Abstract
Robotic surgery enhances the precision of minimally invasive surgery through improved three-dimensional views and articulated instruments. There has been increasing interest in adopting this technology to colorectal surgery and this has recently been introduced to the Irish health system. This paper gives an account of our early institutional experience with adoption of robotic colorectal surgery using structured training. Analysis was conducted of a prospectively maintained database of our first 55 consecutive robotic colorectal cases, performed by four colorectal surgeons, each at the beginning of his robotic surgery experience, using the Da Vinci Si® system and undergoing training as per the European Academy of Robotic Colorectal Surgery (EARCS) programme. Overall surgical and oncological outcomes were interrogated. Fifty-five patients underwent robotic surgery between January 2017 and January 2018, M:F 34:21, median age (range) 60 (35–87) years. Thirty-three patients had colorectal cancer and 22 had benign pathologies. Eleven rectal cancer patients had neoadjuvant chemoradiotherapy. BMI was > 30 in 21.8% of patients and 56.4% of patients had previous abdominal surgery. Operative procedures performed were low anterior resection (n = 19), sigmoid colectomy (n = 9), right colectomy (n = 22), ventral mesh rectopexy (n = 3), abdominoperineal resection (n = 1) and reversal of Hartmann’s procedure (n = 1). Median blood loss was 40 ml (range 0–400). Mean operative time (minutes) was 233 (SD 79) for right colectomy and 368 (SD 105) for anterior resection. Median length of hospital stay was 6 days (IQR 5–7). There was no 30-day mortality, intraoperative complications, conversion to laparoscopic or open, or anastomotic leakage. Median lymph nodes harvest was 15 in non-neoadjuvant cases (range 7–23) and 8 in neoadjuvant cases (2–14). Our early results demonstrate that colorectal robotic surgery can be adopted safely for both benign and neoplastic conditions using a structured training programme without compromising clinical or oncological outcomes. The early learning curve can be time intensive.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Minimal invasive surgery has gained wide acceptance and utilisation in colorectal surgery over the last two decades. Randomised controlled trials have established that the laparoscopic approach is associated with good oncological outcomes, shorter hospital stay, fewer morbidities and less postoperative pain [1, 2]. Long-term outcomes are improved with less postoperative adhesions, fewer incisional hernias and easier reoperation when needed [3,4,5,6,7]. As a result, laparoscopic resection has become the standard of care in colorectal resections in many institutions [8,9,10,11]. However, despite these benefits, adoption of laparoscopy, particularly it’s utilisation in rectal cancer surgery remains around 30–50% in the Western world [12]; this is partly due to limitation of straight instruments particularly in narrow pelvis and longer learning curve associated with learning such techniques [13,14,15,16].
Robotic surgery brings technical advantages over laparoscopic surgery with increased degrees of instrument movement, reduced fulcrum effect and better optics. Robotic systems provide an interface between the operating surgeon and the patient into which enabling technology can be built to overcome ergonomic difficulties and to enhance the precision of surgical procedures. The Da Vinci Robotic® system (Intuitive Surgical Inc., Sunnyvale, CA, USA) is currently the most widely used robotic system and provides a three-dimensional view, using a stable platform for precise dissection. The Endowrist® feature offers a wide range of movement allowing 7 degrees of freedom, 180° articulation and 540° rotation [17, 18]. These attributes allow a vastly more sophisticated range of movements when compared to the straight laparoscopic instruments. This advantage is particularly important in confined spaces such as the lower third of rectum and the pelvic floor.
Weber performed the first robotic colonic resection in 2002, and since then there has been an increasing interest in adopting robotic platforms in many colorectal units [19]. Robotic colorectal surgery was introduced to the Irish health system in 2016, and our hospital is among the first in the country to embrace this technology.
This manuscript describes our institutional experience with the adoption of robotic colorectal surgery for a variety of benign and malignant conditions following the training structure outlined by the European Academy of Robotic Colorectal Surgery (EARCS) [20].
Methods
Study population
Prospective data on 55 consecutive patients who underwent robotic colorectal procedures from January 2017 to January 2018 were analysed. Robotic surgery was performed by four colorectal surgeons who were closely supervised by a robotic trainer for initial part of their experience.
Patients’ selection and preoperative workup
Patients with both benign and malignant conditions were offered robotic surgery. All patients with colorectal cancer had preoperative staging and were discussed in our MDT meeting. Patients with T3/4 rectal cancer, node-positive disease and/or involved or a threatened circumferential mesorectal margin had neoadjuvant therapy. It involved long course chemoradiation regime with surgery performed between 8 and 12 weeks following the completion of down-staging.
Robotic training
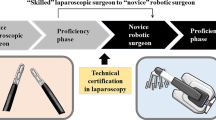
Four surgeons with prior laparoscopic colorectal experience were enrolled into the EARCS robotic training program at different times during the year. Da Vinci Si® robot was used in all patients. Robotic training was as outlined by EARCS [20] and involved the following steps:
-
1.
Theoretical knowledge and case observations:
This step consisted of lecture-based teaching to cover the knowledge of pelvic anatomy and the theory behind Total Mesorectal Excision (TME). It also incorporated live case observation at a faculty member institution.
-
2.
Robotic system and dissection training course:
A 2-day course was conducted at a designated training centre to provide insight and competence with the system and console using a porcine training model for procedure specific training.
-
3.
Hands-on clinical training:
Proctored clinical training was conducted by EARCS faculty members at Beacon Hospital. Surgeons started with benign and malignant right and left sided resections and progressed to rectal resections at a pace directed by the supervising proctor. Five satisfactory rectal resections was a minimum requirement for competence.
-
4.
Final competence assessment and accreditation:
Global Assessment Score (GAS) forms were used for assessment of competence. Upon completion of training, two unedited videos of self-performed robotic low anterior resection (LAR) were submitted for blind assessment by EARCS faculty. Satisfactory performance as independently judged was a requirement for graduation from the programme and EARCS certification. At the time of preparation of this manuscript, two of our surgeons has achieved EARCS accreditation, one is half way through and the other is at an earlier stage of the training pathway.
Ethical consideration
This study was reviewed and approved by the ethics committee in our institute.
Statistical analysis
IBM SPSS version 24 (SPSS Inc., Chicago, IL, USA) and Microsoft Excel 2016™ were used for the statistical analysis. Data variables were expressed as median with range or inter-quartile range (IQR) for non-parametric data and mean with standard deviation for parametric data.
Results
Patient demographics
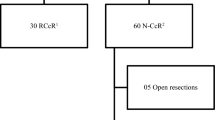
Fifty-five patients underwent robotic colorectal surgery at our hospital between January 2017 and January 2018. There were 34 males (61.8%) and 21 females (38.2%). Median age was 60 years (range 35–87). Median body mass index (kg/m2) was 26.9 (range 17.8–41.6), and 21.8% of patients had BMI greater than 30. Thirty-one patients (56.4%) had previous laparoscopic or open abdominal surgery. Sixty percent (n = 33) of procedures were performed to treat bowel cancer, while the remaining 40% were for benign conditions. Patient characteristics and surgical indications are summarised in Table 1.
Operative and clinical outcomes
Procedures performed included low anterior resection (n = 19), sigmoid colectomy (n = 9), right colectomy (n = 22), ventral mesh rectopexy (n = 3), abdominoperineal resection (n = 1) and reversal of Hartmann’s procedure (n = 1). Median blood loss was 40 ml (range 0–400). Mean operative time was 233 min (SD 79.07) for right colectomy and 368 min (SD 104.65) for anterior resection. The median length of hospital stay was 6 days (IQR 5–7).
There was no mortality within 30 days of surgery and no intraoperative complications or conversion to open or laparoscopic surgery. Readmission within 30 days rate was 7.3% (4 patients). Two patients (3.6%) required reoperation within 30 days from the index procedure; one required relook laparoscopy and the other had evacuation of port site haematoma. One patient had a colonoscopy for a late reactive short-lived anastomotic bleed. Other comorbidities included pelvic collection (n = 2) and wound infection (n = 3). Operative details and outcomes are outlined in Tables 2 and 3.
Oncological outcomes
Thirty-three patients were operated on for colorectal cancer; 19 had rectal cancer (57.6%), 3 had sigmoid cancer (9.1%), and 11 had right-sided colon cancer (33.3%). In the rectal cancer group, 11 patients (57.9%) had neoadjuvant chemoradiotherapy. Preoperative TNM classification is summarized in Table 4.
Postoperative histopathology showed 14 patients had T3 or T4 tumours and median lymph nodes harvested were 15 in non-neoadjuvant cases (range 7–23) and 8 in neoadjuvant cases (range 2–14). In rectal cancer patients, the circumferential margin (CRM) was reported negative (R0) in 18 patients (95%), while 1 patient was reported to have an R1 margin < 1 mm from CRM (this was a 52-year-old male with T3N0M0 low rectal cancer who had LAR following neoadjuvant chemoradiotherapy) Table 5.
Discussion
Colorectal surgery has become increasingly precise over recent decades with the introduction of minimally invasive surgical techniques. The adoption of laparoscopic surgery in colorectal resection has played a key role in more precise surgery and the use of the robotic systems has further enhanced this development. The utilisation of robotic technology can help to overcome the inherent technical difficulties associated with laparoscopic colorectal surgery. The (Intuitive®) robotic platform provides a three-dimensional view, using a stable platform. The robotic wristed instruments allow a more sophisticated range of movements compared to straight laparoscopic instruments, especially during dissection within the confines of the pelvis [15, 16]. There is a growing body of literature that recognises the safety and feasibility of robotic colorectal surgery [21]. For rectal cancer, in particular, it would seem to offer significant advantage to the surgeon. Despite being initially reported in 2002, the introduction of robotic colorectal surgery has been slow due to cost considerations, technical difficulty and technologic limitations.
The successful and safe adoption of any new surgical technique requires structured approach to learning and close supervision which in turn leads to better short term outcomes for patients. Whilst adopting new techniques, the impact of learning curve on patient clinical outcomes remain the most challenging aspect. We present our initial experience with robotic colorectal surgery and compare our surgical and oncological outcomes with the existing literature. Our results have demonstrated the safety and feasibility of incorporation of robotic systems in colorectal surgery using a structured approach. Our data showed there were no intraoperative complications nor conversion to open or laparoscopic surgery, which is in keeping with the reported low conversion rates in the literature [22,23,24]. The median length of stay in hospital was 6 days and the readmission rate was 7.3%. There was no mortality in our series. These findings compare very favourably to previously published data [25].
Our mean operative times were 233 min in right colectomy and 368 min in anterior resection which appear to be longer than some reported series [26, 27]. These prolonged operative times should be viewed in the context of an early learning curve for four different surgeons at variable stages in their robotic training. It worth mentioning that early reports from laparoscopic colectomies showed the operative time was prolonged when compared with open approach; however, this prolongation improved significantly when surgeons were more experienced with the laparoscopic approach [28]. Additionally, this prolongation of operative time did not translate into poor operative outcomes, as shown in our results. This observation concurs with the finding of a previous study by Sunu et al. [29], which concluded that prolongation of operative time in minimally invasive colon and rectal surgery does not adversely affect early postoperative outcomes.
In this series, 33 patients had resection for colonic cancer. Oncological outcomes in terms of R0 resections and the number of harvested lymph nodes were comparable with reported laparoscopic outcomes in the literature [30]. The same favourable results were maintained in the rectal cancer subgroup, which benefits the most from the robotic approach. Twenty-one percent of our patients had BMI greater than 30 and more than half of the rectal cancer patients were males, and 58% received preoperative neoadjuvant chemoradiotherapy. It is well recognized that these factors can make surgery more challenging, yet we observed favourable outcomes in these patients.
Our study demonstrates the safety and feasibility of adopting a robotic platform in colorectal surgery in an Irish healthcare setting. Further studies will follow which will address the long-term outcomes and will compare the robotic approach to laparoscopic approaches.
Conclusion
We believe that the robotic platform has enhanced the precision of minimally invasive colorectal surgery through better views and articulated instruments. We have embraced the EARCS training programme and achieved the introduction of robotic colorectal surgery without undermining clinical or oncological outcomes.
References
Fleshman J, Sargent DJ, Green E et al (2007) Laparoscopic colectomy for cancer is not inferior to open surgery based on 5-year data from the COST Study Group trial. Ann Surg 246:655–664. https://doi.org/10.1097/SLA.0b013e318155a762
Colon Cancer Laparoscopic or Open Resection Study Group, Buunen M, Veldkamp R et al (2009) Survival after laparoscopic surgery versus open surgery for colon cancer: long-term outcome of a randomised clinical trial. Lancet Oncol 10:44–52. https://doi.org/10.1016/S1470-2045(08)70310-3
Nelson H, Sargent DJ, Wieand HS et al (2004) A comparison of laparoscopically assisted and open colectomy for colon cancer. N Engl J Med 350:2050–2059
Abraham NS, Young JM, Solomon MJ (2004) Meta-analysis of short-term outcomes after laparoscopic resection for colorectal cancer. Br J Surg 91:1111–1124
EH A (2009) Laparoscopic colorectal surgery: summary of the current evidence. Ann R Coll Surg Engl 91:541–544
Stephen M. Kavic SMK (2002) Adhesions and adhesiolysis: the role of laparoscopy. JSLS 66:99–109
Moran DC, Kavanagh DO, Nugent E et al (2011) Laparoscopic resection for low rectal cancer: evaluation of oncological efficacy. Int J Colorectal Dis 26:1143–1149. https://doi.org/10.1007/s00384-011-1221-9
Ohtani H, Tamamori Y, Arimoto Y et al (2012) A meta-analysis of the short- and long-term results of randomized controlled trials that compared laparoscopy-assisted and open colectomy for colon cancer. J Cancer 3:49–57. https://doi.org/10.7150/jca.3621
Tjandra JJCMK (2006) Systematic review on the short-term outcome of laparoscopic resection for colon and recto-sigmoid cancer. Color Dis 8:375–388
Maggiori LPY (2013) Is it time for a paradigm shift: laparoscopy is now the best approach for rectal cancer? Transl Gastrointest Cancer 3(1):1–3
Good DW, O’Riordan JM, Moran D et al (2011) Laparoscopic surgery for rectal cancer: a single-centre experience of 120 cases. Int J Color Dis 26:1309–1315. https://doi.org/10.1007/s00384-011-1261-1
Abu Gazala M, Wexner SD (2017) Re-appraisal and consideration of minimally invasive surgery in colorectal cancer. Gastroenterol Rep 5:1–10. https://doi.org/10.1093/gastro/gox001
Lee SW (2009) Laparoscopic procedures for colon and rectal cancer surgery. Clin Colon Rectal Surg 22:218–224. https://doi.org/10.1055/s-0029-1242461
Shearer R, Aly OE, Aly EHGM (2013) Have early post-operative complications from laparoscopic rectal cancer surgery improved over the past 20 years? Color Dis 15:1211–1226
Cecil TD, Taffinder N, Gudgeon AM (2006) A personal view on laparoscopic rectal cancer surgery. Color Dis 8:30–32
Delaney CP, Lynch AC, Senagore AJ, Fazio VW (2003) Comparison of robotically performed and traditional laparoscopic colorectal surgery. Dis Colon Rectum 46:1633–1639
Ballantyne GHMF (2003) The da Vinci telerobotic surgical system: the virtual operative field and telepresence surgery. Surg Clin N Am 83:1293–1304
SH B (2008) Robotic colorectal surgery. Yonsei Med J 49:891–896
Weber PA, Merola S, Wasielewski A et al (2002) Telerobotic-assisted laparoscopic right and sigmoid colectomies for benign disease. Dis Colon Rectum 45:1686–1695
EARCS Training curriculum for robotic colon and rectal surgery. https://earcs.pt/index.php/curriculum. Accessed Apr 2018
Al Asari S, Min BS (2012) Robotic colorectal surgery: a systematic review. Surgery. https://doi.org/10.5402/2012/293894
Rawlings AL, Woodland JH, Crawford DL (2006) Telerobotic surgery for right and sigmoid colectomies: 30 consecutive cases. Surg Endosc 20:1713–1718
Kariv Y, Delaney CP (2005) Robotics in colorectal surgery. Minerva Chir 60:401–416
Giulianotti PC, Coratti A, Angelini M et al (2003) Robotics in general surgery: personal experience in a large community hospital. Arch Surg 138:777–784
Baik SH, Kwon HY, Kim JS et al (2009) Robotic versus laparoscopic low anterior resection of rectal cancer: short-term outcome of a prospective comparative study. Ann Surg Oncol 16:1480–1487
Xu H, Li J, Sun Y et al (2014) Robotic versus laparoscopic right colectomy: a meta-analysis. World J Surg Oncol 12:274. https://doi.org/10.1186/1477-7819-12-274
Sun Y, Xu H, Li Z, Han J, Song W, Wang J, Xu Z (2016) Robotic versus laparoscopic low anterior resection for rectal cancer: a meta-analysis. World J Surg Oncol 14:61
Fielding GA, Lumley J, Nathanson L et al (1997) Laparoscopic colectomy. Surg Endosc 11:745–749. https://doi.org/10.1007/s004649900441
Sunu PV, Mittal NJ (2017) Outcomes after laparoscopic or robotic colectomy and open colectomy when compared by operative duration for the procedure. Am J Surg 215(4):577–580. https://doi.org/10.1016/j.amjsurg.2017.04.020
Van der Pas MH, Haglind E, Cuesta MA et al (2013) Laparoscopic versus open surgery for rectal cancer (COLOR II): short-term outcomes of a randomised, phase 3 trial. Lancet Oncol 14:210–218
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Mohammed Aradaib, Paul Neary, Adnan Hafeez, Reza Kalbassi and Diarmuid O’Riordain declare that they have no conflict of interest. Professor Amjad Parvaiz is a proctor for the European Academy of Robotic Colorectal Surgery which is funded by Intuitive Surgical.
Rights and permissions
About this article
Cite this article
Aradaib, M., Neary, P., Hafeez, A. et al. Safe adoption of robotic colorectal surgery using structured training: early Irish experience. J Robotic Surg 13, 657–662 (2019). https://doi.org/10.1007/s11701-018-00911-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11701-018-00911-0