Fig. 9). Obviously, photosynthetic impairment was shown to be a consequence of both stomatal and non-stomatal limitation; however, spraying drought-stressed plants by EBL and/or Spm maintained the leaf photosynthetic capacity by modification the two kinds of limitation. Additionally, data point out that the highest photosynthetic efficiency was detected when the mixture of EBL and Spm was used. Obtained results confirm that this combined treatment can alleviate drought-induced irreversible harm to plant photosynthetic system.
5 Conclusions
Data reveal that EBL and/or Spm exogenous treatments maintained photosynthetic activities that allow maize (Zea mays L.) to adapt to drought stress. Water deficiency impaired photosynthetic performance; however, combined application of EBL and Spm maintained higher chlorophylls and carotenoids content, sustained both gas exchange and chlorophyll fluorescence systems, enhanced PSI and PSII reaction center activities, up-regulated photosynthetic enzymes activity, increased osmotic adjustment substances accumulation, and finally resulted in improving the efficiency of photosynthetic carbon fixation and consequently promoting the plant’s drought tolerance. Furthermore, combined treatment could be exploited to alleviate the deleterious impact of water stress through not only regulation the photosynthetic ability but also by altering the endogenous phytohormones profile.
References
Ahmed CB, Rouina BB, Sensoy S, Boukhris M, Abdallah FB (2009) Changes in gas exchange, proline accumulation and antioxidative enzyme activities in three olive cultivars under contrasting water availability regimes. Environ Exp Bot 67:345–352
Anjum SA, Tanveer M, Ashraf U, Hussain S, Shahzad B, Khan I, Wang L (2016) Effect of progressive drought stress on growth, leaf gas exchange, and antioxidant production in two maize cultivars. Environ Sci Pollut Res 23:17132–17141
Blasco B, Ríos JJ, Cervilla LM, Sánchez-Rodríguez E, Rubio-Wilhelmi MM, Rosales MA, Ruiz JM, Romero L (2010) Photorespiration process and nitrogen metabolism in lettuce plants (Lactuca sativa L.): induced changes in response to iodine biofortification. J Plant Growth Regul 29:477–486
Cerovic ZG, Plesnicar M (1984) An improved procedure for the isolation of intact chloroplasts of high photosynthetic activity. Biochem J 223:543–545
Chen Y, Wang XM, Zhou L, He Y, Wang D, Qi YH, Jiang DA (2015) Rubisco activase is also a multiple responder to abiotic stresses in rice. PLoS One 10(10):e0140934
Chernyadev II (2009) The protective action of cytokinins on the photosynthetic machinery and productivity of plants under stress (review). Appl Biochem Microbiol 45:351–362
Coombs J, Hall DO, Long SP, Scurlock JMO (1987) Techniques in bioproductivity and photosynthesis. Pergamon, Oxford
Cottenie A, Verloo M, Kiekens L, Velghe G, Camerlynck R (1982) Chemical analysis of plants and soils. In: Laboratory of analytical and agrochemistry. State University, Ghent, pp 14–24
Divi UK, Krishna P (2009) Brassinosteroids confer stress tolerance. In: Weinheim HH (ed) Plant stress biology: genomics goes systems biology. Wiley-VCH, Weinheim, pp 119–135
Divi UK, Rahman T, Krishna P (2010) Brassinosteroid-mediated stress tolerance in Arabidopsis shows interactions with abscisic acid, ethylene and salicylic acid pathways. BMC Plant Biol 10:151
Dobrikova AG, Vladkova RS, Rashkov GD et al (2014) Effects of exogenous 24-epibrassinolide on the photosynthetic membranes under non-stress conditions. Plant Physiol Biochem 80:75–82
Dong Y, Wang W, Hu G, Chen W, Zhuge Y, Wang Z, He M (2017) Role of exogenous 24-epibrassinolide in enhancing the salt tolerance of wheat seedlings. J Soil Sci Plant Nutr 17(3):554–569
Dubois M, Gills K, Hamilton JK, Robers PA, Smith F (1956) Colorimeter method for determination of sugars and related substances. Anal Chem 28:350–356
Dwivedi RS, Randhawa NS (1974) Evolution of a rapid test of the hidden hunger of zinc in plants. Plant Soil 40:445–451
Fan WQ, Zhao MY, Li SL, Bai X, Li J, Meng H et al (2016) Contrasting transcriptional responses of PYR1/PYL/RCAR ABA receptors to ABA or dehydration stress between maize seedling leaves and roots. BMC Plant Biol 16:99
Farooq M, Wahid A, Lee DJ (2009) Exogenously applied polyamines increase drought tolerance of rice by improving leaf water status, photosynthesis and membrane properties. Acta Physiol Plant 31:937–945
Gill MB, Cai K, Zhang G, Zeng F (2017) Brassinolide alleviates the drought-induced adverse effects in barley by modulation of enzymatic antioxidants and ultrastructure. Plant Growth Regul 82:447–455
Gleason SM, Wiggans DR, Bliss CA, Comas LH, Cooper M, DeJonge KC, Young JS, Zhang H (2017) Coordinated decline in photosynthesis and hydraulic conductance during drought stress in Zea mays. Flora 227:1–9
Gururani MA, Venkatesh J, Tran LSP (2015) Regulation of photosynthesis during abiotic stress-induced photoinhibition. Mol Plant 8:1304–1320
Hamdani S, Yaakoubi H, Carpentier R (2011) Polyamines interaction with thylakoid proteins during stress. J Photochem Photobiol B 104:314–319
Hu W, Yan X, Xiao Y, Zeng J, Qi H, Ogweno J (2013) 24-Epibrassinosteroid alleviate drought-induced inhibition of photosynthesis in Capsicum annuum. Sci Hortic 150:232–237
Huang X, Zhou G, Yang W, Wang A, Hu Z, Lin C, Chen X (2014) Drought-inhibited ribulose-1,5-bisphosphate carboxylase activity is mediated through increased release of ethylene and changes in the ratio of polyamines in pakchoi. J Plant Physiol 171:1392–1400
Irigoyen JJ, Emerich DW, Sanchez-Dıaz M (1992) Water stress induced changes in concentrations of proline and total soluble sugar in nodulated alfalfa (Medicago sativa) plants. Physiol Plant 84:55–60
Jiang YP, Cheng F, Zhou YH, Xia XJ, Mao WH, Shi K, Chen ZX, Yu JQ (2012) Cellular glutathione redox homeostasis plays an important role in the brassinosteroid-induced increase in CO2 assimilation in Cucumis sativus. New Phytol 194:932–943
Li Z, Zhang Y, Zhang X, Peng Y, Merewitz E, Ma X, Huang L, Yan Y (2016) The alterations of endogenous polyamines and phytohormones induced by exogenous application of spermidine regulate antioxidant metabolism, metallothionein and relevant genes conferring drought tolerance in white clover. Environ Exp Bot 124:22–38
Lichtenthaler HK (1987) Chlorophylls and carotenoids: pigments of photosynthetic biomembranes. Methods Enzymol 148:350–382
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275
Lu CM, Qiu NW, Wang BS, Zhang JH (2003) Salinity treatment shows no effects on photosystem II photochemistry, but increases the resistance of photosystem II to heat stress in halophyte Suaeda salsa. J Exp Bot 54:851–860
Mehta P, Jajoo A, Mathur S, Bharti S (2010) Chlorophyll a fluorescence study revealing effects of high salt stress on photosystem II in wheat leaves. Plant Physiol Biochem 48:16–20
Miller G, Suzuki N, Ciftci-Yilmaz S, Mittler R (2010) Reactive oxygen species homeostasis and signalling during drought and salinity stresses. Plant Cell Environ 33:453–467
Nehela Y, Hijaz F, Elzaawely AA, El-Zahaby HM, Killiny N (2016) Phytohormone profiling of the sweet orange (Citrus sinensis L., Osbeck) leaves and roots using GC–MS-based method. J Plant Physiol 199:12–17
Osório ML, Osório J, Romano A (2013) Photosynthesis, energy partitioning, and metabolic adjustments of the endangered Cistaceae species Tuberaris major under high temperature and drought. Photosynthetica 51:75–84
Pastenes C, Pimentel P, Lillo J (2005) Leaf movements and photoinhibition in relation to water stress in field-grown beans. J Exp Bot 56:425–433
Popovic RB, Kyle DJ, Cohen AS, Zalik S (1979) Stabilization of thylakoid membranes by spermine during stress induced senescence of barley leaf discs. Plant Physiol 64:721–726
Qin G, Gu H, Ma L, Peng Y, Deng XW, Chen Z, Qu LJ (2007) Disruption of phytoene desaturase gene results in albino and dwarf phenotypes in Arabidopsis by impairing chlorophyll carotenoid, and gibberellin biosynthesis. Cell Res 17:471–482
Radhakrishnan R, Lee I (2013) Spermine promotes acclimation to osmotic stress by modifying antioxidant, abscisic acid, and jasmonic acid signals in soybean. J Plant Growth Regul 32:22–30
Ramel F, Sulmon C, Gouesbet G, Couee I (2009) Natural variation reveals relationships between pre-stress carbohydrate nutritional status and subsequent responses to xenobiotic and oxidative stress in Arabidopsis thaliana. Ann Bot 104:1323–1337
Ribaut JM, Betran J, Monneveux P, Setter T (2012) Drought tolerance in maize. In: Bennetzen JL, Hake SC (eds) Handbook of maize: its biology. Springer, New York, pp 311–344
Sapeta H, Costa JM, Lourenco T, Maroco J, van der Linde P, Oliveira MM (2013) Drought stress response in Jatropha curcas: growth and physiology. Environ Exp Bot 85:76–84
Shakirova F, Allagulova C, Maslennikova D, Fedorova K, Yuldashev R, Lubyanova A, Bezrukova M, Avalbaev A (2016) Involvement of dehydrins in 24-epibrassinolide-induced protection of wheat plants against drought stress. Plant Physiol Biochem 108:539–548
Snedecor GW, Cochran WG (1980) Statistical methods, 7th edn. Iowa State Univ. Press, Ames
Sun C, Gao X, Chen X, Fu J, Zhang Y (2016) Metabolic and growth responses of maize to successive drought and re-watering cycles. Agric Water Manag 172:62–73
Talaat NB (2013) RNAi based simultaneous silencing of all forms of light-dependent NADPH:protochlorophyllide oxidoreductase genes result in the accumulation of protochlorophyllide in tobacco (Nicotiana tabacum). Plant Physiol Biochem 71:31–36
Talaat NB (2019a) Role of reactive oxygen species signaling in plant growth and development. In: Hasanuzzaman M, Fotopoulos V, Nahar K, Fujita M (eds) Reactive oxygen, nitrogen and sulfur species in plants: production, metabolism, signaling and defense mechanisms. Wiley, Chichester, pp 225–266
Talaat NB (2019b) Abiotic stresses-induced physiological alteration in wheat. In: Hasanuzzaman M, Nahar K, Hossain A (eds) Wheat production in changing environments—responses, adaptation and tolerance. Springer Nature Singapore Pte, Ltd, Singapore, pp 1–30
Talaat NB, Shawky BT (2012) 24-Epibrassinolide ameliorates the saline stress and improves the productivity of wheat (Triticum aestivum L.). Environ Exp Bot 82:80–88
Talaat NB, Shawky BT (2013) 24-Epibrassinolide alleviates salt-induced inhibition of productivity by increasing nutrients and compatible solutes accumulation and enhancing antioxidant system in wheat (Triticum aestivum L.). Acta Physiol Plant 35:729–740
Talaat NB, Shawky BT (2016) Dual application of 24-epibrassinolide and spermine confers drought stress tolerance in maize (Zea mays L.) by modulating polyamine and protein metabolism. J Plant Growth Regul 35:518–533
Talaat NB, Shawky BT, Ibrahim AS (2015) Alleviation of drought-induced oxidative stress in maize (Zea mays L.) plants by dual application of 24-epibrassinolide and spermine. Environ Exp Bot 113:47–58
Tiwari BS, Bose A, Ghosh B (1997) Photosynthesis in rice under salt stress. Photosynthetica 34:303–306
Todorova D, Talaat NB, Katerova Z, Alexieva V, Shawky BT (2016) Polyamines and brassinosteroids in drought stress responses and tolerance in plants. In: Ahmad P (ed) Water stress and crop plants: a sustainable approach, vol 2. Wiley, Chichester, pp 608–627
Xia XJ, Huang LF, Zhou YH, Mao WH, Shi K, Wu JX, Asami T, Chen Z, Yu JQ (2009) Brassinosteroids promote photosynthesis and growth by enhancing activation of Rubisco and expression of photosynthetic genes in Cucumis sativus. Planta 230:1185–1196
Yih RY, Clark HE (1965) Carbohydrate and protein content of boron deficient tomato root tips in relation to anatomy and growth. Plant Physiol 40:312–315
Zhang RH, Zhang XH, Camberato JJ, Xue JQ (2015) Photosynthetic performance of maize hybrids to drought stress. Russ J Plant Physiol 62:788–796
Zhang W, Wang C, Dong M, Jin S, Li H (2018) Dynamics of soil fertility and maize growth with lower environment impacts depending on a combination of organic and mineral fertilizer. J Soil Sci Plant Nutr 18(2):556–575
Zhao G, Xu H, Zhang P, Su X, Zhao H (2017) Effects of 24-epibrassinolide on photosynthesis and Rubisco activase gene expression in Triticum aestivum L. seedlings under a combination of drought and heat stress. Plant Growth Regul 81:377–384
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The author declares that she has no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Talaat, N.B. 24-Epibrassinolide and Spermine Combined Treatment Sustains Maize (Zea mays L.) Drought Tolerance by Improving Photosynthetic Efficiency and Altering Phytohormones Profile. J Soil Sci Plant Nutr 20, 516–529 (2020). https://doi.org/10.1007/s42729-019-00138-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42729-019-00138-4