Abstract
This paper aims to present the influence of anode catalyst supports, current collector open ratio, liquid electrolyte layer incorporated membrane, and methanol concentration on the passive Direct methanol fuel cell (DMFC) performance. Current collectors with three different opening ratios of 45.40%, 55.40% and 63.05% were considered in the present study. For the three current collectors, the fuel cell is operated with diluted methanol solution (concentration varied from 1 to 5 M) as the anodic fuel. The experimental results showed that the current collector with 55.40% open ratio at 3 M of methanol concentration produced the MPD compared to the other two open ratios. A liquid electrolyte (LE) layer was inserted in between the two half MEAs to recuce the methanol crossover. It was noticed that the LE-DMFC gave the best performance with all the three current collector having different opne ratios. Similarly, the effect of two anaode catalyst layers was also studied. From the experimental results, it was noticed that the fuel cell performance improved by using the MEA with two layers of anode catalyst of Pt-Ru/Carbon black + Pt-Ru/C.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Portable electronic devices such as personal data assistants, mobile phones, laptops, computers etc., are playing a important role in the day to day life throughout the world (Yunphuttha et al. 2012). The current rechargeable battery technology will not reach the present requirements for charging these devices. The increase in demand for these devices and the scarcity of conventional energy sources will pose a serious challenge. Fuel cell technology gives a feasible solution to reach these challenges. Out of the different types of fuel cells, DMFCs are best suited for such applications by virtue of their higher energy density (Irannejad et al. 2019; Ulas et al. 2018).
DMFCs are of two types, viz., passive and active. In the active DMFC system, the fuel at the anode and the oxidant at the cathode are supplied by a pump and an external compressors, respectively. In the passive DMFC system, the anodic fuel and the oxidant at the cathode are supplied by diffusion and natural convection process, respectively. The passive DMFC is very useful for charging small electronic devices, which has no harmful emissions other than carbon dioxide. The design of the current collectors plays a major role in the passive DMFC system. The current collectors (CC) are fabricated by pressing and cutting operation or by laser cutting technology. In the passive DMFC,CC are made with different shapes of perforations such as circular, rectangular, hexagon, triangular, etc.
Yousefi et al. (2012, 2013) pioneered the study of large active area of single cell passive DMFC. They experimented on the influence of various parameters such as methanol molar concentration, sealing design, and end frame designon the passive DMFC performance. They concluded that the cell performance enhanced with increase in the methanol concentration up to 4 M, and there after the cell performance deteriorated. This decrease in the cell performance was attributed to methanol crossover. Yang et al. (2007) experimentally studied the impact of CC structure on the cell performance. They found that circular CC on the cathode side and parallel CC on the anode side were the best. Borello et al. (2014) tested the performance of two types of perforated CC of OR 36% and 38%. It was noticed that 38% and 36% open ratio current collectors gave their best at 1M, 2 M and 4 M methanol concentration, respectively.
Xue et al. (2015) numerically investigated the effect of geometry of the circular holes on the cathode CC with inclusion of distance between them. They revealed that the CC with small uniformly sized holes was better at the cathode side. Scott et al. (2001) analysed the outcome in use of metal meshes as flow fields for DMFC. It was identified that relatively thick mesh with low voidage along with rough surface was promising. Shrivastava et al. (2014a, b) analysed the effect of adding as Supporting plate for the stainless steel wire mesh current collector (WMCC) in a passive DMFC. Enhancement in cell performance was reported with the incorporation of the supporting plate because of the phenomenon of optimum diffusion layer compression. Mallick and Thombre (2017) and Mallick et al. (2016) experimentally studied the influence of clamping torque along with expanded metal mesh current collectors (EMCC) with supporting plates on the performance of a single passive DMFC. They noticed that uniform clamping torque up to 8 N-m showed the best performance. Falcão et al. (2016) analysed the outcome of meshes, which were placed between the CC and MEA. Their results revealed that the presence of meshes enhanced the performance of the cell. Heidary et al. (2013) experimented the impact of clamping torque on the cell performance. It was identified that the MCO can be reduced through the application of more cathode back pressure. Ning et al. (2017, 2019) fabricated a flexible air breathing proton exchange membrane (PEM)and incorporated it in a PEMFC.The performance of this PEMFC with the modified MEA was studied. They revealed that the cell with this arranement exhibited higher volumetric power density compared to the conventional PEMFC.
Chen and Zhao (2007a) experimentally studied the behaviour of the metal porous CC on the cathode side of a DMFC. They observed enhancement of oxygen diffusion and faster water removal due to the small pore size. Shao et al. (2006) developed a novel design of anode structure, with titanium mesh coated with deposition of Pt-Ru. Their study revealed that the coated deposition has good physical properties and resists methanol crossover, which yielded better fuel cell performance at lower methanol concentrations as compared with conventional porous CCs. Kim et al. (2009) made experimental studies by changing the geometry of the cathode current collector openings, i.e., rectangular, circular and triangular shaped openings. They noticed that the circular opening exhibited the best cell performance. Calabriso et al. (2015) evaluated the performance of a DMFC with stainless steel (SS316) current collectors having circular perforations with OR of 17% and 35%. They observed that the CC with OR of 35% exhibited better performance with peak power density at 2 M of methanol concentration. Braz et al. (2019) experimentally investigated the impact of OR of the perforated CC, (34%, 41% and 64% OR) on the passive DMFC performance. They noticed that the CC with the smaller OR of 34% produced higher power out put than the other two open ratios. Lower open ratio reduced the methanol crossover and increased electrical contacting area. Wang et al. (2017) expanded metal mesh with smaller strand widths gave better passive DMFC performance at lower methanol concentrations and worse at higher methanol concentrations than conventional perforated current collectors. Alipour et al. (2016) experimentally studied the effect of NaOH addition in to the methanol fuel. They observed that NaOH addition methanol fuel increased the maximum power and also reduced the methanol crossover. Addition of NaoH to methanol enhanced the electrochemical reaction kinetics and also increased ionic conductivity through the membrane.
Cai et al. (2011) studied the perofmance of a passive DMFC with LE layer (LE-DMFC).They noticed that the LE-DMFC produced 30% higher maximum power density than the conventional fuel cell with no liquid electrolyte layer. Boni et al. (2019a) experimentally analysed the impact of LE layer thickness on performance of a passive DMFC.They observed the MPD and the MCD were higher for the LE-DMFC compared with the conventional DMFC with no liquid electrolyte layer. Kim et al. (2017) experimented the impact of a thin metal barrier in the middle of two membranes. They observed that the metal barrier could considerably decrease the MCO, and the cell performance enhanced by 37.5% when compared to the reference electrode. Boni (2020) experimentally investigated the impact of different modifications to the cathode current collectors, such as perforated current collectors,wire mesh current collector (WMCC) and WMCC with supporting plate on the performance of an air-breathing direct methanol fuel cell. They revealed that, out of the three different current collectors, wire mesh current collector with supporting plate exhibited better fuel cell performance. Liu et al. (2018) experimentally studied the influence of sulfonated poly (ether ether ketone) (SPEEK) based membrane on the performance of a DMFC. They noticed that SPEEK decreased the MCO and enhanced the fuel cell performance than the conventional Nafion117 membrane. This due to the higher MCO resistance.
From the above literature review it is observed that most of the literature analysed the impact of OR of the WMCC as well perforated CC. The major challenges associated with the passive DMFC are water and methanol crossover, which can be minimized by selecting an appropriate opening ratio for the current collectors and simulataneously maintaining better contact between the MEA and the CC. This can be addressed by suitably modifying the catalyst layers, incorporating lquid electrolyte layer and changing the CC with an OR. The present paper analysed the effect of multiple anode catalyst layers (two layers) along with a liquid electrolyte layer inserted in the middle of a two half MEAs,on the performance of a passive DMFC. The impact of CC with OR on the DMFC performance was also examined by varying the open ratio (45.40%, 55.40% and 63.05%) and comparision of the literature with present work as shown in (Table 1).
Experimentation
Fabrication of the membrane electrode assembly (MEA)
MEA with an reactive area 25 cm2 was made by hot pressing of Nafion117 membrane at 135 °C and 8 MPa for 3 min. Before starting the hot pressing,organic and inorganic impurities were eliminated from the Nafion membrane, by boiling the membrane in 3 wt% H2O2 solution for one hour,subsequent cleansing in deionised (DI) water, and later boiling in 0.5 M H2SO4 solution for 1 h, and lastly boiling it in DI water for 1 h. The cathode and anode backing layers were fabricated of carbon cloths with 20% polytetrafluoroethylene (PTFE). The anode and cathode catalysts were processed by distribution of sufficient quantity of catalyst into the solutions containing DI water, Nafion solution and isopropyl alcohol. Initially the membrane was coated with Pt-Ru/black catalyst on the diffusion layer with a loading of 2.5 mg cm−2.The second layer was coated with Pt-Ru/C catalyst on the first layer of the catalyst with a loading of 1.5 mg cm−2. After the first and second stages, the layer was hot pressed with a temperature of 135 °C. Two different MEAs were used in the present study. their details are shown in Table 2. A single layer catalyst of 60% Pt/C with a loading of 2 mg cm−2 was coated on the cathode side diffussion layer.
Liquid electrolyte incorporated passive DMFC
In a conventional passive DMFC (C-DMFC), the MEA has a single set of components, which has diffusion layers as well as catalyst layers on the anode side and the cathode side along with the membrane. In a liquid electrolyte incorporated passive DMFC (LE-DMFC), the LE layer is placed in between the two half MEAs. The liquid electrolyte layer is made of piled hydrophilic filter papers,which are soaked in the 1 M H2SO4 solution. The schematic of fabriacation of the LE modified memebrane is shown in Figs. 1 and 2. For each membrane,the catalyst and the diffusion layers were attached by hot pressing process. The details of the complete MEA for the LE-DMFC are given in Boni et al. (2019a).
Schematic of Half MEAs with LE layer (Boni et al. 2019b). 1. Anode gas diffusion layer (ADL), 2. anode catalyst layer (ACL), 3. anode membrane, 4. liquid electrolyte layer, 5. cathode membrane, 6. cathode catalyst layer, 7. cathode gas diffusion layer
Preparation of composite MEA a half MEAs before hot pressing, b after hot pressing along with LE layer, c composite MEA (Boni et al. 2019b)
Single cell fixture
The main parts of the passive DMFC consists of anode and cathode end plates, anode and cathode current collectors and the MEA. The anode end plate also acts as the methanol reservoir. It has two holes of 3 mm diameter for inlet of the anodic fuel and for the release of the CO2 produced in the reaction. The cathode end plate is having an open area same size of the active reaction area of the membrane, and is opened to the atmosphere, which enables the supply of oxygen from the atmopspheric air by natural convection. Current collectors (CC) were made of 2 mm SS 316L plates. Unoiform circular holes were drilled on these current colectors. Three diferent open ratios of 45.40%, 55.40% and 63.05% were considered in the present study. Figure 3 shows the photo of the three current collectors. PTFE gaskets of 0.23 mm thickness were placed between the CC and MEA on both the cathode side and the anode side. SS316L current collector with different OR are used for collecting electrons. All parts of the cell are assembled by using nuts and bolts with a torque of 5 N-m. The exploded view of the passive DMFC is illustrate in Fig. 4.
Exploded view of the passive DMFC (Boni et al. 2019a). 1. Anode methanol reservoir, 2. anode current collector, 3. PTFE gasket for anode side, 4. MEA, 5. PTFE gasket for cathode side, 6. cathode current collector, 7. cathode end plate
Experimental set-up and test conditions
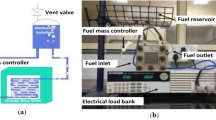
A DC electronic load bank was operated to record the current–voltage data. For recording the voltage, at an time laps of one minute was considered between two successive readings to arrive at the stable voltage. Before performing the experiment, the newly fabricated MEA was activated for 12 h of duration at 1 M of methanol molar concentration. All the tests were conducted at room temperature. The experimental set-up of the passive DMFC is shown in Fig. 5.
Uncertainity analysis
Uncertainity analysis is to measure the errors related with the experiments. The uncertainty of the DC Electronic load bank specified by manufacturer is depicted in the Table 3. The maximum value of uncertainty in the power by using this test rig is 0.31% (Fig. 5; Table 3).
Results and discussion
In this experimental work, the influence of the anode catalyst supports, CC with OR and LE layer on the performance of a passive DMFC was experimentally studied. In the first phase of experimentation, experiments were carried out to analyse the impact of the anode catalyst supports. In the second phase, tests were conducted to study the impact of OR of the perforated current collector (PCC). The effect of the anodifc fuel concentration was also studied by varying the methanol concentration from 1 M to5 M. Experiments were conducted with the objective of identifying the effect of methanol concentration for a given OR of the CC, and identifying the optimum combination of open ratio and methanol concentration to give the best fuel cell performance. The influence of methanol concentration on the performance of fuel cell with different CC with OR is shown in Figs. 7, 8 and 9.
Effect of anode catalyst supports
Experiments were performed to calculate the impact of anode supports on the performance of a DMFC with using a single layer of anode catalyst (MEA-1) and two layers of anode catalyst (MEA-2), as shown in Table 2. Figure 6 depicts the polarisation curves of the MEA-1 and MEA-2 incorported fuel cell with a current collector with 45.40% open ratio, and supplied with diluted methanol of 4 M concentration as the anodic fuel. It can be observed from the figure that the MEA-2 (i.e., two layers of anode catalyst) incorporated fuel cell gave better performance. It can be explained that the two layer anode catalyst has higher electrochemical activity than the single layer catalyst. It can be visualized that intially the methanol fuel on the anode side enters Pt-Ru/back catalyst,where fast reactions take place and maximum amount of fuel is consumed. The remaining amount of the fuel then enters the second layer of Pt-Ru/C, where some more quantity of fuel is consumed, and then the remaining amount of the fuel will be transported over the membrane to the cathode side. This unreacted methanol fuel passes from the anode side to the cathode side of the membrane is called as the MCO. It can be understood that the MCO would be lower in the case of MEA-2, which is having two layers of catalyst. Hence, it gives better performance. The MCD and MPD produced by the fuel cell with MEA-2 are 41.6 mA cm−2 and 3.36 mW cm−2, respectively.
Effect of current collector open ratio
Figure 7 depicts the performance characteristics of a passive DMFC using current collector of open ratio 45.40% at different methanol concentrations. It can be noticed from the figure that as the methanol concentration is increased, the cell performance increased. It can be noticed that the MCD and MPD of the cell increased with increase inthe methanol concentration. The MPD and MCD produced by the passive DMFC with current collector of 45.40% open ratio at 5 M methanol concentration is 3.612 mW cm−2 and 44 mA/cm−2, respectively. Similarly, Figs. 8 and 9 illustrate the performance characteristics of the passive DMFC using CC with OR of 55.40% and 63.05%, respectively. It can be noticed from Fig. 8 that the effect of methanol concentration is not monotonous, as in the case of 45.40% current collector, i.e., the cell performance does not increase continuously with increase the methanol concentration. Initially, the cell performance improved with increase in the methanol concentration up to 3 M, and increasing the methanol concentration further from 3 to 5 M, the cell performance deteriorated. The MCD and MPD for the fuel cell with currrent collector of 55.40% open ratio are 40.8 mA cm−2and 3.872 mW cm−2, respectively. It can be noticed from Fig. 9 that for the fuel cell with current collector of 63.05% open ratio,the impact of methanol concentration on the cell performance is not monotonous. Initially, the cell performance enhanced with increase in the methanol concentration from 1 to 4 M, and then decreased with further increase in the methanol concentration from 4 to 5 M. The MPD and MCD obtained by the cell are 2.448 mW cm−2 and 36 mA cm−2 at 4 M of methanol concentration. It can be seen that there is no uniform impact of methanol concentration on the cell performance with different current collector open ratios. The optimum value of the methanol concentration which gives the best cell perforamce depends on the OR of the CC also.
It can be explained that increase of the CC with OR has a mixed impact on the cell performance. On one hand, increase in the CC with OR increases the area for passage of the reactants and hence promotes the mass transfer of the reactants. There by it enhances the rate of reaction and improves the cell performance. Similarly, it also facilitates easy removal of the products of reaction (CO2 and H2O) from the reaction sites. This is a favourable effect for increasing the cell performance. On the other hand, increase in the open ratio of the current collector, leads to increased MCO from the anode to the cathode, causing mixed over potential on the cathode side reaction area. Because of this mixed overpotential, the fuel utilization rate decreases, and the unreacted methanol obstructs oxygen transport on the cathode reaction sites. This adversely affects the cell performance. Similarly, increae in the OR of the CC reduces the contact area of the collector with reaction sites. This decreases the current collector’s ability to conduct more electrons and thus adversely influence the cell performance. Simulataeously, the electrical impedance of the cell is also influenced by the OR of the CC.
On the other hand, increase in the methanol concentration increases the diffusion of methanol through the anode diffusion layer and the anode catalyst layer, and thus makes available more amount of methanol for the reaction near the membrane. On the negative side of its effect is when the concentration of methanol is more the probability for the MCO from the anode to the cathode is more. This causes increased mixed over potential loses and results in the deterioration of the cell performance. Thus, the cell performance is a manifestation of the cumulative impact of favourable and adverse effects due to the OR of the CC and the methanol concentration.
Figure 10a, b show the variation of the MCD and MPD with methanol concentration for the three different ORs of the CC. The MPD and MCD increases with increase in methanol concentration from 1 to 5 M for 45.40% current collector. For 55.40% open ratio current collector, the MPD and the MCD increase with increase in methanol concentration from 1 to 3 M and then decreases. For the current collector with an OR of 63.05%, the MCD and MPD increase with increase in the methanol concentration from 1 to 4 M and then decreases. It can be noticed that in the present range of methanol concentrations of 1–5 M and for the current collector open ratios of 45.40%, 55.40% and 63.05%, the fuel cell with current collector of 55.40% open ratio exhibited the best performance of maximum values of current density and power density at 3 M methanol concentration. At 5 M of methanol concentration, the 45.40% open ratio current collector based fuel cell produced the MCD and the MPD compared to other two current collectors. Among three current collectors of different open ratios, the fuel cell with current collector of 55.40% open ratio produced maximum values of power density and current density of 40.8 mA cm−2and 3.872 mW cm−2, respectively. Present experimental results are compared with literature as depicted in Table 4.
Effect of liquid electrolyte layer
Figure 11a–c show the polarisation characteristics of a passive DMFC with and without incorporation of LE layer for the fuel cell employing current collectors of three different open ratios at 5 M of methanol concentration. It can be observed from the figure that in all the three cases of CC with OR, the performance of the fuel cell improved by incorporating the liquid electrolyte layer. It can be explained that in a passive DMFC, the cell performance is strongly affected by methanol and water cross-over. The incorporation of the liquid electrolyte layer considerably reduces this cross over nad hence improves the cell performance. In general, the incorporation of an additional layer increases the ohmic resistance and impairs the cell performance. The cumulative effect of reduction in the MCO and increase in the ohmic losses of the cell determine the overall effect of incorporating a liquid electrolyte layer on the fuel cell performance.
Figure 12 illustrate the variation of the current density with respect to time variation at constant voltage of 0.25 V for the fuel cell fitted with current collector of 45.40% open ratio. It can be observed that current density drop is more for the conventional DMFC compared to the LE-DMFC. This can be attributed to the methanol crossover. Methanol crossover losses are more in the C-DMFC. The methanol crossing the membrane due to crossover reaches the cathode and reacts with oxygen to generate water (in the form of bubbles). These water bubbles resist the oxygen flow in to the cathode reaction sites and deteriorates the cell performance with time (Fig. 13).
Besides the above observations, formation of CO2 and water bubbles were observed on the anode and cathode side as observed in Fig. 13a, b at 25 mA cm−2. During the anodic reaction process, electrons, protons and carbon dioxide bubbles are produced near the anode. The produced electrons transports over the external circuit and reach the cathode. The protons pass through the membrane and reach the cathode side. At cathode side the electrons,protons and oxygen combine and produced water in the form of bubbles. More number of CO2 gas bubbles were generated at higher current densities and higher methanol concentrations due to greater reaction rates. The CO2 gas bubbles resist the methanol flow to the anode reaction sites. Similarly, on the cathode side, the water bubbles block the flow of oxygen to the cathode side reaction sites. Both of them deteriorate the cell performance with time.
Figure 14a shows the equivalent circuit of the fuel cell. Figure 14 shows the EIS curves of the fuel cell fitted with the CC of three different OR at 3 M methanol concentration. From the equivalent circuit, the charge transfer resistance (Rcharge) on the anode side, the ohmic resistance (Rohmic), and the mass transfer resistance of O2 (Rmass) on the cathode side can be observed. Nyquist plot is plotted for the real and the imaginary impedance components in X-axis and Y-axis, respectively. Ohmic losses (Rohmic),charge transfer resistance (Rcharge) on the anode side and mass transfer resistance of O2 (Rmass) on the cathode side are observed in high frequency region, medium frequency region and low frequency regions of the Nyquist plot, respectively. From the figure, it is observed that as the impedance arcs of the Nyquist plot decrease with decrease in the voltage drop. The larger arc is observed for the fuel cell with current collector of 63.05% OR with CC fitted fuel cell. Ohmic resistance losses almost same for all the three types of the current collectors. Charge transfer and mass transfer losses are lower in the current collector for the 55.40% fitted fuel cell. Mass transfer losses on the cathode side influenced by MCO, this is resists the oxygen flow in to the reaction sites.
Conclusion
The present work describes an experimental investigation of the impact of anode catalyst supports, OR of the CC and the incorporation of LE layer in the MEA on the performance of a passive DMFC. Experiments were carried out current collectors of three different open ratios, i.e. 45.40%, 55.40% and 63.05%. With each of these three current collectors the methanol concentrationwas varied from 1 to 5 M. It was observed the optimum value of current collector open ratio depends on the methanol concentration also. Finally, it was observed from the experiemtnal results that the OR of the CC, the anode catalyst supports and the LE layer has significant effect on the passive DMFC performance. Based on the experimental results, the following conclusions are drawn:
-
In the comparison of the single layer catalyst support and the two layer catalyst support, the fuel cell with the two layer anode catalyst (MEA-2) gave better fuel cell performance.
-
The fuel cell performance is influenced by the quantity of methanol fuel entering the anode catalyst layer,and it is goverened by either increase in the OR of CC or increase of methanol concentration.
-
The current collector open ratio has a mixed impact on the cell performance. The optimum value of open ratio was found to vary with the methanol concentration also. Thus, at 3 M methanol concentration, the CC with OR of 55.40% exhibited better cell performance, while at 5 M methanol concentration, the CC with an OR of 45.40% gave the best cell performance.
-
Within the present range of experiments of methanol concentration from 1 M to 5 M and three current collector open ratios of 45.40%, 55.40% and 63.05%, the fuel cell gave the best performance at 3 M methanol concentration with the CC with an OR of 55.40%.
Abbreviations
- LE:
-
Liquid electrolyte
- CC:
-
Current collector
- OR:
-
Open ratio
- MEA:
-
Membrane electrode assembly
- MPD:
-
Maximum power density
- MCD:
-
Maximum current density
- MOR:
-
Methanol oxidation reaction
- ORR:
-
Oxyen reduction reaction
References
Alipour A, Rowshanzamir S, Javad M (2016) Investigation of NaOH concentration effect in injected fuel on the performance of passive direct methanol alkaline fuel cell with modified cation exchange membrane. Energy 94:589–599
Boni M (2020) Performance evaluation of an air breathing—direct methanol fuel cell with different cathode current collectors with liquid electrolyte layer, (December 2019), pp 1–10. https://doi.org/10.1002/apj.2465
Boni M, Rao SS, Srinivasulu GN (2019a) Influence of intermediate liquid electrolyte layer on the performance of passive direct methanol fuel cell. Int J Green Energy 00(00):1–10. https://doi.org/10.1080/15435075.2019.1671419
Boni M, Rao SS, Srinivasulu GN (2019b) Influence of intermediate liquid electrolyte layer on the performance of passive direct methanol fuel cell. Int J Green Energy 16(15):1475–1484. https://doi.org/10.1080/15435075.2019.1671419
Borello D, Calabriso A, Cedola L, Del Zotto L, Santori SG (2014) Development of improved passive configurations of DMFC with reduced contact resistance. Energy Procedia 61:2654–2657. https://doi.org/10.1016/j.egypro.2014.12.268
Braz BA, Moreira CS, Oliveira VB, Pinto AMFR (2019) Electrochimica Acta Effect of the current collector design on the performance of a passive direct methanol fuel cell. Electrochim Acta 300:306–315. https://doi.org/10.1016/j.electacta.2019.01.131
Cai W, Li S, Yan L, Feng L, Zhang J, Liang L et al (2011) Design and simulation of a liquid electrolyte passive direct methanol fuel cell with low methanol crossover. J Power Sources 196(18):7616–7626. https://doi.org/10.1016/j.jpowsour.2011.05.006
Calabriso A, Cedola L, Zotto LD, Rispoli F, Santori SG (2015) Performance investigation of passive direct methanol fuel cell in different structural configurations. J Clean Prod 88:23–28. https://doi.org/10.1016/j.jclepro.2014.06.087
Chen R, Zhao TS (2007a) Porous current collectors for passive direct methanol fuel cells. Electrochim Acta 52(13):4317–4324. https://doi.org/10.1016/j.electacta.2006.12.015
Chen R, Zhao TS (2007b) Porous current collectors for passive direct methanol fuel cells 52(October 2006):4317–4324. https://doi.org/10.1016/j.electacta.2006.12.015
Falcão DS, Pereira JP, Pinto AMFR (2016) Effect of stainless steel meshes on the performance of passive micro direct methanol fuel cells. Int J Hydrog Energy 41(31):13859–13867. https://doi.org/10.1016/j.ijhydene.2016.05.059
Heidary H, Abbassi A, Kermani MJ (2013) Enhanced heat transfer with corrugated flow channel in anode side of direct methanol fuel cells. Energy Convers Manage 75:748–760. https://doi.org/10.1016/j.enconman.2013.08.040
Irannejad L, Javad S, Mojtaba A (2019) Platinum nanospheres electrodeposited on titanium oxide/titanium modified electrode for improved electrocatalytic activity of methanol electrooxidation. Chem Pap 73(9):2153–2164. https://doi.org/10.1007/s11696-019-00727-8
Kim SH, Cha HY, Miesse CM, Jang JH, Oh YS, Cha SW (2009) Air-breathing miniature planar stack using the flexible printed circuit board as a current collector. Int J Hydrog Energy 34(1):459–466. https://doi.org/10.1016/j.ijhydene.2008.09.088
Kim S, Jang S, Kim SM, Ahn CY, Hwang W, Cho YH et al (2017) Reduction of methanol crossover by thin cracked metal barriers at the interface between membrane and electrode in direct methanol fuel cells. J Power Sources 363:153–160. https://doi.org/10.1016/j.jpowsour.2017.07.071
Liu AX, Zhang Y, Deng S, Cheng H (2018) SC. Chin Chem Lett. https://doi.org/10.1016/j.cclet.2018.09.021
Mallick RK, Thombre SB (2017) Performance of passive DMFC with expanded metal mesh current collectors. Electrochim Acta 243:299–309. https://doi.org/10.1016/j.electacta.2017.04.113
Mallick RK, Thombre SB, Motghare RV, Chillawar RR (2016) Analysis of the clamping effects on the passive direct methanol fuel cell performance using electrochemical impedance spectroscopy. Electrochim Acta 215(September):150–161. https://doi.org/10.1016/j.electacta.2016.08.080
Ning F, He X, Shen Y, Jin H, Li Q, Li S et al (2017) Flexible and lightweight fuel cell with high specific power density. ACS Nano. https://doi.org/10.1021/acsnano.7b01880
Ning F, Shen Y, Bai C, Wei J, Lu G, Cui Y, Zhou X (2019) Critical importance of current collector property to the performance of flexible electrochemical power sources. Chin Chem Lett 30(6):1282–1288. https://doi.org/10.1016/j.cclet.2019.02.032
Scott K, Argyropoulos P, Yiannopoulos P, Taama WM (2001) Electrochemical and gas evolution characteristics of direct methanol fuel cells with stainless steel meshow beds. Cell. https://doi.org/10.1023/A:1017559124395
Shao ZG, Lin WF, Christensen PA, Zhang H (2006) Ti mesh anodes prepared by electrochemical deposition for the direct methanol fuel cell. Int J Hydrog Energy 31(13):1914–1919. https://doi.org/10.1016/j.ijhydene.2006.05.003
Shrivastava NK, Thombre SB, Motghare RV (2014) Wire mesh current collectors for passive direct methanol fuel cells. J Power Sources. https://doi.org/10.1016/j.jpowsour.2014.09.010
Shrivastava NK, Thombre SB, Mallick RK (2014) Effect of diffusion layer compression on passive DMFC performance. Electrochim Acta 149:167–175. https://doi.org/10.1016/j.electacta.2014.10.080
Ulas B, Caglar A, Kivrak A, Kivrak H (2018) Atomic molar ratio optimization of carbon nanotube supported PdAuCo catalysts for ethylene glycol and methanol electrooxidation in alkaline media. Chem Pap. https://doi.org/10.1007/s11696-018-0601-9
Wang A, Yuan W, Huang S, Tang Y, Chen Y (2017) Structural effects of expanded metal mesh used as a flow field for a passive direct methanol fuel cell. Appl Energy 208(July):184–194. https://doi.org/10.1016/j.apenergy.2017.10.052
Xue YQ, Guo H, Shang HH, Ye F, Ma CF (2015) Simulation of mass transfer in a passive direct methanol fuel cell cathode with perforated current collector. Energy 81:501–510. https://doi.org/10.1016/j.energy.2014.12.063
Yang WM, Chou SK, Shu C (2007) Effect of current-collector structure on performance of passive micro direct methanol fuel cell. J Power Sources 164(2):549–554. https://doi.org/10.1016/j.jpowsour.2006.11.014
Yousefi S, Shakeri M, Ganji DD, Sedighi K (2012) Experimental investigation of a passive direct methanol fuel cell with 100 cm2 active areas. Electrochim Acta 85:693–699. https://doi.org/10.1016/j.electacta.2012.08.045
Yousefi S, Shakeri M, Sedighi K (2013) The effect of cell orientations and environmental conditions on the performance of a passive DMFC single cell. Ionics 19(11):1637–1647. https://doi.org/10.1007/s11581-013-0889-y
Yunphuttha C, Bunjongpru W, Porntheeraphat S (2012) Fabrication of a micro-direct methanol fuel cell using microfluidics. Chem Pap 66(12):1137–1145. https://doi.org/10.2478/s11696-012-0230-7
Acknowledgements
The authors acknowledged the financial support provided by DST-SERB,Govt. of India and TEQIP-II-CoE, National Institute of Technology, Warangal, Telagana, India.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Boni, M., Surapaneni, S.R., Golagani, N.S. et al. Experimental investigations on the effect of current collector open ratio on the performance of a passive direct methanol fuel cell with liquid electrolyte layer. Chem. Pap. 75, 27–38 (2021). https://doi.org/10.1007/s11696-020-01277-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11696-020-01277-0