Abstract
Background
The most common technique described for robotic ventral hernia repair (RVHR) is intraperitoneal onlay mesh (IPOM). With the evolution of robotics, advanced techniques including retro rectus mesh reinforcement, and component separation are being popularized. However, these procedures require more dissection, and longer operative times. In this study we reviewed our experience with robotic ventral/incisional hernia repair (RVHR) with hernia defect closure (HDC) and IPOM.
Methods
Retrospective chart review and follow-up of 31 consecutive cases of ventral/incisional hernia treated between August 2011 and December 2018. Demographics, operative times, blood loss, length of stay (LOS), hernia size, location, and type, mesh size and type, recurrence, conversion to open ventral hernia repair (OVHR) and complications including bleeding, seroma formation and infection were analyzed.
Results
Mean age was 63.9 years old, with median BMI of 31.24 kg/m2. Median hernia area was 17 cm2. Mean operating time was 142.61 min (SD 59.79). Mean LOS was 1.46 days (range 1–5), with 48% being outpatient, and overnight stay in 32% for pain control. Conversion was necessary in 12.9% cases. Complication rate was 3% for enterotomy. Recurrence was 14.81% after a mean follow-up of 26.96 months. There was significant association of recurrence with COPD history (P = 0.0215) and multiple hernia defects (P = 0.0376).
Conclusion
Our recurrence rate (14.81%) compares favorably to those reported in literature (16.7%) for LVHR with HDC and IPOM. Our experience also indicates that IPOM is associated with satisfactory outcomes, low conversion and complications rates, and short LOS.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
Historically, ventral/incisional hernias have been repaired using an open approach (OVHR) but in recent years, there has been a shift to minimally invasive techniques including laparoscopic ventral hernia repair (LVHR) and robotic ventral hernia repair (RVHR). Studies have demonstrated that application of robotic technology to ventral hernia repair is safe and feasible [1]. RVHR has become implemented by surgeons due to its advantageous ergonomics especially for fascial closure and mesh fixation without the use of tackers. Although the use of mesh has reduced recurrence of ventral hernias, the number is still high, affecting approximately 5 million Americans with a cost of 2.5 to $3 billion for every 250,000 ventral hernia repairs performed each year in the United States.
The most common technique described in the literature is intraperitoneal onlay mesh (IPOM) reinforcement. However, with recent advances and availability of robotics, different techniques including primary close with transabdominal preperitoneal (TAPP) mesh reinforcement, retro rectus mesh reinforcement (Rives-Stoppa), and components separation with Transversus Abdominis release (TAR), are being reported and popularized in the literature. However, these procedures are more complex, require significantly more dissection, surgical expertise, and longer operative times. As RVHR is becoming more widespread, it is crucial to determine which technique provides feasibility, security, and also positive outcomes, in term of improvements of length of stay (LOS), complications, and recurrence rates. The purpose of this study was to characterize our experience with RVHR with fascial hernia defect closure (HDC) and IPOM reinforcement.
Methods
We performed a retrospective chart review and follow-up of 31 consecutive cases of RVHR with HDC and IPOM reinforcement, completed between August 2011 to December 2018 using the CERNER® system and paper-based charts, and created a worksheet database in Microsoft® Excel® 2019 software version 1808, with age, gender, comorbidity, American Society of Anesthesiologists (ASA) classification, height, weight, operatives times, blood loss, LOS, size and type of mesh, size, location, and type of hernia, and postoperative outcomes including complications rate, conversion and recurrence. Follow-up data was obtained through chart review and standardized phone calls for those patients who have no follow-up within the last year. We performed a descriptive statistical analysis using Microsoft® Excel®, and a stratified data analysis using Graphpad™ Prism® software version 7.05.237, for Chi square and Fisher exact test calculations. A P value < 0.05 was considered statistically significant. This study was performed after obtaining approval from the University of Miami Institutional Review Board (IRB), and the Jackson Health System Office of Research.
Surgical technique
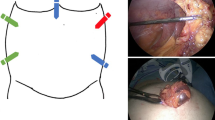
The patient is placed on the table in the supine position. After adequate general anesthesia is attained, the abdomen is prepped and draped in the usual sterile manner. Pneumoperitoneum is established by Veress needle. The abdomen is initially insufflated to 15 mmHg. When using Airseal™, pressure can be decreased as needed to 8 mmHg especially for defect closure. Trocars are placed as described in the Intuitive™ manual. An example is shown in Fig. 1a, b.
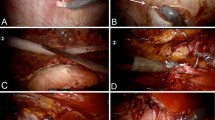
The robot is docked and the surgeon proceeds to the console as in Fig. 1c. Adhesions to the anterior abdominal wall and the hernia sac are cleared and the hernia contents reduced, Fig. 2a. The peritoneal sac is either left in situ or dissected as shown in Fig. 2b. After completion of the dissection, the hernia defect is measured, and closed approximating the fascial edges of the hernia defect with non-absorbable 0 barbed suture (V-Loc™ PBT, Medtronic Covidien, or Stratafix™ Ethicon, Johnson & Johnson) in two layers as depicted in Fig. 2c. This is reinforced with several interrupted figure of eight sutures with non-absorbable 0 polypropilene (Prolene™, Ethicon, Johnson & Johnson). An appropriately sized mesh is tailored to overlap all margins of the defect by 5 cm. The mesh is introduced through the 12-mm trocar into the abdominal cavity. The mesh is secured circumferentially to the abdominal wall with absorbable double arm 2–0 barbed suture (Stratafix™, Ethicon, Johnson & Johnson) and the final aspect is shown in Fig. 2d. The trocars are removed under direct vision. The fascia of the 12-mm port site is routinely closed with 0 Prolene™ and a suture passer; skin incisions were closed in subcuticular fashion. Patients were examined postoperatively at 1 week, 3 months, 1 year, and thereafter, as clinically indicated. Follow-up was achieved by reviewing medical records of clinic visits, and a structured phone interview performed by two reviewers. All patients with a minimum of 7-day follow-up were included in the analysis for follow-up results.
Results
A total of 31 charts of cases of robotic ventral hernia repair were retrospectively reviewed using either CERNER™ system or paper-based charts. Procedures were performed by two robotic surgeons, between August 2011 to December 2018 in a low-volume community hospital in the city of Miami, and data was used for analysis.
Patients characteristics
Demographic features and perioperative data of the 31 patients are listed in Table 1. There was a predominance of female patients over males (68% vs. 32%). Mean age was 64.3 years old (range 39–85). Median BMI was 31.24 kg/m2 (range 22.89–47.99), 52% of patients were ASA class II and 42% ASA class III. Full list of comorbidities is listed on Table 2.
Operative results
Incisional hernias were more common than “primary” ventral hernias (80.65% vs. 19.35%). We found 6 (19.35%) patients with multiple hernia defects and 2 (6.45%) patients with recurrence from previous hernia repairs. The most common hernia location was periumbilical (32%), followed by midline (25%). Overall, median hernia area was 17 cm2 (range 9–198). Detailed information regarding hernia type, size, characteristics and location is listed in Table 3. For analysis purposes, patients were classified by hernia type and size according to the European Hernia Society (EHS) [2].
Most common mesh used for reinforcement was Ventralight™ ST Echo PS™ (Bard, BD) in a total of 22 patients. Mean operative time was 142.61 min (SD 59.79). Hospital length of stay (LOS) was 1.46 days (range 1–5), with 48.38% of outpatient cases, and 32.25% overnight stay in cases where postoperative pain control was needed. Conversion to open surgery was necessary in 4 (12.9%) patients, in 2 patients after extensive intraabdominal adhesions, in one patient after a large defect of 18 × 11 cm composed by several fascial defects was found, and in one patient after a small bowel enterotomy during hernia sac lysis. The latter, is listed in Table 4 as a complication (3.23%). Other complications associated to minimally invasive abdominal wall hernia repair procedures such as ileus, seroma, surgical site infection and sepsis, urinary retention, respiratory distress, deep venous thrombosis, bowel obstruction, infectious colitis, renal failure, anemia or death were not found in this series.
Although it was not considered a postoperative complication, 2 (6%) patients complained of mild discomfort during follow-up evaluations that did not require additional intervention. After a mean follow-up of 26.96 months, a recurrence rate of 14.81% was observed.
Discussion
While reviewing our experience, a number of questions came to mind regarding the current utility of IPOM as a first line option for RVHR, especially when detractors sustain their claims on the fact that the mesh stays in direct contact with abdominal viscera and compromises security for the risk of adhesions, fistulas, perforations, etc. Nevertheless, we had the opportunity to visualize how the mesh was completely epithelized 6 months after an IPOM RVHR, during a laparoscopic surgery, not related to previous hernia repair, of one of our patients. In Fig. 2e, it is shown how the mesh is completely protected by a lining of peritoneum, disregarding any possibility of adhesion to intrabdominal viscera. Hence, we asked ourselves, should IPOM be abandoned in favor of TAPP and retro rectus mesh reinforcement for all cases of robotic ventral/incisional hernia repair? If not, what are the indications to robotic IPOM? Despite its detractors [3], IPOM is the approach that most general surgeons are using today when performing both LVHR and RVHR, since TAPP requires longer operative times and extensive tissue dissection.
The introduction of robotic technology has addressed some of the limitations of laparoscopy, improving dexterity, ergonomics, and increasing the surgeon ability to close fascial hernia defects with ease [1, 4, 5]. Martin‑del‑Campo et al. [6] evaluating LVHR with and without HDC found that the addition of closure was not associated with a significant increase in operative time. However, longer operative times have been seen in robotic surgery cases in general [7], a tendency also reported by Chen et al. [8] and Warren et al. [5] after comparing LVHR to RVHR. Our median surgical time was 142 min (range 84–396), due to a small number of cases (12%) exceeding 3 h duration. Majority of the patients (83%) had an operating time in the range of 84–185 min (mean 130 min) which compares to the published literature.
In this series, we performed primary closure of all hernia defects, after the reduction of the hernia sac content, facilitated by a safe and easy lysis of adhesions with the robotic instruments. We firmly believe that approximating the fascial edges by suture prior to fixation of the mesh, enables a more physiologic and anatomic hernia repair. In a previous experience [9] comparing closure versus non-closure of hernia defect during LVHR with mesh, we found a lower recurrence rate in the closure group (6.25%) versus non-closure (19.18%), a finding also corroborated by other publications [6, 10, 11]. It is important to note that intracorporeal laparoscopic closure of fascial defects is a surgical practice not broadly performed due to its technical complexity and demands for advanced laparoscopic skills [12], nonetheless, current availability of robotic surgery equipment even in small community hospitals is turning the balance in favor of more robotic procedures being performed [13].
We agree with statements affirming that RVHR offers the ergonomic movements and dexterity of wristed instrumentation, allowing the surgeon to secure the mesh to the abdominal wall with a circumferential absorbable suture with ease. In our experience, this advantageous feature of the robotic platform has significantly reduced the incidence of postoperative pain complains related to mesh fixation with absorbable tacks, a well-known issue in patients with laparoscopic hernia repairs.
In this series, we had a mean LOS of 1.46 days (range 1–5), that compares favorably to literature reports [12, 14,15,16]. Authors with large case series such as Gonzalez et al. [12] reported a mean LOS of 1 day for RVHR. Recent reports describe shorter LOS for RVHR vs. LVHR, with a comparable perioperative morbidity [8]. However, authors such as Coakley et al. [15] and Armijo P et al. [16] report no difference in LOS comparing RVHR versus LVHR.
Gonzalez et al. [12] presented 368 patients who underwent a RVHR with a total complication rate of 8.4%, including urinary retention (3.8%), ileus (2.4%), seroma formation (1.4%) and bowel injury (0.5%). In a systematic review of 11 publications of LVHR with primary fascial close and IPOM, Nguyen et al. [11] found a 4.3–27.8% seroma rate and a 4.8–16.7% recurrence rate. In our experience we had a patient (3.23%) with an enterotomy. Our recurrence rate (14.81%) compares favorably to that reported in the literature (16.7%) for LVHR with HDC and IPOM [11], but is higher than the recurrence reported in other studies of RVHR [12]. We believe that our recurrence rate was attributed mainly to patient conditions more than to the technique itself. After a stratified analysis of our data, we found a significant association between positive history of chronic obstructive pulmonary disease (COPD) and recurrence (P = 0.0215). Similarly, significant association between multiple hernia defects repair vs. single hernia defect repair and recurrence was observed (P = 0.012). In contrast, we found that recurrence was not associated with hernia size (P = 0.603), body mass index (BMI) (P = 0.3357), positive history of cancer (P = 0.3173), or dyslipidemia (P = 0.1313). Inclusion of a larger number of cases is warranted to address any other confounding variables that may play a role in recurrence. Heniford et al. [17] in a large series of 850 cases of LVHR, found that bigger hernia size was significantly associated with higher recurrence rates and that patients who had a complication were more than 3 times more likely to have a hernia recurrence (P < 0.05), while morbid obesity patients showed a trend toward higher chances of complications that did not reach statistical significance (P = 0.09). Licari et al. [18] described a greater incidence of comorbidities such as diabetes (37%), dyslipidemia (16%), hypertension (51%) and COPD (16%) among patients with recurrence after ventral hernia repair.
An important aspect to have in mind when planning a RVHR with HDC and IPOM is hernia defect size. Years of experience with LVHR and now RVHR have taught that an 8–10 cm fascial defect diameter is the maximal cutoff point to decide between an OVHR and a RVHR in carefully selected patients. Dealing with large hernias results in additional technical challenges, derived from handling bigger meshes and working under too much tension on the abdominal wall. We recommend to decrease pneumoperitoneum pressure to 8 mmHg with the Airseal™ system, during the hernia defect closure, and to use a 15 × 20 cm mesh in large hernias with lengths up to 10 cm, to provide a 5 cm mesh overlap.
In conclusion, we still recommend implementation of RVHR with HDC and IPOM, given it feasibility, security, and good results. Given that the main drawback was the small number of cases presented in this study, data obtained in a low-volume community hospital is far from been generalizable for larger institutions. However, is important to keep in mind that our main goal was answering a simple question: should we continue to implement RVHR IPOM or should we move into more complex robotic procedures? The answer is evident, RVHR IPOM is both secure and effective, and can be offered as a first line of treatment in carefully selected patients. We recommend to analyze judiciously each particular case, and give proper importance to comorbidities at the time of selecting the most appropriate ventral hernia repair approach. Randomized clinical trials comparing robotic TAPP and IPOM ventral/incisional hernia repair are still needed to gather more clinical evidence regarding outcomes and indications.
References
Kudsi OY, Paluvoi N, Bhurtel P, McCabe Z, El-Jabri R (2015) Robotic repair of ventral hernias: preliminary findings of a case series of 106 Consecutive cases. Am J Robot Surg 2(1):22–26
Muysoms FE, Miserez M, Berrevoet E, Campanelli G et al (2009) Classification of primary and incisional abdominal wall hernias. Hernia 13(4):407–414
Sarli L, Pietra N, Choua O et al (1997) Laparoscopic hernia repair: a prospective comparison of TAPP and IPOM techniques. Surg Laparosc Endosc 7(6):472–476
Gonzalez AM, Romero RJ, Seetharamaiah R et al (2015) Laparoscopic ventral hernia repair with primary closure versus no primary closure of the defect: potential benefits of the robotic technology. Int J Med Robot 11(2):120–125
Warren JA, Cobb WS, Ewing JA et al (2017) Standard laparoscopic versus robotic retromuscular ventral hernia repair. Surg Endosc 31:324–332
Martin-Del-Campo LA, Miller HJ, Elliott HL, Novitsky YW (2018) Laparoscopic ventral hernia repair with and without defect closure: comparative analysis of a single-institution experience with 783 patients. Hernia 22(6):1061–1065
Turchetti G, Palla I, Pierotti F, Cuschieri A (2012) Economic evaluation of da Vinci assisted robotic surgery: a systematic review. Surg Endosc 26(3):598–606
Chen YJ, Huynh D, Nguyen S et al (2017) Outcomes of robot-assisted versus laparoscopic repair of small-sized ventral hernias. Surg Endosc 31(3):1275–1279
Zeichen MS, Lujan HJ, Mata WN et al (2013) Closure versus non-closure of hernia defect during laparoscopic ventral hernia repair with mesh. Hernia 17(5):589–596
Rea R, Falco P, Izzo D et al (2012) Laparoscopic ventral hernia repair with primary transparietal closure of the hernial defect. BMC Surg 12(1):33–37
Nguyen DH, Nguyen MT, Askenasy EP et al (2014) Primary fascial closure with laparoscopic ventral hernia repair: systematic review. World J Surg 38(12):3097–3104
Gonzalez A, Escobar E, Romero R et al (2017) Robotic-assisted ventral hernia repair: a multicenter evaluation of clinical outcomes. Surg Endosc 31(3):1342–1349
Stoikes N, Webb D, Voeller G (2016) Robotic hernia repair. Surg Technol Int 29:119–122
Carbonell AM, Warren JA, Prabhu AS et al (2018) Reducing length of stay using a robotic-assisted approach for retromuscular ventral hernia repair: a comparative analysis from the Americas Hernia Society Quality Collaborative. Ann Surg 267(2):210–217
Coakley KM, Sims SM, Prasad T et al (2017) A nationwide evaluation of robotic ventral hernia surgery. Am J Surg 214(6):1158–1163
Armijo P, Pratap A, Wang Y et al (2018) Robotic ventral hernia repair is not superior to laparoscopic: a national database review. Surg Endosc 32(4):1834–1839
Heniford BT, Park A, Ramshaw BJ, Voeller G (2003) Laparoscopic repair of ventral hernias: nine years’ experience with 850 consecutive hernias. Ann Surg 238(3):391–399 (discussion 399–400)
Licari L, Salamone G, Campanella S et al (2019) Use of the KSVM-based system for the definition, validation and identification of the incisional hernia recurrence risk factors. G Chir 40(1):32–38
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Dr. Lujan is a consultant for Intuitive Surgical, Inc. Dr. Fuenmayor, Dr. Plasencia, Dr. Karmaker, Dr. Mata and MS. Vecin declare that they have no conflict of interest.
Research involving human participants
Approval from the University of Miami Institutional Review Board and Jackson Health System Office of Human Research were obtained before the development of this study. This research posed a minimal risk to its human subjects.
Informed consent
Patients treatment has already occurred at the time this study was developed. Being a retrospective data study, informed consent was not needed.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Fuenmayor, P., Lujan, H.J., Plasencia, G. et al. Robotic-assisted ventral and incisional hernia repair with hernia defect closure and intraperitoneal onlay mesh (IPOM) experience. J Robotic Surg 14, 695–701 (2020). https://doi.org/10.1007/s11701-019-01040-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11701-019-01040-y