Abstract
Brassinosteroids promote the growth of plants and are effective in alleviating adverse effects of abiotic stresses such as salinity and drought. Under saline conditions, improvement in grain yield is more important than simple growth. Previously it was found that although foliar application of brassinosteroids improved growth of wheat plants, it did not increase grain yield. In present study, influence of root applied 24-epibrassinolide was assessed in improving growth and yield of two wheat cultivars. Plants of a salt tolerant (S-24) and a moderately salt sensitive (MH-97) were grown at 0 or 120 mM NaCl in continuously aerated Hoagland’s nutrient solution. Different concentrations of 24-epibrassinolide (0, 0.052, 0.104, 0.156 μM) were also maintained in the solution culture. Exogenous application of 24-epibrassinolide counteracted the salt stress-induced growth and grain yield inhibition of both wheat cultivars. Of the varying 24-epibrassinolide concentrations used, the most effective concentrations for promoting growth were 0.104 and 0.052 μM under normal and saline conditions, respectively. However, root applied 0.052 μM 24-epibrassinolide enhanced the total grain yield and 100 grain weight of salt stressed plants of both cultivars and suggested that total grain yield was mainly increased by increase in grain size which might have been due to 24-epibrassinolide induced increase in translocation of more photoassimilates towards grain. Growth improvement in both cultivars due to root applied 24-epibrassinolide was found to be associated with improved photosynthetic capacity. Changes in photosynthetic rate due to 24-epibrassinolide application were found to be associated with non-stomatal limitations, other than photochemical efficiency of PSII and photosynthetic pigments. Leaf turgor potential found not to be involved in growth promotion.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Agricultural productivity is severely affected due to soil salinity. The damaging effects of salt stress on crop growth and productivity are due to its ionic and osmotic stress which severely depresses various physiological and biochemical processes (Munns 2005). Of these, photosynthetic capacity, a major determinant of growth, is significantly inhibited in plants subjected to salinity stress (Ashraf 2004). A positive relationship between photosynthetic capacity and growth under salt stress has been reported in a number of plant species, e.g., wheat (Raza et al. 2006), Panicum antidotale (Ashraf 2003), six Brassica diploid and amphiploid species (Ashraf 2001), Spinacea oleracea (Robinson et al. 1983). However, suppression in photosynthetic capacity by increased salt stress was ascribed to lower stomatal conductance, inhibition in specific metabolic processes in carbon uptake, perturbation in photochemica1 capacity, or a combination of these (Dubey 2005). Thus, the final biological or economical yield can be increased by increasing the rate of photosynthesis (Nátr and Lawlor 2005).
Despite the suppression of photosynthetic capacity due to salt stress, changes in endogenous concentrations of plant hormones were also observed in different plant species (Ashraf and Foolad 2005). Of plant hormones, a considerable attention has been paid to brassinosteroids (BRs) as plant hormones in a number of textbooks of botany or comprehensive reviews of plant development (Clouse and Sasse 1998; Mussig 2005; Haubrick and Assmann 2006). In view of the information presented in these reviews, BRs can regulate a number of physiological processes such as cell elongation and division, ATPase activity, prevented photosynthetic pigment loss, and enhanced carboxylation (Sasse 1997; Mussig 2005; Haubrick and Assmann 2006), which results in enhanced crop growth under stressful conditions. In our previous studies, it was found that foliar application of 24-epibrassinolide improved salt tolerance in wheat by enhancing growth but not yield (Shahbaz et al. 2008) and suggested that uptake and translocation 24-epibrassinolide through the leaves might have less effective in modulating some important physiological processes that improve grain yield. In view of all the afore-mentioned reports, it was hypothesized that root applied BRs might have a modulating effect on some important physiological processes that improve grain yield of wheat plants subjected to salt stress. Thus, the primary objective of the present study was to assess whether the exogenous application of 24-epibrassinolide through the rooting medium could improve the growth and yield in wheat plants subjected to salt stress. Moreover, to draw the relationship between growth and other physiological attributes, thus physiological basis of BRs-induced growth improvement was explored.
Materials and methods
Seed of a salt tolerant (S-24) and a moderately salt sensitive cultivar (MH-97) of spring wheat were obtained from the University of Agriculture, and Ayyub Agricultural Research Institute in Faisalabad, Pakistan. A hydroponic experiment was conducted during the winter 2004–2005 in a net-house of the University of Agriculture (latitude 31°30 N, longitude 73°10 E and altitude 213 m), with 10/14 light/dark period with maximum PAR measured at noon ranged 800–1100 μmol m−2 s−1 PPFD, a day/night temperature cycle of 26/15°C and 65 ± 5% relative humidity. Seeds of both cultivars were surface sterilized with 5% sodium hypochlorite solution for 5 minutes and then thoroughly rinsed with distilled water. Seed (100 seeds of each cultivar; 25 seeds per Petri dish) of both cultivars were germinated for 7 days on filter paper moistened with half-strength Hoagland’s nutrient solution containing 24-epibrassinolide (0, 0.052, 0.104, 0.156 μM in the rooting medium) under non-saline (0 mM NaCl) or saline conditions (150 mM NaCl). Seven-day old young wheat seedlings were transferred on styrofoam supports with holes. The styrofoam supports were then placed over plastic tanks (1.5 × 2.5 × 0.10) containing 20 l of each treatment solution as described earlier. The seedlings were allowed to grow in hydroponics for 45 days. Nutrient solution was replaced every week. All the treatment solutions were continuously aerated. The experiment consisted of four replicates in a completely randomized (CRD) design arranged. After 45 days, following physiological attributes were measured.
Water relations
The 2nd leaf from the top of each plant was used for the measurement of water relations. The leaf from each plant was excised at 7.00 a.m., and the leaf water potential measurements were made with a Scholander type pressure chamber (Arimad-2, ELE International, Tokyo, Japan). A proportion of the leaf used for water potential measurements, was frozen into 2 ml polypropylene tubes by placing them in liquid N for 2 minutes and then kept at −40°C in an ultra-low freezer for two weeks, after which time the plant material was thawed and the frozen sap was extracted by crushing the material with a glass rod. After centrifugation (8000 x g) for four minutes, the sap osmotic potential was determined using a vapor pressure osmometer (Wescor 5520, Wescor Inc., Logan, Utah, USA). Turgor pressure was calculated by subtracting the leaf water potential values from those of leaf osmotic potential.
Chlorophyll concentration
The chlorophyll ‘a’ was determined according to the method of Arnon (1949). Fresh leaves (0.2 g) were cut and extracted overnight with 80% acetone at 0–4°C. The extracts were centrifuged at 10,000 x g for 5 minutes. Absorbance of the supernatant was read at 645, 663 and 480 nm using a spectrophotometer (Hitachi-U2001, Tokyo, Japan).
Chlorophyll fluorescence
The polyphasic rise of fluorescence transients (OJIP) were measured with a Plant Efficiency Analyzer (PEA, Handsatech Instruments Ltd., King’s Lynn, UK) according to Strasser et al. (1995). The fluorescent transients were recorded during 60 sec pulse of red light of 3000 μmol (photon) m−2 s−1 provided by an array of six light emitting diodes (peak 650 nm). All the samples were dark adapted for 30 minutes prior to fluorescence measurements. The following original data were retained: maximal fluorescence (Fm), minimum fluorescence (F o), variable fluorescence (F v). From these data, maximum quantum efficiency of PSII was calculated as F v/F m.
Gas exchange parameters
Measurements of gas exchange attributes were made on 2nd intact leaf from the top of each plant using an ADC LCA-4 portable infrared gas analyzer (Analytical Development, Hoddesdon, UK). These measurements were made from 10.30 to 12.30 h under the following conditions: leaf surface area, 11.25 cm2; ambient temperature, 45 ± 3°C; ambient CO2 concentration, 352 μmol mol−1; temperature of leaf chamber varied from 37.2 to 47.2°C; leaf chamber gas flow rate (U), 251 μmol s−1; molar flow of air per unit leaf area (Us) 221.06 mol m−2 s−1; RH (relative humidity) of the chamber ranged from 35.4 to 41.2 %; PAR (photosynthetically active radiation, Qleaf) at leaf surface during noon was maximum up to 918 μmol m−2 s−1; ambient pressure 98.8 kPa.
After 45 days, plants were harvested. Plant roots were removed from the hydroponic system and washed in cold LiNO3 solution isotonic with the corresponding treatment in which plants were growing. Plants were separated into shoots and roots and then blotted dry before recording their fresh weights. All plant parts were dried at 65°C until constant dry weight, and dry weights were recorded.
Statistical analysis of data
The data were subjected to analysis of variance using a COSTAT computer package (Cohort Software, Berkeley, California). The mean values were compared with the least significance difference (LSD) test following Snedecor and Cochran (1980).
Results
Salt stress caused a significant reduction in shoot fresh and dry weight, and shoot length of both wheat cultivars (Table 1). Although cv. S-24 exhibited higher shoot fresh and dry weight than the MH-97 under saline conditions, these cultivar differences were diminished at different concentrations of 24-epibrassinolide in shoot dry weight (Fig. 1). The adverse effects of salt stress on the growth of both cultivars were alleviated in terms of shoot fresh and dry weights, particularly when 0.052 μM 24-epibrassinolide was applied. Furthermore, under non-saline conditions exogenous application of 0.104 μM 24-epibrassinolide caused a significant increasing effect on shoot fresh and dry weights (Fig. 1). Salt stress also caused a marked reduction in shoot length of both cultivars and cultivars differed significantly (Table 1; Fig 1). Although exogenously applied 24-epibrassinolide had a significant effect on shoot length (Table 1), this effect was only visible on salt stressed plants of MH-97 (Fig 1).
Imposition of salt stress reduced the grain yield, number of grains and 100 grain weight of both cultivars (Table 1). Different concentrations of 24-epibrassinolide applied through rooting medium improved all these yield attributes in both non-stressed and salt stressed plants of both wheat cultivars (Fig 1). However, this 24-epibrassinolide induced improving effect on these yield attributes was more pronounced in total grain yield (Fig 1). In addition, 0.052 μM 24-epibrassinolide increased number of grains only in salt stressed plants of MH-97, whereas other concentrations of 24-epibrassinolide did not change the number of grains in both wheat cultivars. Similarly, 0.052 μM 24-epibrassinolide increased the 100 grain weight of salt stressed plants of both wheat cultivars (Fig. 1).
All gas exchange attributes such as net CO2 assimilation rate (P N), stomatal conductance (g s), transpiration rate (E) etc. were significantly reduced in both cultivars due to salt stress except water use efficiency (measured as P N/E) (Table 2). However, addition of 0.052 and 0.104 μM 24-epibrassinolide caused a maximum increase in net CO2 assimilation rate in S-24 and MH-97, to the non-saline rooting medium, respectively (Fig. 2). In contrast, exogenous application of 0.052 μM 24-epibrassinolide caused a significant increase in net CO2 assimilation rate of both cultivars under saline conditions. Addition of 0.104 μM 24-epibrassinolide to the rooting medium caused a maximum increase in stomatal conductance (g s) in both cultivars under non-saline conditions, whereas under saline conditions the same was true at 0.052 μM 24-epibrassinolide. However, 0.104 μM 24-epibrassinolide caused a significant increase in transpiration rate in MH-97, whereas it did not affect transpiration rate of S-24 under non-saline conditions (Fig. 2). Furthermore, transpiration rate was significantly reduced in both cultivars at the highest concentration of 24-epibrassinolide under saline conditions. In contrast, sub-stomatal CO2 (C i) was slightly increased in both cultivars due to the addition of 24-epibrassinolide under saline conditions (Fig. 2). Water use efficiency (P N/E) of both cultivars was significantly increased under both non-saline and saline conditions due to exogenous application of 24-epibrassinolide, particularly at 0.052 μM.
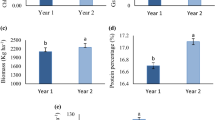
Salt stress or addition of epibrassinolides did not affect leaf chlorophyll ‘a’ of both cultivars (Table 3; Fig. 3). Similarly, quantum yield of photosystem II (PSII) (measured as F v/F m) was also not affected due to salt stress or 24-epibrassinolides (Table 3; Fig. 3).
Chlorophyll contents (mg/g F.wt) and maximal quantum yield of PSII (F v/F m) of two spring wheat cultivars differing in salinity tolerance when grown for 45 days at various levels of 24-epibrassinolide under normal or saline conditions (Number of replicates n = 4; vertical lines are standard errors)
The growth medium salinity significantly lowered the leaf water potential, osmotic potential (more negative values) and turgor potential of both cultivars (Table 3; Fig. 4). However, the adverse effect of salt stress on these water relation attributes was more pronounced on cv. MH-97 than on cv. S-24. Addition of epibrassinolides to the rooting medium caused a further decrease in leaf water potential of salinized S-24 plants at all epibrassinolides levels, whereas that of MH-97 plants it remained almost unaffected (Fig. 4). Similarly, leaf osmotic potential of salanized plants of both cultivars was further decreased due to exogenously applied epibrassinolide through the rooting media (Fig. 4). In contrast, addition of 0.104 μM 24-epibrassinolide slightly reduced the leaf turgor of salanized S-24 plants, whereas in MH-97 plants 0.104 μM and 0.156 μM of 24-epibrassinolide increased the leaf turgor potential (Fig. 4).
Discussions
In the present study, salt stress reduced the growth at the vegetative stage of both wheat cultivars and the inhibitory effect of salt stress was more pronounced on MH-97 than on S-24. However, this reduction in growth was alleviated in both cultivars with the addition of 0.052 μM 24-epibrassinolide to the rooting medium. These results can be related to some earlier studies in which it has been observed that BRs has a role in growth promotion under normal or stress conditions in wheat (Anuradha and Rao 2003), Brassica juncea (Hayat et al. 2000) and chickpea (Ali et al. 2007). In the present study, the most effective dose of epibrassinolide under non-saline conditions was found to be 0.104 μM, whereas 0.052 μM was an effective concentration in improving growth under saline conditions. However, cv. S-24 showed a better response in terms of growth to effective concentration of BRs, which is in contrast to the findings of Sairam (1994) who reported that the drought-tolerant variety showed a higher response to BR application under water stress conditions compared that of drought susceptible wheat variety. Similarly, Shahbaz et al. (2008) reported that ameliorative effect of foliar applied BRs was more in salt tolerant wheat cultivars compared with that of salt sensitive cultivar. These contrasting results can be explained in view of the arguments of different researchers that these growth promoting effects depends on type of species, plant developmental stage, concentration of epibrassinolide, and mode of application (Amzallag 2002; Fariduddin et al. 2003; Ali et al. 2007). Furthermore, this growth promotion effect of BRs on wheat under normal or stress conditions probably through their auxin like hormonal effect on cell division and cell enlargement, or BRs induced turgor-driven cell expansion occurs due to enhanced activity of aquaporins (Morillon et al. 2001), or their role in enhancing photosynthetic capacities through a network of gene regulations (Mussig 2005; Haubrick and Assmann 2006).
Grain yield is one of the most important determinants in appraising crop productivity under stressful environments. Undoubtedly, grain yield depends on both number and size of grains (Grieve et al. 1992). From the results of the present study, it could be suggested that salt-induced reduction in grain yield and improvement in grain yield with root applied 24-epibrassinolide was mainly due to increase in grain size. In view of some earlier studies, the improving effect of 24-epibrassinolide on grain yield may have been due to greater translocation of photoassimilates to grains during the grain filling stage thereby increasing grain weight. For example, exogenous application of BRs in bean enhanced sink strength and phloem unloading (Petzold et al. 1992). While working with cucumber, Nakajima and Toyama (1999) showed that root applied 24-epibrassinolide promoted transport of 14C-labeled sucrose from the primary leaf to the epicotyl. In another study, while monitoring the effect of brassinolide on the distribution of starch and sucrose to different organs of rice plants, Fujii and Saka (2001) found that brassinolide caused more accumulation of starch in the grains at the expense of the leaf sheaths and culms, where sucrose levels decreased to a great extent. Extracellular invertases are very important for the supply of carbohydrates to sink tissues. In tomato, Goetz et al. (2000) found that exogenous application of BRs caused enhancement of cell-wall-bound invertase activity with a concurrent increase in sucrose uptake. Furthermore, they also found tissue-specific induction of mRNA for extra-cellular invertase. From these findings it is suggested that EBL-induced increase in growth and grain yield may have been due to more supply of carbohydrates through activation of appropriate enzymes.
The decline in growth in many plant species subjected to stressful environment is often associated with a reduction in photosynthetic capacity as has been observed in the present study. However, root applied BRs improved the photosynthetic rate which is in agreement with some earlier reports in which it has been observed that BRs can improve photosynthetic rate in mustard (Hayat et al. 2000), and mungbean (Fariduddin et al. 2003). The BRs induced improvement in photosynthetic rate might have been due to stomatal or non-stomatal factors or combination of these (Dubey 2005). Since photosystem II (PSII) plays a key role in the response of leaf photosynthesis to environmental perturbation (Baker 1991; Dubey 2005). Until now, there has been little evidence to show that epibrassinolide is directly involved in the regulation of photosynthesis. Recently, Yu et al. (2004) have demonstrated that exogenous application of epibrassinolide improved the photosynthetic capacity in Cucumis sativus through increase in PSII quantum yield. However, in the present study, quantum yield of PSII measured as F v/F m was not affected either due to salt stress or exogenously applied epibrassinolide. Thus, an increase in photosynthetic capacity of both wheat cultivars at varying levels of epibrassinolide under non-saline or saline conditions cannot be related to their photochemical properties.
Since, BRs has a role in stomatal conductance (Hayat et al. 2000; Fariduddin et al. 2003), it can be expected that BRs application might have promoted A through stomatal factors. Net photosynthetic rate (A) was positively associated with sub-stomatal CO2 (C i) and stomatal conductance (g s), indicating that BRs-induced increase in photosynthetic capacity was due to overcoming stomatal limitations. However, in cv. MH-97 an increase or decrease in g s of both wheat cultivars at varying levels of BRs under saline conditions were not accompanied by a significant corresponding change in C i, suggesting that stomatal conductance was not the sole factor for BRs-induced changes in photosynthesis. Non-stomatal limitations to photosynthetic rate may include photosynthetic pigments, rubisco enzyme concentration and activity, and use of assimilation products (Dubey 2005). Of the above-mentioned variables, only photosynthetic pigments were determined in the present study. However, parallels between rate of photosynthesis and chlorophyll ‘a’, cannot be easily drawn. Thus, improved photosynthetic rate with exogenously applied BRs of both cultivars under non-saline or saline conditions cannot be related to photosynthetic pigments measured in the present study. In view of Yu et al. (2004) it is plausible to propose that exogenous application of BRs increased the capacity of CO2 assimilation in the Calvin cycle by an increase in the initial activity of rubisco.
Growth promotive effect of BRs might have also been due to its role in ion homeostasis, which is necessary for various biochemical or physiological processes controlling growth. For example, BRs has a role in turgor-driven cell expansion by enhancing activity of aquaporins (Morillon et al. 2001), or in proton pumping and modulation of stress tolerance (Sakurai et al. 1999). However, exogenous application of BRs had a further decreasing effect (more negative values) on both leaf osmotic potential (ψs) and leaf water potential (ψw) of both wheat cultivars. Furthermore, leaf turgor potential was only improved in salt moderately sensitive cv. MH-97 due to BRs-induced osmoregulatory changes. However, there was no positive relationship between leaf turgor potential and growth indicating that leaf turgor did not control the growth. Furthermore, exogenous application of BRs did not change the accumulation of Na+ and K+ in the leaves of both cultivars (data not shown). Thus, BRs-induced improvement in growth under saline conditions by modulating water or ion-homeostasis cannot be generalized.
In conclusion, salt-induced reduction in growth was ameliorated by the exogenous application of BRs in both cultivars, which was associated with improved photosynthetic capacity. BRs-induced improvement in photosynthetic capacity of both cultivars was due to combination of stomatal and non-stomatal factors. However, this improvement was not due to its protective effect on photosynthetic pigments. Furthermore, ameliorative effect of BRs was not associated with BRs-induced changes in water homeostasis, thus, detailed insights of complex interactive effects of BRs on biochemical and physiological processes associated with photosynthesis by regulating plant hormones, or signal transduction pathways need to be elucidated.
References
Ali B, Hayat S, Ahmad A (2007) 28-Homobrassinolide ameliorates the saline stress in chickpea (Cicer arietinum L.). Environ Exp Bot 59:217–223. doi:10.1016/j.envexpbot.2005.12.002
Amzallag GN (2002) Brassinosteroids and metahormones: evidence for their specific influence during critical period in sorghum development. Plant Biol 4:656–663. doi:10.1055/s-2002-37397
Anuradha S, Rao SSR (2003) Applications of brassinosteroids to rice seeds (Oryza sativa L.) reduce the impact of salt stress on growth, prevented photosynthetic pigment loss and increased nitrate reductase activity. Plant Growth Regul 40:29–32. doi:10.1023/A:1023080720374
Arnon DT (1949) Copper enzyme in isolated chloroplasts, polyphenaloxidase in Beta vulgaris. Plant Physiol 24:1–15
Ashraf M (2001) Relationships between growth and gas exchange characteristics in some salt-tolerant amphidiploid Brassica species in relation to their diploid parents. Environ Exp Bot 45:155–163. doi:10.1016/S0098-8472(00)00090-3
Ashraf M (2003) Relationships between leaf gas exchange characteristics and growth of differently adapted populations of Blue panic grass (Panicum antidotale Retz) under salinity or waterlogging. Plant Sci 165:69–75. doi:10.1016/S0168-9452(03)00128-6
Ashraf M (2004) Some important physiological selection criteria for salt tolerance in plants. Flora 199:361–376
Baker NR (1991) A possible role for photosystem II in environmental perturbations of photosynthesis. Physiol Plant 81:563–570. doi:10.1111/j.1399-3054.1991.tb05101.x
Clouse SD, Sasse JM (1998) Brassinoseroides essential regulators of plant growth and development. Annu Rev Plant Physiol Plant Mol Biol 49:427–451. doi:10.1146/annurev.arplant.49.1.427
Dubey RS (2005) Photosynthesis in plants under stressful conditions. In: Pessarakli M (ed) Hand book photosynthesis, 2nd edn. CRC Press, Taylor and Francis Group, New York, pp 717–737
Fariduddin Q, Ahmad A, Hayat S (2003) Photosynthetic response of vigna radiata to presowing seed treatment with 28-homobrassinolide. Photosynthetica 41:307–310. doi:10.1023/B:PHOT.0000011968.78037.b1
Fujii S, Saka H (2001) Distribution of assimilates to each organ in rice plants exposed to low temperature at the ripening stage and effect of brassinolide on the distribution. Plant Prod Sci 4:134–136
Goetz M, Godt DE, Roitsch T (2000) Tissue-specific induction of the mRNA for an extra-cellular invertase isoenzyme of tomato by brassinosteroids suggests a role for steroid hormones in assimilate partitioning. Plant J 22:515–522. doi:10.1046/j.1365-313x.2000.00766.x
Grieve MC, Scott ML, Francois EL, Mass VE (1992) Analysis of main-spike yield components in salt-stressed wheat. Crop Sci 32:697–703
Haubrick LL, Assmann SM (2006) Brassinosteroids and plant function: some clues, more puzzles. Plant Cell Environ 29:446–457. doi:10.1111/j.1365-3040.2005.01481.x
Hayat S, Ahmad A, Mobin M, Hussain A, Faridduddin Q (2000) Photosynthetic rate, growth and yield of mustard plants sprayed with 28-homobrassinolide. Photosynthetica 38:469–471. doi:10.1023/A:1010954411302
Morillon R, Catterou M, Sangwan RS, Sangwan BS, Lassalles JP (2001) Brassinolide may control aquaporin activities in Arabidopsis thaliana. Planta 212:199–204. doi:10.1007/s004250000379
Munns R (2005) Genes and salt tolerance: bringing them together. New Phytol 167:645–663
Mussig C (2005) Brassinosteroid-promoted growth. Plant Biol 7:110–117. doi:10.1055/s-2005-837493
Nakajima N, Toyama S (1999) Effects of epibrassinolide on sugar transport and allocation to the epicotyl in cucumber seedlings. Plant Prod Sci 2:165–171
Nátr L, Lawlor DW (2005) Photosynthetic plant productivity. In: Pessarakli M (ed) Hand book of photosynthesis, 2nd edn. CRC Press, New York, pp 501–524
Petzold U, Peschel S, Dahse T, Adams G (1992) Stimulation of C14-sucrose export in Vicia faba plants by brassinosteroids, GA3 and IAA. Acta Bot Neerl 41:469–479
Raza SH, Athar HR, Ashraf M (2006) Influence of exogenously applied glycinebetaine on the photosynthetic capacity of two differently adapted wheat cultivars under salt stress. Pak J Bot 38(2):341–351
Robinson SP, Downton WJS, Millhouse JA (1983) Photosynthesis and ion content of leaves and isolated chloroplasts of salt-stressed spinach. Plant Physiol 73:238–242
Sakurai A, Yokota T, Clouse SD (1999) Brassinosteroids. Steroidal plant hormones. Springer, Tokyo
Sasse JM (1997) Recent progress in brassinosteroid research. Physiol Plant 100:696–701. doi:10.1111/j.1399-3054.1997.tb03076.x
Shahbaz M, Ashraf M, Athar HR (2008) Does exogenous application of 24-epibrassinolide ameliorate salt induced growth inhibition in wheat (Triticum aestivum L)? Plant Growth Regul 55:51–64. doi:10.1007/s10725-008-9262-y
Snedecor GW, Cochran GW (1980) Stat Method, 7th edn. The Iowa State University Press, Ames
Strasser RJ, Srivastava A, Govindjee (1995) Polyphasic chlorophyll ‘a’ fluorescence transients in plants and cyanobaderia. Photochem Photobiol 61:32-42. doi:10.1111/j.1751-1097.1995.tb09240.x
Yu JQ, Huang LF, Hu WH, Zhou YH, Mao WH, Ye SF et al (2004) A role of brassinosteroid in regulation of photosynthesis in Cucumus sativus. J Exp Bot 55:135–1143. doi:10.1093/jxb/erh124
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ali, Q., Athar, HuR. & Ashraf, M. Modulation of growth, photosynthetic capacity and water relations in salt stressed wheat plants by exogenously applied 24-epibrassinolide. Plant Growth Regul 56, 107–116 (2008). https://doi.org/10.1007/s10725-008-9290-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10725-008-9290-7