Abstract
A single cell passive air-breathing liquid feed direct methanol fuel cell (DMFC) is designed and fabricated. Furthermore, the effects of cell orientation and environmental conditions such as temperature and relative humidity on the performance of such passive DMFC are tested experimentally. The obtained results indicate that both environmental temperature and relative humidity have significant effects on the performance of fabricated fuel cell. The experimental data contained within this work shows that under lower relative humidity and higher temperature, the passive air-breathing direct methanol fuel cell has higher power output and better performance. According to experimental results, flooding has a vital role on the cell performance in various relative humidity and temperatures. The results also show that cell orientation has a strong effect on the performance of passive DMFC. The best power output and performance were achieved under vertical orientation.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Direct methanol fuel cell (DMFC) has received much attention because of its high theoretical energy density, using liquid methanol as fuel, employing polymer electrolyte membrane, low pollution (green), and working under ambient conditions [1–7]. A DMFC is able to directly convert the electrochemical energy into electricity by using aqueous or vaporized methanol at the anode side and oxygen as the oxidant at the cathode side. According to the reactant delivery modes, it can be categorized into two types, including passive and active DMFCs [8, 9]. In passive DMFCs, fuel and oxidant are supplied passively without any moving parts (contrary to active) as a result, fuel cell system becomes more simple and compact (with low weight) and also the parasitic energy losses due to ancillary devices eliminate significantly. Through these advantages, the passive DMFCs have been considered as a more promising and reliable power sources for portable applications [10–12]. Also, it should be mentioned that although the performance of active DMFCs is higher than that of passive DMFCs, the passive system is simpler and has lower weight, and as a result, it is regarded as one of the most promising candidates to provide sustainable power output for portable applications [13, 14]. In recent years, numerous researches were conducted about different passive DMFCs.
For example, Tang et al. [15] studied the effects of structural aspects on the performance of a passive air-breathing DMFC. Consequently, the maximum power density of 10.7mWcm−2 was achieved by selecting a 28.3 % open ratio for the cathode and anode current collectors and also by using a membrane electrolyte assembly (MEA) with non-bonded diffusion layer.
Guo and Faghri [16] developed a 1 (W) passive DMFC. A peak power output of 1.5 W was achieved with the dilute methanol solution feed.
On the other hand, as reported by Yousefi et al., in high-power portable devices like laptop or hand phone, one approach to use passive DMFC as a power source instead of stacking could be single passive DMFC with higher active area. For example, Yousefi et al, designed, manufactured, and investigated a passive direct methanol fuel cell with 100-cm2 active areas [12]. Finally, with an optimized design, a maximum power output of 520 mW (5.2 mWcm−2) was achieved under ambient conditions.
S. Basri et al.[17] also proved that the voltage of passive direct methanol fuel cells affect the design parameters while the current is influenced by the active areas of passive direct methanol fuel cell.
Also, R. Chen and Zhao et al. investigated both the effects of methanol concentration and cell orientation on the performance of an air-breathing passive DMFC with 4-cm2 active area. They concluded that the better performance with higher methanol concentrations is mainly attributed to the increase in the cell operating temperature which was caused by the exothermic reaction between permeated methanol and oxygen on the cathode side. They also indicated that stronger natural convection in the vertical orientation caused the improved performance in the vertical orientation, and at this orientation, the fuel utilization is lower as a result of the increased rate of methanol crossover. It should be emphasized that all of the previous researches were limited to passive DMFCs with active areas lower than 36 cm2 and obtained results were not validated with higher active areas.
It should be noted that because of the inherent properties of passive DMFCs, which must operate passively with unforced air and without any auxiliary devices (unlike active cases), the performance of passive DMFC is too sensitive to the variations of environmental operating conditions. These environmental conditions could be ambient pressure, dynamic behavior of the place where fuel cell is located, surrounding temperature, relative humidity, etc. As a result, in order to develop the portable applications of passive DMFCs (such as vehicles or laptop, etc.) and also to produce reliable power output in various operating environments, these environmental operating conditions should be investigated carefully and experimentally. Hence, as discussed earlier, because of the poor studies about the effects of environmental conditions on the performance of air-breathing passive DMFC, the objective of this work is to study the effect of two parameters in environmental conditions such as surrounding temperature and relative humidity on the performance of such fabricated passive DMFC. Furthermore, the effect of cell orientation on the performance of such fuel cell with a new active area is investigated experimentally.
Material and methods
Membrane electrolyte assembly
The pretreated Nafion 117 was employed in current experiments. The pretreatment approach included boiling the membrane in 5 % vol. H2SO4 and washing in deionized water for 2 h, respectively. Then, membrane was kept in the deionized water before the fabrication of MEA. Single side ELAT electrodes were used in both anode and cathode sides, where type (A) E-TEK carbon cloth was used as the backing support layer with a 30 % wt. polytetrafluoroethylene (PTFE) wet-proofing treatment. The catalyst loading on the cathode side was 2.0 mgcm−2 using 40 %wt. Pt on Vulcan XC-72 and the catalyst loading on the anode was 4.0 mg cm−2 with Pt-Ru black (1:1 a/o). Furthermore, 0.8 mg cm−2 dry Nafion® ionomer was applied onto the surface of anode and cathode electrodes. Finally, MEA with an active area of 10 × 10 cm2 was formed by hot pressing at 140 °C and 6 MPa for 2 min. More detailed MEA fabrication procedures and information can be found elsewhere [18, 19].
Single cell fixture and instrumentation
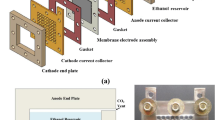
Figure 1 shows a schematic diagram of the configuration of the single cell passive air-breathing DMFC. For the visualization of the entire cell, the transparent acrylic Plexiglas material was used to fabricate the methanol solution reservoir and cathode window end frame by using milling and polishing techniques. The use of transparent acrylic Plexiglas material as a fixture not only allowed the visualization of the internal activities of the cell but also provided sufficient rigidity to support the unit cell. A built-in reservoir with a volume of approximately 50 mL was machined to store the methanol solution. Two through holes were drilled at the top of the methanol solution reservoir for fuel injection and CO2 exhaust. Both the anode and cathode current collectors were made of two 1 mm 316-L stainless steel plates with perforated hole-arrays distributed within the active areas. In this study, a plurality of holes with a diameter of 1 mm was drilled inside the active areas of both current collectors. A hollow frame window was cut in the cathode endplate to expose the cathode compartment to the surrounding air. Consequently, methanol solution diffuses into the anode catalyst layer from the fuel reservoir, while oxygen from surrounding air diffuses into the cathode catalyst layer through the hollow frame window passively. A silicon-rubber strip was used to seal the gaps between CC and the end plate, while an in-house PTFE film served as the gasket between CC and the edges of the MEA. Fig. 2 show the entire fabricated passive DMFC during the experiments.
Schematic of the passive air-breathing DMFC [15]
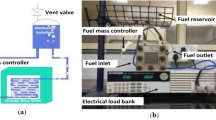
A commercial Chromo (2.6 kW) electronic load interfaced to a computer was employed to control the condition of discharging and record the voltage–current curves (or polarization curve). For measuring each set of polarization curves, a waiting period of more than 40 s after fuel injection was used to reach stable voltage. The internal resistance of the passive DMFC was also measured by Arbin BT2000 built-in function. The internal resistances reported in this work are the average data over appropriate applied pulses. The temperature of the fabricated fuel cell was measured by a K-type thermocouple used together with an Agilent® Data Acquisition/switch unit. It must be noted that the sensor of thermocouple was installed in fuel reservoir in order to monitor cell operating temperature. A thermo vacuum oven chamber was fabricated for use as an environmental chamber during the temperature and relative humidity testing. The temperature and relative humidity inside the environmental chamber (vacuum oven) were monitored and recorded with a humidity and temperature transmitter. The transmitter error range for relative humidity and for temperature Measuring were ±1.0 % and ±0.10 °C respectively. The error range of measuring the mass of methanol and deionized water solution during the test was within ±0.2 gr. Prior to experimental testing, to improve the proton conductivities of PEM and electrodes, the MEA was hydrated by filling the reservoir with 1 wt.% methanol solution and allowing the cell to stand for about 4 h.
Before each test, the MEA was activated under a constant load for about 4 h, and also, to eliminate the effects of previous tests, the MEA was washed in distilled water. All experiments were conducted at 1 atm (ambient condition).
Results and discussions
Effects of cell orientation
Figure 3 shows the transient discharging voltage of fabricated DMFCs with different cell orientations at a constant current of 300 mA. It should be noted that here, all experiments were conducted at 25 °C, 1 atm and the relative humidity of 45 % (ambient condition). As shown in Fig. 3, the fabricated cell with horizontal orientation with anode facing upward and vertical orientation shows better performance due to the easy removal of CO2 bubbles generated at anode and produced water at cathode, than horizontal orientation with anode facing downward. In horizontal orientation with anode facing downward, due to the buoyancy force and gravity force, a large amount of generated CO2 bubbles accumulate at the anode catalyst layer and also, a large amount of generated water accumulate at the cathode catalyst layer (flooding), which both block the paths for methanol and oxygen transferring to the reaction sites (the removal of CO2 and water bubbles become rather difficult) and consequently, cell performance decreases significantly [20].
As shown in Fig. 3, the performance of passive cell with vertical orientation and that of passive DMFC with horizontal orientation with anode facing upward were similar, so these two orientations were compared separately. Figure 4 shows a comparison of the performance of fabricated cell with vertical orientation and horizontal anode facing upward orientation. It can be seen that vertical orientation always yielded better performance than the horizontal orientation with anode facing upward. As shown in Fig. 5, under a constant load and 2.5-M methanol solution, vertical orientation produced higher output voltage than that of horizontal orientation with anode facing upward.
The cell operating temperature was measured for further analysis, when the passive DMFC was differently oriented. Figure 6 shows the variations of the cell operating temperature with 2.5-M methanol solution and under a constant load at 600 mA. Apparently, the vertical orientation exhibited a higher operating temperature than the horizontal orientation with anode facing upward. The higher operating temperature in vertical orientation was mainly caused by the exothermic reaction between the permeated methanol and oxygen on the cathode [20]. This can also be concluded from Fig. 7 because open circuit voltage (OCV) values reflect the methanol crossover degree. This phenomenon is related to the fact that the rate of methanol crossover becomes higher in vertical orientation than the methanol crossover in horizontal orientation with anode facing upward and accordingly, because of a higher mixed potential, the OCV values decrease in vertical orientation. Thus, the exothermic reaction between this higher rate of permeated methanol and oxygen on the cathode reaction sites generates more heat and therefore causes a higher operating temperature in vertical orientation than the horizontal orientation with anode facing upward.
This explains why the fuel cell with vertical orientation yielded a higher performance in all conditions. On the other hand, the higher operating temperature in the vertical orientation led to the improved electrochemical kinetics of methanol oxidation and oxygen reduction reactions and as a result, caused a better performance in vertical orientation than other orientations. The increased operating temperature as a result of the increased methanol crossover could be the most important reason that yields a higher performance of the fabricated cell operated at the vertical orientation. As shown in Fig. 3, the fabricated passive DMFC with anode facing downward results in the worst performance due to flooding in cathode and CO2 bubbles accumulation in anode. Also, the vertical orientation always yields the best performance than the horizontal orientations. The improved performance in the vertical cell orientation was caused by the increased operating temperature as a result of a higher rate of methanol crossover (which was resulted from Fig. 7), which finally resulted in higher electrochemical kinetics of methanol oxidation and oxygen reduction reactions. So, as discussed earlier, it can be concluded that the cell orientation has a significant effect on the cell performance.
Effects of environmental conditions
Here, the effects of the variations of environmental temperature and environmental relative humidity on the performance of fabricated cell would be studied experimentally. Figures 8 and 9 show the performance of the fabricated passive DMFC operated under different environmental temperatures which varies from 10 to 50 °C. It must be noted that in current tests, all experiments were conducted with 3-M methanol solution at 1 atm and 50 % relative humidity. It is obvious that the power density of passive DMFC, increased by increasing environmental temperature from 10 to 50 °C. Hence, a maximum power density of 3.47 mWcm−2 was obtained at highest environmental temperature which was 50 °C (highest temperature among other temperatures). Also, to investigate the effect of environmental temperature on the operating conditions of fabricated cell, the effect of temperature on the output voltage of cell were tested under a constant load (0.6 A). As shown in Fig. 10, for any given test, the highest environmental temperature which was 50 °C produced the highest output voltage under a constant load.
Consequently, it is found by observing the results in Figs. 8, 9, 10 that the performance of fabricated cell at highest surrounding temperature (50 °C) was better than the cell performance at 30 °C surrounding temperature and also the cell performance at 30 °C surrounding temperature was better than the cell performance at 10 °C surrounding temperature.
The reason for this behavior can be attributed to the fact that the higher environmental temperature leads to the improved electrochemical kinetics of methanol oxidation and oxygen reduction reactions, which finally resulted in the better performance of the fabricated passive DMFC [1, 12, 20–22], as shown in Figs. 8, 9, 10. Also, as discussed in the previous literatures, the internal resistance of the fabricated passive DMFC decreases by increasing cell temperature, which can also improve the performance of fuel cell [12, 23]. This phenomenon could also be concluded from Table 1, which shows the variations of internal cell resistance under various surrounding temperatures (10, 30, 50 °C). As shown in Table 1, the internal resistance of cell decreased with increasing in surrounding environmental temperature.
Also, it should be noticed that increasing surrounding environmental temperature, leads to higher rate of the evaporation of water which is generated at cathode side. As a result, the rate of flooding phenomenon would be reduced significantly, and so, more oxygen would be supplied to the cathode catalyst sites and thus the cell performance would be improved dramatically [1, 12, 24–26]. This subject is obvious in Fig. 11.
Figure 11 shows that decreasing the temperature lead to decreasing mass loss of water from fuel cell due to a lower rate of water evaporation. As a result, lower temperatures exacerbate and increase the rate of water flooding at the cathode side. So, even more parts of the cathode pores for supplying oxygen to the cathode catalyst sites would be blocked due to this higher rate of flooding water. Consequently, the cell performance would be decreased significantly (see Figs. 8 to 11).
Figures 12, 13 show the performances of the fabricated cell at different relative humidity which varies from 20 to 90 %. It must be noted that in current tests, all experiments were conducted with 3-M methanol solution at 1 atm and 25 °C. It is obvious that power density of the cell increased by decreasing relative humidity from 90 to 20 %. Hence, a maximum power density of 3.1 mWcm−2 was obtained with 3-M methanol solution under the lowest relative humidity which was 20 %. Also, to investigate the effect of varying relative humidity on the operating conditions of fabricated fuel cell, the effects of relative humidity on the output voltage of fuel cell were tested under a constant load (0.6 A). As shown in Fig. 14, the 20 % relative humidity caused the highest output voltage under a constant load and 90 % relative humidity caused the worst performance.
This means that increasing relative humidity has an unfavorable and adverse effect on the performance of passive DMFC. This could be related to the fact that because the rate of the water evaporation decreased significantly with increase in surrounding relative humidity, the flooding becomes increasingly severe. This fact could be concluded from Fig. 15 too.
Figure 15 shows the variations of the mass of water in which loss from the cell is due to evaporation for various relative humidity. Given the water content at the beginning and end of each test and the total mass of water consumed, the mass of water that were removed from the fuel cell during the test were calculated. The difference in the total mass of water removed from the fuel tank and the mass of water reacted at the anode is the mass of water lost due to water crossover. The mass of water consumed is closely related to both the operating temperature and the relative humidity [1]. For any given relative humidity, the mass of water consumed during the test increased with increasing temperature. Also, the mass of water reacted at the anode increased with increasing temperature because the electrochemical kinetics of methanol oxidation and oxygen reduction reactions increases with increasing temperature. However, some content of the water consumed during each test was lost due to water crossover and not reacted at the anode again. Water lost to crossover ultimately is removed from the system by evaporation to the environment or by drainage of liquid water. As discussed earlier, for all tests conducted, as shown in Fig. 11, the mass of water lost due to evaporation increased with the increasing environmental temperature.
Also, the relative humidity played an important role in the rate of water removal from the fuel cell. For all tests performed at 25 °C, the rate of water removal from the fuel cell decreased with increasing relative humidity. In low relative humidity environments, water evaporates at a faster rate than higher relative humidity environments [1]. For example, for the tests performed at 25 °C, the mass of water removed from cell due to evaporation at 50 % relative humidity was lower than 10 % relative humidity. This trend is obvious in Fig. 15. As a result, higher relative humidity causes higher rate of flooding at cathode side due to lower water evaporation rate and as a result, higher relative humidity increases the rate of water flooding at the cathode side. So more parts of the cathode pores for supplying oxygen to the cathode catalyst sites would be blocked due to this higher rate of flooding water. Consequently, the cell performance would be decreased significantly. This trend is also obvious in Figs. 12 to 14.
Figure 16 shows that for all polarization tests performed at a given relative humidity (4-M methanol solution, 1 atm), as temperature increases, the power density of fabricated passive DMFC increases and also the power density of fabricated passive DMFC at a given temperature decreases as relative humidity increases. As discussed previously, these trends could be attributed to this fact that flooding rate decreased with increasing temperature and flooding rate increased with increasing relative humidity.
Finally, maximum power density of 7.41 mWcm−2 was obtained with 4-M methanol solution at 20 % relative humidity and 50 °C surrounding environmental temperature. So, it should be noticed that environmental conditions like relative humidity and temperature have a significant effect on the performance of passive direct methanol fuel cells. Also, water removal systems to decrease flooding at cathode side could play a vital role on the performance of such fuel cells under various environmental conditions.
Conclusions
A passive air-breathing direct methanol fuel cell was fabricated and tested to study its behavior when varying the cell orientation and environmental conditions, such as relative humidity and surrounding temperature. According to the experimental results, it was observed that the cell orientation has significant effect on the cell performance. The fabricated passive DMFC with horizontal orientation with anode facing downward yielded the worst performance due to higher flooding in cathode side and CO2 bubbles accumulation in anode side. Also, the vertical orientation always yielded the best performance than other orientations. It was demonstrated that the improved performance in the cell vertical orientation was caused by the increased operating temperature as a result of a higher rate of methanol crossover.
The experimental results also revealed that both environmental temperature and relative humidity had strong effects on the performance of passive air-breathing direct methanol fuel cell. As discussed earlier, increasing the temperature was shown to improve the performance and current production capabilities of the fuel cell. Higher environmental temperature leads to the improved electrochemical kinetics of methanol oxidation and oxygen reduction reactions, decreasing internal resistance within the fuel cell and decreasing flooding at cathode side, which finally, all of them resulted in better performance of fabricated passive DMFC in higher environmental temperatures. For example, best performance was obtained by highest environmental temperature. Also, increasing relative humidity proved to decrease the performance and current production capabilities of fabricated fuel cell. For example, lowest performance was obtained by highest relative humidity. The experimental results suggested that flooding was the major factor that reduces the fuel cell performance when operating at higher relative humidity. The highest peak power density achieved in this study was 7.41 mWcm−2 with 4-M methanol solution at 50 °C temperature and 20 % relative humidity.
Therefore, from both fuel efficiency and power production perspective, because flooding appears to be one of the largest barriers to passive direct methanol fuel cells application, it is very important to prevent or manage flooding at the cathode side. Future research work could include testing and evaluating new ways of preventing and managing flooding phenomenon in different environments. Although studying the effect of environmental pressure on the performance of such passive DMFCs would be helpful.
References
Woolard D (2010) Performance characterization of a passive DMFC over a range of operating temperatures and relative humidity. Virginia polytechnic Ins-M.S. thesis(2010)
Achmad F, Kamarudin SK, Daud WRW, Majlan EH (2011) Passive direct methanol fuel cells for portable electronic devices. Appl Energy 88:1681
Chu D, Jiang R (2006) Effect of operating conditions on energy efficiency for a small passive direct methanol fuel cell. Electrochim Acta 51:5829–5835
Jianyu C, Mei C, Ji C (2010) Novel hydrogen production technologies and applications. Hydrog Energy 35:4622
Chen R, Zhao TS (2007) A novel electrode architecture for passive direct methanol fuel cells. Electrochem Commun 9:718
Dicks A, Larminie J (2002) Fuel cell systems explained. John Wiley & Sons Ltd, New York. ISBN 978-0-470-84857-9
Chang H, Kim JR, Cho JH, Kim HK, Choi KH (2002) Solid State Ionics 148:301
Wei Y, Yong T, Zhenping W, Minqiang P (2010) Operational characteristics of a passive air-breathing direct methanol fuel cell under various structural conditions. J Hydrog Energy 36:1–13
Kamarudin SK, Daud WRW, Ho SL, Hasran UA (2007) Overview on the challenges and developments of micro-direct methanol fuel cells (DMFC). J Power Sources 163:743–754
Shrivastava NK, Thombre SB (2011) Barriers to commercialization of passive direct methanol fuel cells: a review. IJEST 3:6000–6007
Kamarudin SK, Achmad F, Daud WRW (2009) Overview on the application of direct methanol fuel cell (DMFC) for portable electronic devices. J Hydrog Energy 34:6902–6916
Sasan Y, Ganji DD (2012) Experimental investigation of a passive direct methanol fuel cell with 100 cm2 active areas. Electrochim Acta 85:693–699
Guo Z, Faghri A (2006) Development of planar air breathing direct methanol fuel cell stacks. J Power Sources 160:1183–1194
Eccariusa S, Krausea F, Beardb K et al (2008) J Power Sources 182:565
Tang Y, Yuan W, Pan M, Tang B, Li Z, Wan Z (2010) Effects of structural aspects on the performance of a passive air-breathing direct methanol fuel cell. J Power Sources 195:5628–5636
Guo Z, Faghri A (2008) Development of a 1 W passive DMFC. Int Commun Heat Mass Transf 35:225–239
Basri S, Kamarudin SK, Daud WRW, Ahmad MM (2010) Non-linear optimization of passive direct methanol fuel cell (DMFC). J Hydrog Energy 35:1759–1768
Ren S, Sun G (2006) Organic silica/Nafion® composite membrane for direct methanol fuel cells. J Membr Sci 282:450–455
Wang L, Northwood DO, Nie X, Housden J, Spain E, Leyland A, Matthews A (2010) Corrosion properties and contact resistance of TiN, TiAlN, and CrN coatings in simulated proton exchange membrane fuel cell environments. J Power Sources 195:3814–3821. doi:10.1016/j.jpowsour.2009.12.127
Chen R, Zhao TS, Liu JG (2006) Effect of cell orientation on the performance of passive direct methanol fuel cells. J Power Sources 157:351–357
Lai QZ, Yin GP, Wang ZB, Du CY, Zuo PJ, Cheng XQ (2008) Influence of methanol crossover on the fuel utilization of passive direct methanol fuel cell. Fuel Cells 8:399–403
Liu JG, Zhao TS, Chen R, Wong CW (2005) The effect of methanol concentration on the performance of a passive DMFC. Electrochem Commun 7:288–294
Guo Z, Faghri A (2006) Miniature DMFCs with passive thermal-fluids management system. J Power Sources 160:1142–1155
Xu C, Faghri A, Li X, Ward T (2010) Methanol and water crossover in a passive liquid-feed direct methanol fuel cell. Int J Hydrog Energy 35:1769–1777
Park JY, Lee JH, Kang SK, Sauk JH, Song I (2008) A small mono-polar direct methanol fuel cell stack with passive operation. J Power Sources 178:181–187
Kim D, Cho EA, Hong SA, Oh IH, Ha HY (2004) Recent progress in passive direct methanol fuel cells at KIST. J Power Sources 130:172–177
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yousefi, S., Shakeri, M. & Sedighi, K. The effect of cell orientations and environmental conditions on the performance of a passive DMFC single cell. Ionics 19, 1637–1647 (2013). https://doi.org/10.1007/s11581-013-0889-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11581-013-0889-y