Abstract
Experimental investigation is carried out on passive direct methanol fuel cell, to study with the selected combination of different anode and cathode current collectors which have high electrical and thermal conductivity together with corrosion resistance compatibility properties. These collectors are fabricated with an opening ratio of 45.3% on Stainless Steel Grade-316L, Nickel-201 and brass (70% Cu-30% Zn) and experimented at 5 M concentration of methanol solution. Polarisation curves and maximum power density curves have been drawn with the experimental results for performance comparison using Ni-SS, Ni-Brass, SS-Ni and SS-brass anode and cathode combinations of current collectors. Comparative studies for maximum power and current densities are investigated and represented on bar charts for identifying the better combination of anode and cathode materials. Performance of cell is found best with the combination using Nickel-201 as anode and brass as cathode. With this combination, the maximum power density developed is 7.157 mW cm−2, and the maximum current density produced is 65.6 mA cm−2 at 5 M concentration.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Passive direct methanol fuel cell
- Current collector
- Corrosion resistance material
- Electrical conductivity
- Thermal conductivity
- Nickel
- Stainless steel
- Brass
1 Introduction
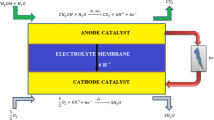
With increase in societal demand for electricity every year across the globe, fuel cell technology is evolving out as one of the protuberant energy resources among the accessible alternative energy resources in place of fossil fuels which are going to be lost within next few decades. Fuel cells are similar to conversion devices like a battery; it converts the chemical energy of reactants into electricity leaving other reaction compounds as by-products [1]. However, fuel cell differs from a battery in that as long as the fuel and oxygen are supplied, it produces electric power continuously. Out of the other well-known fuel cells classified based on proton conducting membrane electrolyte, polymer exchange electrolyte membrane-based cell working with direct liquid feed methanol as fuel and air as an oxidant emerges out as an electric power source for the applications [2] of portable electronic appliances like mobile phones, laptops, tape recorders, Walkman, toys, computers, cell phones, emergency lights, including material handling equipment like forklifts, cargo loaders, etc., and also for space application systems [3]. As a fuel, liquid methanol is relatively inexpensive and easily available and has more specific energy density, quick refuelling and good transportation and better storage facility. Further, fuel cell characteristics are ultimately affected by significant aspects such as choice and make use of suitable materials and its novel designing. These fuel cells facilitate to operate at low temperatures and pressures without additional liquid electrolyte requirement [4]. pDMFC can also be operated at ambient pressure and temperatures conditions. It has other advantages like clean by-products, extremely no/low emission of oxides of nitrogen and sulphur, operates quietly, not having any moving parts and extra fuel processing to meet demand requirement and high energy. Compact cell design of pDMFC makes it easy to handle. Schematic representation of passive direct methanol fuel cell is represented in Fig. 1.
To have better reaction kinetics, pDMFC makes use of ruthenium and platinum as catalyst on the anodic side of the membrane to break the chemical bonds in the methane water solution to form carbon dioxide, hydrogen ions (protons) and free electrons as shown in Eq. (1). In the cell, the liberated electrons flow from the anodic side of the cell through an external circuit to the cathodic side, and the protons are transported through the proton conducting electrolyte membrane. At the cathode, the electrons and hydrogen ions react with oxidant to form water as shown in Eq. (2). The liberated heat of reaction is mostly released to surroundings through cathode side current collector. Overall chemical reaction of the cell is shown in Eq. (3).
Anode End Reaction:
Cathode End Reaction:
Overall Fuel Cell Reaction:
2 Literature Survey
Passive direct methanol fuel cell is getting importance across the globe as an electrical power source due to high-energy density of fuel. Among the fuel cell components, current collector material properties and their compatibility in water–methanol solution are influencing cell durability, performance and effectiveness.
Braz et al. [5] has studied the optimization process of passive direct methanol fuel cell with various current collector materials. It is indicated that to ascertain DMFC commercially, an optimum balance between its price, competence and durability should be achieved. Current collectors are accountable for about 70–80% of the system weight, and different current collector materials were tested to balance price and weight reduction. Performance of the fuel cell and its duration were identified using polarisation measurements. A serious novelty of this study is the use of an innovative identification and quantification of performance. The utmost power density of 5.23 mW cm−2 was achieved using Titanium as anode current collector and Stainless Steel as cathode current collector at a methanol concentration of 7 M. The durability tests showed a lifetime about 200 h and a reduction in efficiency of fuel cell by 41% from original value.
Tabbi et al. [6], in their investigation, identified that the automobile industry is encouraging the use of metals as current collector plates as metals having small thickness and weight as well as good conductivity both thermally and electrically. Using stainless steel would reduce the cost, but non-coated SS from investigation still has some challenge with surface-insulating layer of chromium oxide (Cr2O3).
Seema et al. [7] has made comprehensive review on recent material development of passive direct methanol fuel cell and emphasis on the performance activity, cost, durability and stability aspects. Each component with their material development along with basic desirable characteristics is reported in this paper. This paper has also reviewed all possible materials of passive DMFC component, which might make the passive DMFC compact and feasible energy source in future.
Mallick et al. [8] in their study on critical review of current collectors for passive direct methanol fuel cells has emphasis on the important aspects such as profile of the current collectors including materials of construction of the current collectors. A number of current collectors of passive DMFC have been selected and reviewed thoroughly. However, very less research works have been found concerning to decrease in the weight of the current collectors as the current collector majorly contributes on the total weight of pDMFC and affects the gravimetric energy density of the fuel cell.
3 Objective
After going through the literature study, it is inferred that the materials of the current collectors influence the performance of the pDMFC. Required properties of the current collector materials are high electrical conductivity at operating zone, thermal conductivity to optimise and to maintain the thermal stability of cell during operation and high corrosion resistance having compatibility in dilute methanol environment. After considering the desirable properties of the bipolar plates, this experimental study has been taken up to identify the better current collector materials combination among Nickel-201, brass and SS-316L current collectors in anode–cathode ends.
4 Problem Description
Current collectors of passive direct methanol fuel cell play as a key component, and the performance of the fuel cell depends on its material of construction, dimensions and novel design with shape factors. The weight of the current collectors contributes almost 3/4 of the total weight of the cell [9]. Hence, the gravitational power density is significantly affected by the selection of current collector materials and its design aspects.
The required characteristics of materials [10] of the current collectors in pDMFC are as follows:
-
1.
Good electrical conductivity or very low electrical resistivity at operating zone of the direct liquid feed methanol cell [11].
-
2.
High thermal conductivity to optimise and to maintain the thermal stability of cell during operation [12].
-
3.
Desirable mechanical properties like high tensile strength and flexural rigidity of materials [13].
-
4.
Better fabrication and machinability processes of materials [14].
-
5.
Corrosion resistance in methanol environment at various concentrations and wide range of operating temperatures [15].
-
6.
Longer durability and life [16].
-
7.
Low density of materials [17].
-
8.
Easily available at cheaper cost [18].
-
9.
Less contact resistance with the diffusion layers [19].
-
10.
Even distributing and transport area of reactants [20].
The functions of the anode and cathode side current collectors are relatively different; however, they have some of the common aspects like uniform spreading of chemical reactants, maintaining cell structure support, disposal of reaction by products and providing the electrical connectivity with adjacent cells in case of stacking of cells. At the anode, current collector allows the passage of transporting methanol solution and carbon dioxide. Further it collects the electric current from MEA, whereas the cathodic end current collector provides transportation of water, collects the current from cathodic end diffusion layer and receives the oxidant from ambient air.
Details of the materials compositions are given in Table 1 [21], and properties of the materials are provided in Table 2.
5 Experimental Set-Up
To evaluate performance of passive DMFC with the combination of Nickel-201, brass and SS-316L current collectors, a single direct methanol fuel cell fixture is selected. For carrying out this experimental testing, different anode and cathode current collector materials, fabricated with 2.00 (± 0.02) mm thickness sheets, are used. The circular openings of 100 numbers, in 10 by 10 matrix pattern, are made using 3.8-mm diameter drill. Fabrication drawing detail of the current collector is shown in Fig. 2.
Nafion-117 solid electrolyte is used as permeable membrane in membrane electrode assembly. The anode catalyst layer (ACL) is made up of Pt-Ru (1:1)/C with a catalyst loading of 4 mgcm−2, and on cathode catalyst layer (CCL), it is made up of Pt/C with a catalyst loading of 2 mg cm−2. To prevent methanol solution and oxidant leakages, PTFE sealing gaskets are provided in between current collectors and MEA components of the cell. The fabricated active area of the cell is 5.0 cm × 5.0 cm. Methanol solution with 5 M concentration has been prepared to use in this experiment. The required clamping of the cell assembly is made using M8 fasteners, and uniform tightening of the bolts is ensured using a torque wrench which is pre-set at 5Nm value. The experimental set-up of the DMFC is shown in Fig. 3.
6 Experimental Methodology
To evaluate performance of passive DMFC with the combination of Nickel-201, brass and SS-316L current collectors, four set-ups of anode and cathode combinations as referred in Table 3 with single direct methanol fuel cell fixture are chosen. As brass is getting reacted with dilute methanol with the formation of metal methoxides, the use of brass as current collector material in anode side is not considered. As Ni and SS material are performed better at 5 M, experiments have been carried out at this concentration of methanol solution.
While performing the experiment, the first set of voltage and current readings has been taken by varying current characteristic conditions using Nickel-201/SS-316L (set-up-I) materials as current collectors at 5 M methanol concentration. This experiment further repeated with the other mentioned set-up-II, Nickel-brass; set-III Stainless Steel-Nickel and with set-up-IV Stainless Steel-brass current collectors, and corresponding voltage and current characteristics have been noted. Total experiment has been repeated thrice at this 5 M concentration methanol solution to get repeatability and consistency in the readings. Mean value of the three readings of observations corresponding to current–voltage is taken for analysis of the cell characteristics.
7 Experimental Results and Analysis
7.1 Polarisation and Power Density Characteristics
In the experimental set-up-I, Nickel-201 material as anode and SS316L as cathode current collector have been used. Cell is tested with 5 M methanol solution concentration at ambient conditions. In this experiment, the highest power density recorded is 6.720 mW cm−2 at current density of 32.0 mA cm−2. During testing, the maximum current density that recorded is 62.4 mA cm−2 at 5 M methanol concentration.
In the experimental set-up-II, Nickel-201 material as anode and brass as cathode current collector have been used. Cell is tested with 5 M methanol solution concentration at ambient conditions. In this experiment, the maximum power density recorded is 7.157 mW cm−2 at a current density of 33.6 mA cm−2. During testing, the maximum current density recorded is 65.6 mA cm−2 at 5 M methanol concentration.
In the experimental set-up-III, SS316L material as anode and Nickel-201 as cathode current collector have been used. Cell is tested with 5 M methanol solution concentration under ambient conditions. In this experiment, the peak power density recorded is 4.704 mW cm−2 at a current density of 24.0 mA cm−2. During testing at the same 5 M methanol concentration, the largest current density recorded is 46.4 mA cm−2.
In the experimental set-up-IV, SS316L material as anode and brass as cathode current collector have been used. Cell is tested with 5 M methanol solution concentration in ambient conditions. In this experiment, the highest power density recorded is 3.397 mWcm−2 at a current density of 17.6 mA cm−2. During testing, the maximum current density recorded is 34.4 mA cm−2 at 5 M methanol concentration.
Polarisation curves (voltage–current density characteristics) of the four set-ups of pDMFC configuration are plotted as shown in Fig. 4. Initially when current density is zero, the cell generated voltage is maximum (open circuit voltage), and as the current density increases, the cell voltage decreases to zero. From Fig. 4, Nickel-brass combination as anode and cathode is performing better with the highest current density as revealed in polarisation characteristics.
Power density curves (power density versus current density characteristics) of these four set-ups of pDMFC configuration are plotted as shown in Fig. 5. Power density of the cell increases from zero to a maximum value, and further, it decreases to zero with increase in the current density. Nickel-brass combination as anode and cathode is performing better with maximum power density as revealed from the drawn characteristics.
The combined voltage and power density superimposed characteristics against the current density are plotted as shown in Fig. 6.
7.2 Comparison of Maximum Power Density and Maximum Current Density
Results of the four set-ups with current collectors against current density and power density are taken for analysis. Bar charts of current collectors’ combinations as anode–cathode materials versus maximum power density produced (refer to Fig. 7) and current density (ref to Fig. 8) are drawn. Anode–cathode combination of Nickel-brass showed better current density and power density among these four set-ups, and SS-brass combination showed the least performance. Better performance of Nickel is due to higher conductivity and higher resistance to methanol solution, whereas brass has superior conductivity but lack of compatibility with methanol solution. For short-term applications, Ni-brass combination is satisfactory, but for long-term applications, Ni-SS is better, as Nickel and SS materials have better compatibility in methanol environment compared to brass.
8 Conclusions
In the commercialisation process of the passive direct methanol fuel cell (pDMFC), market demands for efficient systems with optimisation of components performance with respect to durability and effectiveness. The desirable qualities of the current collector materials are excellent electrical conductivity and high thermal conductivity to optimise and to maintain the thermal stability of cell during operation and high corrosion resistance with compatibility in dilute methanol environment. These aspects are experimentally investigated with the combination of anode and cathode current collectors, fabricated with an opening ratio of 45.3% with combination set-ups, set-up-I, Nickel-Stainless steel; set-up-II, Nickel-brass; set-up-III Stainless Steel-Nickel and with set-up-IV Stainless Steel-brass materials. The anode–cathode combination of set-up-II, Nickel-brass showed the best current density (46.4 mA cm−2) and power density (7.157 mW cm−2), and set-up-IV, SS-brass combination showed the least performance with current density (34.4 mA cm−2) and power density (3.397 mW cm−2). Superior performance of Nickel is due to good electrical conductivity and better corrosion resistance to dilute methanol solution, whereas brass has the best electrical conductivity among the selected materials but suffers lack of compatibility with methanol solution. For short-term durations, Ni-brass combination performance is found satisfactory, but for long-term applications Ni/SS-316L is better as these materials have excellent compatibility and corrosion resistance in methanol environment compared to brass. In future investigations, materials that are suitable in dilute methanol environment either bare or with electrical conducting coatings may be used with combination of current collectors.
Abbreviations
- CC:
-
Current collector
- CD:
-
Current density
- MEA:
-
Membrane electrode assembly
- Ni:
-
Nickel
- PD:
-
Power density
- PTFE:
-
Polytetrafluoroethylene
- pDMFC:
-
Passive direct methanol fuel cell
- SS:
-
Stainless Steel
References
O’Hayre R, Cha S-W, Colella W, Prinz FB (2016) Fuel cell fundamentals, 3rd edn. John Wiley & Sons Inc., New Jersey
Raghavaiah NV, Naga Srinivasulu G, HariPrasad I (2020) Review of challenges in direct methanol fuel cell and contemporary status. Res Appl Thermal Eng 3(2):1–8. https://doi.org/10.5281/zenodo.3989515
Kamarudin SK, Achmad F, Daud WRW (2009) Overview on the application of direct methanol fuel cell (DMFC) for portable electronic devices. Published by Elsevier Ltd., International Association for Hydrogen Energy. https://doi.org/10.1016/j.ijhydene.2009.06.013
Boni M, Srinivasa Rao S, Naga Srinivasulu G (2020) Performance evaluation of an air breathing–direct methanol fuel cell with different cathode current collectors with liquid electrolyte layer. Asia-Pac J Chem Eng 2020:2465. https://doi.org/10.1002/apj.2465
Braz BA, Oliveira VB, Pinto AMFR (2020) Optimization of a passive direct methanol fuel cell with different current collector materials. Energy 208:118394
Wilberforce T, Ijaodola O, Ogungbemi E, El Hassan Z, Thompson J (2018) Effect of bipolar plate materials on performance of fuel cells. Module in Mater Sci Mater Eng. https://doi.org/10.1016/B978-0-12-803581-8.11272-X
Munjewar SS, Thombre SB, Mallick RK (2017) A comprehensive review on recent material development of passive direct methanol fuel cell, Ionics vol 23, pp 1–18
Mallick RK, Thombre SB, Shrivastava NK (2015) A critical review of the current collector for passive direct methanol fuel cells. J Power Sources 285:510–529
Yuan W, Tang Y, Yang X, Liu B, Wan Z (2012) Structural diversity and orientation dependence of a liquid-fed passive air-breathing direct methanol fuel cell. Int J Hydrogen Energ 37:9298–9313
Raghavaiah NV (2019) Overview of pressure vessel design using ASME boiler and pressure vessel code section viii division-1 and division- 2. Int J Res Eng Sci Manage 2(6):525–526
YangaW M, ChoubC SK (2007) Shua 2007, Effect of current-collector structure on performance of passive micro direct methanol fuel cell. J Power Sources 164(2):549–554. https://doi.org/10.1016/j.jpowsour.2006.11.014
Dohle H, Mergel J, Stolten D (2002) [2002], Heat and power management of a direct-methanol-fuel-cell (DMFC) system. J Power Sources 111(2):268–282
Huang J, Baird DG, McGrath JE (2005) Development of fuel cell bipolar plates from graphite filled wet-lay thermoplastic composite materials. J Power Sources 150:110–119. https://doi.org/10.1016/j.jpowsour.2005.02.074
Abraham BG, Chetty R (2021) Design and fabrication of a quick-fit architecture air breathing direct methanol fuel cell. Int J Hydrogen Energ 46(9), pp 6845–6856
Song SQ, Liang ZX, Zhou WJ, Sun GQ, Xin Q, Stergiopoulos V, Tsiakaras P (2005) Direct methanol fuel cells: The effect of electrode fabrication procedure on MEAs structural properties and cell performance. J Power Sources 145(2):495–501. https://doi.org/10.1016/j.jpowsour.2005.02.069
Cha H-C, Chen C-Y, Shiu J-Y (2009) Investigation on the durability of direct methanol fuel cells. J Power Sources 192(2):451–456. https://doi.org/10.1016/j.jpowsour.2009.03.028
Kuan Y-D, Lee S-M, Sung M-F (2014) Development of a direct methanol fuel cell with lightweight disc type current collectors. Energies 7:3136–3147. https://doi.org/10.3390/en7053136
Sgroi M, Zedde F, Barbera O, Stassi A, Sebastián D, Lufrano F, Schuster M (2016) Cost analysis of direct methanol fuel cell stacks for mass production. Energies 9(12):1008. https://doi.org/10.3390/en9121008
Braz BA, Oliveira VB, Pinto AM (2020) Experimental evaluation of the effect of the anode diffusion layer properties on the performance of a passive direct methanol fuel cell. Energies13:5198. https://doi.org/10.3390/en13195198
Shrivastava NK, Harris TA (2017) Direct methanol fuel cells. encyclopedia of sustainable technologies, pp 343–357. https://doi.org/10.1016/b978-0-12-409548-9.10121-6
Properties of Some Metals and Alloys (2021) Nickel Institute, https://nickelinstitute.org/media/1771/ propertiesofsomemetals and alloys_297_pdf.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2023 The Author(s), under exclusive license to Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Raghavaiah, N.V., Srinivasulu, G.N. (2023). Experimental Investigation on Passive Direct Methanol Fuel Cell with Dissimilar Current Collector Materials. In: Mehta, H.B., Rathod, M.K., Abiev, R., Arıcı, M. (eds) Recent Advances in Thermal Sciences and Engineering. Lecture Notes in Mechanical Engineering. Springer, Singapore. https://doi.org/10.1007/978-981-19-7214-0_1
Download citation
DOI: https://doi.org/10.1007/978-981-19-7214-0_1
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-19-7213-3
Online ISBN: 978-981-19-7214-0
eBook Packages: EngineeringEngineering (R0)