Abstract
The global sensing science in the past couple of years has seen brilliant successes in the designs and syntheses of diverse fluorescent and colourimetric chemosensors of ultra-high selectivities and sensitivities for the tracking of metal ions in environmental and biological systems. Amongst the most widely employed fluorophores for the development of fluorescent and colourimetric chemosensors is the 1, 8-naphthalimide fluorophore, which is distinctive due to its possession of outstanding photophysical properties unequalled by other fluorophores. Many reported literatures are replete with employment of 1, 8-naphthalimide as a unique fluorophore for the construction of chemosensors for the monitoring of metal ions (such as Cu2+, Hg2+, Cr3+, Fe3+, Zn2+, Ag+, Pd2+, Al3+, Ba2+, Au3+, and Bi2+, and/or a combination of any of them) with remarkable results documented from various labs. This review summarises recent advances in the development of representative fluorescent and colourimetric 1, 8-naphthalimide-based chemosensors reported within the past 7 years. It is believed that gaining insights into the various highlighted examples would help to refine our knowledge of the field and pave the way for further advancement in the constructions of fluorescent and colourimetric 1, 8-naphthalimide-based chemosensors of improved sensing parameters and practical application values.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Over the past two decades of the advent of the supramolecular chemistry, research efforts have been directed towards the development of fluorescent chemosensors as vibrant tools for sensing various heavy metal ions and anions of environmental and biological significance, and hydrogen ion (i.e. proton) in biological systems (Gunnlaugsson et al. 2006; Lodeiro and Pina 2009; Duke et al. 2010; Georgiev et al. 2011; Marinova et al. 2011). Inasmuch as the fluorescent technique is a very useful sensing tool, it has enjoyed wide application in the research areas of clinical diagnostics, biotechnology, molecular biology and biochemistry, and materials and environmental sciences (Mason 1999; Lakowicz et al. 2006). The fluorescent signalling method offers the multiple advantages of high sensitivity and selectivity, real-time monitoring, local observation, simplicity of operation, inexpensiveness of equipment, and fast response time (Lee et al. 2015; Carter et al. 2014; Zhang et al. 2014a; Kim et al. 2012), and non-destructibility, that overrule the merits of extant traditional bulk methods of high-performance liquid chromatography, mass spectrometry, and atomic absorption spectroscopy. A closely related detection technique to fluorescence method is colourimetric method, which has also been used as a powerful sensing tool because it can afford naked eye detection even before the use of spectrophotometric analysis. The method has been successfully used in diagnostic assays like blood-glucose monitoring and early pregnancy tests (Bicker et al. 2011).
Fluorescent chemosensors
Also known as probes, generally, chemical sensors or chemosensors are molecules that are capable of detecting matter or energy with the concomitant output of a signal that could be measured. Sensors which upon interaction with a species under test (i.e. an analyte) give fluorescence modulations are grouped collectively as fluorescent chemosensors. Typically, chemosensors are made up of three components and are usually designed on the ‘fluorophore–spacer–receptor’ paradigm (Bryan et al. 1989) as illustrated in Fig. 1. Given their differences, each component of a chemosensor serves distinct role as briefly explained below (Parkesh et al. 2011; de Silva et al. 1995a; Wasielewski 1992):
The fluorophore
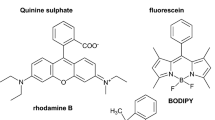
The fluorophore is the signalling moiety and is responsible for the transduction of the interaction between the receptor and the species being detected (i.e. analyte) into a readable signal of change in fluorescence. Chemosensors owe their colours to the structural features of the fluorophore moieties they embed. Commonly encountered fluorophores include boron-dipyrromethene (BODIPY), rhodamine, fluorescein, pyrene, anthracene, naphthalimide, and coumarin (Deng et al. 2017; Liu et al. 2017b; Papalia et al. 2017; Dey et al. 2017; Jiang et al. 2017; Mironenko et al. 2017; Erdemir and Kocyigit 2017; Fu et al. 2017a; Hou et al. 2017; Goncalves et al. 2017a; Sun et al. 2017; Saura et al. 2017; Gupta et al. 2017; Liu et al. 2017b; Gonçalves et al. 2017b; Huang et al. 2017).
The spacer
The spacer links and keeps both the fluorophore and receptor at a convenient distance to allow for the feasibility of photoinduced electron transfer (PET) process which dictates the tunable fluorescence property of fluorescent chemosensors. Chemosensors necessarily may or may not anchor spacer. Most commonly encountered length of a spacer is a few carbon chains usually between a single to a double carbon chain.
The receptor
This is also known as the recognition unit, and it serves the purpose of binding to the analyte in a way that allows for selectivity and effectiveness.
The process whereby chemosensors interact with an analyte species to give off an energy that could be measured is known as signal ‘transduction’. The fluorescence mechanism is one such desirable transduction mechanism since the emission wavelength always displays higher value than that of the excitation wavelength. Low concentrations of analyte substances are required for such signalling transduction. Figure 2 gives a diagrammatic illustration of typical fluorescence signal transduction mechanism (de Silva et al. 1995a).
Ideally, fluorescent chemosensors must fulfil two stringent requirements: on the one hand, there must be enough affinity between the receptor unit and the relevant analyte; on the other hand, there must be no interferences from the other rival substances under the same investigation (Valeur and Leray 2001).
The 1, 8-naphthalimide fluorophore
To date, several fluorophores have been widely used in the construction of fluorescent chemosensors, including boron-dipyrromethene (BODIPY), rhodamine, fluorescein, pyrene, anthracene, naphthalimide, coumarin, etc.). Amongst them, 1, 8-naphthalimide stands out as a much sought-after, exquisite fluorophore owing to its superior features of strong absorption band in the visible region (a wide range usually between 350 and 450 nm but can extend up to 650 nm), outstanding photostability, high fluorescent quantum yield (a wide range typically between 0.20 and 0.80), and large Stokes’ shift (a wide range usually between 3500 and 6500 cm−1), besides the possibility of an easy modification of its structure (Lippert et al. 2011; Shao et al. 2015; Lee et al. 2013; Du et al. 2012; Huang et al. 2014a; Lee et al. 2014; Shaki et al. 2010). 1, 8-naphthalimide contains a very strong naphthalene ring structure so that the interaction of its molecules with solvents or other solute molecules is reduced, making the external transfer energy to also be lowered, which is very beneficial to the emission of fluorescence. The presence of an electron donor conjugated system in its molecular structure allows for electrons in its system to be easily excited by the external light or electric field so as to produce a strong fluorescence (Grabchev et al. 1995). Owing to its conjugated electron system (i.e. π electron system), the 1, 8-naphthalimide structure could be easily modified via different synthetic approaches. This property can then be taken advantage of to interact with various substituents, thereby generating varied fluorescent transduction and properties. Figure 3 gives the structure of the 4-amino-1, 8-naphthalimide, which is the more commonly employed architectural block, although the 3-amino-1, 8-naphthalimide unit is also occasionally used (de Silva et al. 1996).
Having been validated to be a fantabulous fluorophore, there have been several instances of engagement of 1, 8-naphthalimide as a key component in the design of fluorescent dyes for polymer materials (Bojinov and Grabchev 2003), laser active media (Gruzinskii et al. 1998), fluorescent markers in biology (Stewart 1981), anticancer agents (Ott et al. 2008) and medicinal analgesics (de Souza et al. 2002), fluorescence switches and sensors (Bojinov et al. 2009), light emitting diodes (Liu et al. 2006), electroluminescent materials (Zhu et al. 2003) liquid crystal displays (LCDs) (Grabchev and Chovelon 2003a), ion probes (Cosnard and Wintgens 1998), logic gates (de Silva et al. 1997b), and organic photoconductive materials (Law 1993).
Over the years, there are a plethora of fluorescent chemosensors built from the 1, 8-naphthalimide fluorophore for the monitoring of metal ions. Such exist in previous publications (de Silva et al. 1995b; Rurack et al. 2000; Burdette et al. 2001; Grabchev et al. 2003b; Gunnlaugsson et al. 2003; He et al. 2003; Fan et al. 2005; Bricks et al. 2005; Wang et al. 2005; Liu et al. 2005; Xu et al. 2005; Anikin and Fedko 2006; Cosnard and Wintgens 1998; Xu et al. 2006; Lu et al. 2007; Chovelon et al. 2007; Mu et al. 2007; Parkesh et al. 2007; Staneva et al. 2007; Grabchev et al. 2004; Bojinov et al. 2008; Grabchev and Chovelon 2008; Duan et al. 2008; Tamanini et al. 2009; Li et al. 2009; Bojinov and Panova 2009; Nandhikonda et al. 2009). It should be made known that it is not the attempt of this review to revisit those pretty ‘old’ references but to report the ‘newer’ ones within the past seven years, i.e. between the years 2010 and 2017. Enough references, 76 articles thereabout, have been reviewed in strength so as to bring to the research spotlight the tremendous works that have done in this field but at the same time ensure conciseness of the report. Moreso, the works reported focus on some representative metal ions, including but not limited to Cu2+, Hg2+, Zn2+, Ag+, Pd2+ and Au3+, and/or a combination of them. Restriction has been placed on the 1, 8-naphthalimide as the fluorophore, with various receptors been successfully coupled with it. All reported compounds either make use of the fluorescence method or combined fluorescence and colourimetry method in their detection modes. To the best of the author’s knowledge, such a recently published review of various 1, 8-naphthalimide-based chemosensors for different cations within the 7-year duration has not been reported.
Metal-specific 1, 8-naphthalimide-based fluorescent chemosensors
Cation sensing has been one of the principal goals and pursuits of researchers in the field of supramolecular chemistry owing to the indisputable roles and impacts of cations in our day-to-day life. The environmental and biological relevance of cations have been recognised (de Silva et al. 1997a; Xiao et al. 2007; Que et al. 2008; Kim et al. 2008; Xu et al. 2010c; Zhang et al. 2011b; Kim et al. 2012). This section maps out selected fluorescent and colourimetric 1, 8-naphthalimide-based chemosensors developed so far for cations.
Cu2+ ion-selective chemosensors
The research team of Fu et al. made remarkable success in the synthesis of the chemosensor 1 that bears 1, 8-naphthalimide Schiff base and photochromic diarylethene units (Fig. 4) (Fu et al. 2017b). This compound which exhibited both fluorescent and colourimetric signalling modes effectively discriminated Cu2+ ion from other cations present together in acetonitrile solution with association constant and detection limit calculated to be 3.13 × 104 and 2.4 × 10−6 mol L−1, respectively. Insight into the Job’s plot analysis yielded a 1:1 binding ratio. The synthesised compound was also capable of monitoring F−. Despite all its other interesting features, the sensing mechanism of the reported compound was analytically irreversible.
In their recent work, the research group of Gao developed the water-soluble fluorescent chemosensor 2, which anchors 1, 8-naphthalimide and two [12]aneN3 as fluorophore moieties (Gao et al. 2016). The compound (Fig. 5) displayed high selectivity and sensitivity for Cu2+ ion monitoring in coexistence with several other ions investigated under the same conditions in Tris–HCl buffer system. Furthermore, the chemosensor experienced a quenching of its fluorescence upon binding with Cu2+ ion (a 127-fold dampening). As given by the titration experiment and Job’s plot, its stoichiometric ratio of binding with Cu2+ ion was 1:2. In further experiment, the resultant complex 2-Cu2+ was employed for the sensing of Adenosine-5′-triphosphate (ATP). The detection limits of chemosensor 2 towards Cu2+ ion and 2-Cu2+ complex towards ATP were obtained to be 1.3 × 10−8 and 8.5 × 10−9 M, respectively, while the respective quantum yields of complex 2-Cu and complex 2-Cu with ATP were calculated to be 0.0014 and 0.1588. The most interesting features of 2 are its regeneration potency (i.e. capability to reversibly detect Cu2+ ion) upon the addition of ATP and its ability for Cu2+ ion and ATP monitoring in living cell samples.
Stimulated by the interesting fluorescent properties of 1, 8-naphthalimide fluorophore, Chen et al. designed and synthesised the simple but effective fluorescence ‘turn on’ chemosensor 3 (Chen et al. 2016). Noteworthy is that the chemosensor displayed an ultra-high sense of affinity towards Cu2+ in the mixture of other various metal ions tested in acetonitrile/water (50/50, v/v, 10 mM HEPES buffer, pH 7.4) solution. The binding ratio of the interaction of 3 and Cu2+ was established to be 1:1, and the detection limit was calculated as 0.0326 μM. The binding of the chemosensor with Cu2+ was demonstrated to be reversible. Finally, the chemosensor (Fig. 5) was applied for Cu2+ imaging in living cells.
Compound 4, which anchors a 1, 8-naphthalimide unit as the fluorophore group and a Schiff base unit as the recognition group, was developed by Xu’s research group as an efficient chemosensor for Cu2+ tracking (Fig. 5) (Xu et al. 2017). The chemosensor’s performance was optimal at pH = 7.2. In Tris–HCl (pH = 7.2) buffer–DMF (1 : 1, v/v) solution, chemosensor 4 displayed unique selectivity for Cu2+ ion amongst other co-existed alkali, alkaline earth, and transition metal ions with a marked reduction in the fluorescence intensity of 4. Job plot and fluorescence titration experiments revealed the formation of a 1:1 complex between 4 and Cu2+ ion. The chemosensor worked best for Cu2+ quantification within the linear range of 0.5–5 μM with detection limit and association constant of 0.23 μM and 1.328 × 106 M−1, respectively obtained.
He et al. fabricated a new naphthalimide-based fluorescent chemosensor, 5, for the analytical detection of Cu2+ ion (Fig. 6) (He et al. 2015). In the absence of Cu2+ ion, 5 displayed strong greenish fluorescence. Upon the addition of 2 equiv. of Cu2+ ion to 5 in CH3CN:H2O (4:1, v/v) solution, there was disappearance of the greenish fluorescence with a simultaneous lowering of the emission intensity (a 30-fold quenching). The addition of other metal ions left a mild influence on the fluorescence intensity of 5. Results of the Benesi-Hildebrand plot and ESI–MS spectra gave a 1:2 stoichiometric binding ratio of 5 with Cu2+. The detection limit of 5 for Cu2+ detection was estimated to be 64 ppb. The compound was successfully assessed for practical detection of Cu2+ in living cells.
Hu and co-workers described a semicarbazide-based naphthalimide, 6, as a colourimetric, fluorescent chemosensor for Cu2+ monitoring (Fig. 6) (Hu et al. 2015). In the presence of Cu2+ in buffer water/acetonitrile (80:20, v/v; pH 7.4), the compound underwent a significant enhancement in its fluorescence intensity. Importantly, 6 displayed high sensitivity and selectivity for Cu2+ over other tested alkali, alkaline-earth metals, and transition metal ions. The sensing of Cu2+ with 6 worked best within the linear range of 1.0 × 10−7–100.0 × 10−7 mol L−1 (R2 = 0.9983). The calculated detection limit was down to the level of 5.2 × 10−8 mol L−1.
Yu’s group constructed a simple ‘off–on’ fluorescent chemosensor 7 that bears the naphthalimide group (Yu et al. 2014b) with a detection limit and an association constant of 0.025 and 3.0 × 10−4 μM, respectively. 7 was effective for the detection of Cu2+ ion in ethanol–water solution (3:2, v/v, 50 mm HEPES, pH 7.4), amidst other tested metal ions and anions. 7 (Fig. 6) showed large fluorescence enhancement with Cu2+ ion, with linearity in the 0.05–1.5 μM range (R = 0.999). Following these results, the practicality of the chemosensor for real-time monitoring of Cu2+ was demonstrated by its potency to track Cu2+ ion in real water samples.
Chen et al. concerted their efforts to develop the naphthalimide derivative 8 that serves as a fluorescent chemosensor for Cu2+ detection (Chen et al. 2013b). In the presence of Cu2+ in acetonitrile–water (70:30, v/v) buffer solution of 3-(N-morpholino) propane sulfonic acid (MOPS, 10 mM, pH = 7.0), the fluorescence intensity of 8 was escalated (Fig. 7) in the order of a 4.5-fold enhancement. The linear detection range of 8 with Cu2+ lied between 4 μM to 7 μM with a detection limit of 0.15 μM calculated.
Lan and his team members intelligently designed two structurally similar fluorescent ‘turn-on’ naphthalimide-appended chemosensors for quantitative detection of Cu2+ among other metal ions, viz. K+, Ag+, Ca2+, Mg2+, Zn2+, Pb2+, Ni2+, Mn2+, Co2+, Cd2+, Hg2+, Fe2+, Fe3+ and Cr3+ (Fig. 7) (Lan et al. 2012). Upon Cu2+ chelation in acetonitrile solution, the fluorescence intensities of chemosensors 9 and 10 experienced tremendous uplift. The two-step binding mode of 9 with Cu2+ ion established the stoichiometric ratio of the chemosensor and analyte ion to be 1:1 and 1:2, which was further supported by ESI–MS results. The stability constants of 9 and 10 were obtained as 4.35 × 105 and 8.13 × 104, respectively. Desirably, Cu2+ monitoring by 9 and 10 was demonstrated to be reversible.
Georgiev and his lab members reported the design and synthesis of the blue-emitting, photostable, photoinduced electron transfer (PET) 1, 8-naphthalimide-based chemosensor 11 (Georgiev and Bojinov 2012). The group found that 11 (Fig. 8) switched between ‘‘off’’ and ‘‘on’’ states in the pH range of 9–6. So as to observe the discriminatory ability of the chemosensor, the team examined the fluorescence property of 11 in DMF solution using an array of various metal ions. A sharp fluorescence enhancement was observed upon Cu2+ addition (the fluorescence enhancement was of the order of 18.6) in the presence of metal ions (Cu2+, Pb2+, Cd2+, Ni2+, Co2+, Fe3+ and Zn2+) and protons. Other co-existing metal ions and proton induced no notable change on the fluorescence intensity of 11.
Yu et al. reported the novel ‘off–on’ type fluorescent chemosensor 12 that anchors both naphthalimide and rhodamine B units, as an effective chemosensor capable of distinguishing Cu2+ ion in an assembly of other cations (Yu et al. 2011). In ethanol–water (1:9, v: v, 50 mM HEPES, pH 7.0) solution, there was an increase in the fluorescence intensity of 12 upon treatment with 10 equiv. of Cu2+. The calculated detection limit was suitably low down to the level of 0.18 μM. Study of the binding ratio of 12 and Cu2+ revealed a 1:1 stoichiometry mode (Fig. 8). The compound was reversible in its sensing nature and was able to visualise Cu2+ in living cells in biological systems.
Xu et al. in 2010 reported the fluorescent chemosensor 13 that bears a naphthalimide unit connected to a piperazine ring. The reported compound could sensitively and selectively detect Cu2+ amongst other investigated cations (Fig. 9) (Xu et al. 2010d). Free 13 displayed a dynamic excimer emission in polar solvents, which results from the naphthalimide dimer formed in the excited state. Meanwhile, complex 13-Cu2+ exhibited static excimer emission which arises from naphthalimide dimer in the ground state. Job’s plot furnished a 1:2 complexation ratio of 13 and Cu2+ in 13-Cu2+. A dramatic increase in the fluorescence intensity of 13 towards Cu2+ in aqueous solutions (CH3CN:HEPES = 1:1, v/v) was observed, but not in the case of other metal ions. The calculated dissociation constant (Kd) of 13 with Cu2+ was 3.4 × 10−4 M.
Xu et al. in 2010 again developed two 4, 5-disubstituted-1, 8-naphthalimide derivatives, 14 and 15, which shows a good response for Cu2+ monitoring (Fig. 9) (Xu et al. 2010b). Compound 14 behaved as a fluorescent chemosensor while compound 15 acts as a colourimetric chemosensor. A significant enhancement in the fluorescence intensity of 14 at 478 nm in 100% aqueous solution took place upon the addition of Cu2+, well distinct from that of the fluorescent emission of 14 centred at 534 nm. The results that proceeded from the spectroscopic investigations showed that compound 15 could sense Cu2+ ion through massive quenching of its fluorescence intensity and concomitant colour change from primrose yellow to pink.
Hg+ ion-selective chemosensors
The analytical capacity of the 1, 8-naphthalimide-based compound 16 as a fluorescent chemosensor for Hg2+ monitoring was appraised by La’s group. The group further demonstrated that the compound also possessed colourimetric sensing properties towards CN− and F−, acting as both cationic- and anionic-specific multianalyte chemosensor (Fig. 10) (La et al. 2016). Results revealed that the compound experienced an amplification of its fluorescence signal intensity upon the addition of Hg2+ while other investigated anions and cations left only rather benign changes in the fluorescence signal intensity of the chemosensor. There was linearity of response of the chemosensor in its monitoring of Hg2+, with the detection limit and association constant determined to be 2.4 × 10−7 and 4.12 × 105 M, respectively. Job’s plot analysis gave a binding ratio of 1:1, which was further evidenced by 1H NMR results.
Li’s research group devised two naphthalimide-appended fluorescent chemosensors, 17 and 18 (Fig. 10) for Hg2+ detection (Li et al. 2016a). Compounds 17 and 18 were capable of Hg2+ ion detection over a wide pH span of 7.0–10.0. In 10 μM solution of 17 in phosphate buffer (pH 7.5) containing various metal ions, only Hg2+ could suppress the fluorescence intensity of 17 by about 90%; meanwhile, other competitive cations collectively impressed only mild effects on the fluorescence intensity of 17. Compound 18 showed a similar observation as with compound 17. The linear range of detection of Hg2+ by 17 was between 2 and 10 μM, and the detection limits of 17 and 18 for Hg2+ tracking were 2.1 and 3.1 μM, respectively.
Vonlanthen and his team members explored the Hg2+-sensing properties of the PET naphthalimide-based chemosensor 19 (Vonlanthen et al. 2014). No fluorescence enlargement was observed at pH 5.5 or lower, but there was noticed significant fluorescence enhancement towards Hg2+ in 9:1 H2O/CH3OH. Job’s plot established a 1:1 stoichiometric ratio between chemosensor 19 and Hg2+ ion (Fig. 11). It is only fair to note that the compound was successfully applied for Hg2+ imaging in living mammalian cells.
Un and co-workers brought into being a simple but effective fluorescent chemosensor that utilises the 1, 8-naphthalimide unit for a ‘turn on’ detection of Hg2+ ion (Fig. 12) (Un et al. 2014a). Titration of aqueous solution (THF–H2O, 1:1, pH 7.4, 10 mM Tris–HCl) of this compound in co-existence with several other metal ions induced a notable enhancement of fluorescence towards Hg2+ only. The fluorescence detection was linear within the range of 1–30 μM. The corresponding detection limit and association constant were estimated as 6.28 × 10−8 M and 5.4 × 104 M−1, respectively. From real-world application standpoint, the fluorescence imaging of Hg2+ in living cells by 20 was successfully demonstrated.
Moon and co-workers introduced a thionaphthalimide-based chemosensor 21 and its two monothio derivatives, 22 and 23 (Fig. 12), responsive for Hg2+ monitoring via an ‘off–on’ modality (Moon et al. 2013). Upon the addition of Hg2+ to chemosensor 21 in 30% aqueous CH3CN solutions, there was enlargement of the fluorescence intensity of 21 at 537 nm. Meanwhile, other investigated background metal ions left no tremendous effect on the fluorescence intensities of chemosensors 22 and 23. The estimated detection limit of the reported 21 for the sensing of Hg2+ ions was 2.7 μM.
The laboratory of Zhang et al. reported a new fluorescent molecule 24 (Fig. 13), for the recognition of Hg2+ among several other metal ions, including Na+, K+, Ca2+, Mg2+, Cu2+, Zn2+, Cr2+, Pb2+, Ni2+, Fe2+, Mn2+, Co2+, and Cd2+ (Zhang et al. 2013a). Successive addition of Hg2+ in EtOH/H2O (1/2, v/v) to solution of the chemosensor led to sharp increment in the fluorescence intensity of 24 while no obvious change was observed for other tested cations. Their findings indicated the existence of a 1:1 binding stoichiometry between Hg2+ and 24 in complex 24-Hg2+ (from Job’s plot analysis).
Li et al. made a report of the PET naphthalimide-based chemosensor 25 that anchors a hydrophilic hexanoic acid group, for the recognition of Hg2+ (Fig. 13) (Li et al. 2012a). Experimental data showed that the compound was most suitable for Hg2+ tracking within a linear range of 2.57 × 10−7–9.27 × 10−5 M. Job’s plot deciphered a 1:1 binding mode of chemosensor 25 and Hg2+ with a detection limit of 4.93 × 10−8 M estimated. In Tris–HNO3 buffer solution of pH 7.0, the chemosensor displayed great enhancement in its fluorescence intensity upon Hg2+ addition in coexistence with other metal ions. The response time of Hg2+ detection by 25 was less than 1 min. 25 was reversible in its sensing mechanism. Noteworthy is that the chemosensor was successfully applied for Hg2+ determination in hair samples.
Yang et al. described a new PET fluorescent chemosensor that incorporates the naphthalimide structure as fluorophore unit (Fig. 13) (Yang et al. 2012). Compound 26 retained excellent affinity for Hg2+ in the presence of a family of environmentally and biologically significant metal ions. In methanol–water (1:9, v/v) solution, there was enlargement of the fluorescence intensity of 26 (about fourfold increment) upon the gradual addition of Hg2+. The response of chemosensor 26 towards Hg2+ was linear within the concentration range of 0–10 μM. The detection limit and association constant were estimated as 63 nM and 1.11 × 105 M−1, respectively. Of note is that the chemosensor can reversibly respond to Hg2+ detection.
Li and his teammates prepared the ‘turn-on’ fluorescent chemosensor 27 effective for Hg2+ tracking amidst a host of other cations (Li et al. 2012b). The addition of 1.0 equivalent of Hg2+ to THF solution of 27 led to a 110-fold increment of the fluorescence intensity, in contrast to other examined metal ions that did not modulate the fluorescence intensity of 27. The compound functioned best for Hg2+ monitoring within the pH range of 5.0–9.0. The fluorescence titrations of 27 with Hg2+ were further conducted under optimised conditions (acetone/water = 1/1, v/v, pH = 7.0), with a 100-fold fluorescence enhancement observed. Further experiments established that chemosensor 27 (Fig. 14) was outstanding for imaging HL cells by a confocal laser scanning microscopy.
Liu and co-workers developed a new chemosensor 28, which contains both rhodamine B and naphthalimide units (Liu et al. 2012). The compound detected Hg2+ in a wide pH range of 5.7–11.0. Upon the addition of 1 equiv. of Hg2+, weak fluorescence emission was observed at 585 nm in 2:1 (v/v) MeOH/water solution (10 mM Tris–HCl, pH 7.0). The compound (Fig. 14) worked optimally within the linear range of 2–10 mM. Job’s plot analysis yielded a maximum at 0.5 mol fraction, implying the formation of a 1:1 complex of 28 with Hg2+.
Kumar et al. obtained the 1, 8-naphthalimide-appended fluorescent chemosensor 29 (Fig. 14) (Kumar et al. 2011) whose binding behaviour and fluorescence response were studied towards different metal ions in mixed aqueous media (THF/H2O; 9.5:0.5). Results showed that the addition of the investigated cations (except for Hg2+) did not give rise to any significant change in the fluorescence intensity of 29. This clearly demonstrates that the compound has excellent affinity for Hg2+ over these ions. The detection limit of 29 for Hg2+ was calculated to be 2 × 10−6 mol L−1. Job’s plot method of continuous variation gave a stoichiometric ratio of 1:1. The binding behaviour of Hg2+ ion to chemosensor 29 was analytically reversible. The potential biological application of the chemosensor was assessed for Hg2+ monitoring ion in prostate cancer (PC3) cell lines.
Cr3+-selective chemosensors
Yu et al. generated the 1, 8-naphthalimide-based chemosensor 30 for singular and reversible detection of Cr3+ (Fig. 15) (Yu et al. 2016). Fluorescence titration experimental results revealed gradual addition of Cr3+ to solution of the chemosensor (1.0 × 10−5 M, water/ethanol = 6:4, v/v) in the presence of the other cations amplified the fluorescence intensity of 30. In contrast, the addition of other cations did not modulate any significant change in the fluorescence intensity of 30. Cr3+ sensing with compound 30 was desirably linear in the range 0–5.5 × 10−5. The calculated detection limit was found to be as low as 0.60 ppm. Above all, the ability of the chemosensor to monitor biological samples of HeLa cells was successfully demonstrated.
Xue et al. reported the use of the colourimetric, fluorescent 1, 8-naphthalimide-based chemosensor 31 that simultaneously bears rhodamine B and diarylethene units (Xue et al. 2015). The chemosensor upheld strict Cr3+ monitoring ability. Addition of various metal ions (10 equiv.) to the chemosensor in acetonitrile (2.0 × 10−5 mol L−1) solution led to insignificant modulation of the initially weak fluorescence intensity of 31 at 370 nm, except in the case of Cr3+. The mentioned compound 31 possessed vibrant regeneration property as evidenced by its reversibility nature in the detection of Cr3+, whereby free 31 was unbound from complex 31-Cr3+ (Fig. 15).
Wu and co-workers published their development of the PET fluorescent chemosensor 32 that anchors the naphthalimide architecture (Fig. 15) (Wu et al. 2014). The designated compound was highly sensitive and selective for Cr3+ tracking amidst other investigated metal ions in THF/H2O solution (85/15, v/v) through a fluorescence ‘turn on’ mode. A 1:1 binding mode between 32 and Cr3+ was furnished by MALDI-TOF–MS analysis. The fluorescence detection response of Cr3+ determination by 32 was desirably linear in the 20–120 μM range. Meanwhile, the detection limit and association constant were determined to be 0.20 μM and 2.4 × 104 M−1, respectively.
Fe3+ ion-selective chemosensors
In the attempt to construct chemosensors that could detect Fe3+ effectively, Li and co-workers designed the fluorescence enhancement chemosensor 33 (Fig. 16) that bears coumarin and naphthalimide (Li et al. 2014). In THF-H2O (v/v, 1:1) solution, the chemosensor exerted a high selectivity for Fe3+ over other investigated metal ions with a massive fluorescence intensity enlargement at 456 nm. Job’s plot gave the binding ratio of compound 33 and Fe3+ in the 33-Fe3+ complex as 1:1 (Fig. 16). The association constant and detection limit were calculated to be (2.589 ± 0.206) × 103 M−1 and 0.388 mM, respectively.
Chereddy and co-workers introduced the PET-operated naphthalimide-based fluorescent chemosensor 34 capable of singular detection of Fe3+ in coexistence with other cations (Fig. 17) (Chereddy et al. 2014). While the addition of Fe3+ amplified the fluorescence intensity of 34 in Tris–HCl–CH3CN solution (v/v, 1:1; 0.01 M Tris–HCl–CH3CN; pH 7.4), the addition of other rival cations impacted collective insignificant effect on the fluorescence intensity of 34. The binding constant was calculated as 1.04 × 105 M−1 while the detection limit was determined to be 3.0 × 10−8 M. Ultimately, the reported compound 34 was reversible in its Fe3+ detection mechanism.
Yang and teammates showed that the fluorescent chemosensor 35 that bears three 1, 8-naphthalimide units in its structure could rapidly monitor Fe3+ (Yang et al. 2013). The addition of various competitive metal ions to 35 in DMF/H2O (v/v, 4:1 and 2:3) did not affect the fluorescence intensity of 35 much, except for Fe3+ that impinged an escalation on the fluorescence intensity of the compound. Based on the fluorescence titration, the calculated detection limit and binding constant were 4.69 × 10−7 M and 2.406 × M−1, respectively. The result obtained from Job plot indicated a 1:1 binding of 35 and Fe3+ in the complex 35-Fe3+ (Fig. 17).
Xu’s research group generated the optode membrane kind of a naphthalimide derivative 36 having terminal double bond (Fig. 18) (Xu et al. 2013). The reported compound was highly sensitive and selective for Fe3+ sensing amongst other tested cations. The incremental addition of Fe3+ to 36 in 0.05 mol/L Tris/HCl (pH 6.02) lowered the fluorescence intensity of the chemosensor rapidly. Strikingly, 36 showed excellent sensing properties as validated by its wide linear tracking range of 1.0 × 10−5–1.0 × 10−3 M and low detection limit of 4.5 × 10−6 M. The experimental optimum working pH range was between 5.00 and 8.00. The developed compound was reversible in its sensing mechanism and above all, it was successful in Fe3+ monitoring in pharmaceutical preparation samples.
Staneva and his peers proved that the poly(propylene amine) dendrimer 37 (Fig. 19) that incorporates four 4-(N, N-dimethylaminoethyloxy)-1, 8-naphthalimide units, could be effective as a chemosensor for Fe3+ detection in acetonitrile solution (Staneva et al. 2012). It was reported that the compound, upon Fe3+ addition, exhibited large fluorescent amplification (of the order of 44.95). Meanwhile, its fluorescence intensity remained unchanged upon the addition of other metal ions under similar testing condition. Furthermore, good linearity of response of Fe3+ monitoring by 37 was observed within the concentration range of 2 × 10−7–4.10−6 mol L−1. Finally, the detection limit of 37 with Fe3+ was estimated as 2 × 10−7 mol L−1.
Zn2+ ion-selective chemosensors
The fluorescent chemosensor 38 that operates through a dual PET-ICT mechanism (Fig. 20) (Wei et al. 2015) was developed by Wei and co-workers in 2015. The initially weak fluorescence of this compound, positioned at the emission wavelength of 465 nm underwent amplification upon the incremental addition of Zn2+ in neutral aqueous solution (10 mM Tris–HCl buffer, pH 7.2, containing 1% CH3CN), while the addition of other rival ions left no significant change in the fluorescence of the compound. The linearity of detection of Zn2+ by the chemosensor was demonstrated to be in the range 0–120.0 µM. The calculated detection limit and association constant were 7.2 × 10−9 M and 6.27 × 104 M−1, respectively. Consequently, the developed compound was utilised to image Zn2+ in living HeLa cells.
Liu and his research group members developed the fluorescent chemosensor 39, which contains 4-amino-1, 8-naphthalimide as fluorophore and iminodiacetic acid as receptor (Fig. 20) (Liu et al. 2014). The gradual addition of Zn2+ to solution of 39 in 20 mM HEPES buffer (pH 7.4) caused an increment in the fluorescence emission intensity of the chemosensor (in the order of a 50-fold increase). Compound 39 was successfully applied to image Zn2+ in living cells. However, the addition of other metal ions imposed no monumental fluorescence change. Chemosensor 39, whose calculated dissociation constant reached the level of 2.4 × 10−5 M, was successfully applied to bioimage Zn2+ in living cells.
The 1, 8-naphthalimide derivative that functions as a turn-on fluorescent chemosensor 40 for Zn2+ detection was prepared by Zhao’s research group (Zhao et al. 2013). In aqueous medium (CH3CN/HEPES, v/v = 6:4, pH 7.4), the ligand interacted with Zn2+ with notable fluorescence increment of about 13-fold increase ensuing from the process, whereas other cations did not significantly alter the fluorescence of the chemosensor. The values of the detection limit and association constant of 40 towards Zn2+ are 1.03 × 106 M and 3.02 × 103 M−1, respectively. The reported compound (Fig. 20) was successfully applied to image Zn2+ in A549, BEAS-2B, CHO, HeLa, and HepG2 cells.
Hanaoka et al. fabricated the water-soluble, fluorescence ‘off–on’ 4-amino-1, 8-naphthalimide-based chemosensor 41 that utilises ICT mechanism in its detection mode of Zn2+ (Fig. 21) (Hanaoka et al. 2010). In HEPES buffer (100 mM, pH 7.4), the compound displayed high affinity for Zn2+ (in co-existence with other cations) with a significant signal amplification of 21.7-fold. The apparent dissociation constant of 41 for Zn2+ detection was estimated as 1.1 nM. Desirably, the chemosensor was effective for Zn2+ bioimaging in cultured HeLa cells in 10 μM HBSS buffer.
Xu and co-workers described the fluorescence ‘off–on’ PET chemosensor 42 whose structure comprises 1, 8-naphthalimide unit as fluorophore and di-2-picolylamine unit as receptor (Fig. 21) (Xu et al. 2010a). In CH3CN-HEPES (v/v, 1:9, HEPES 0.5 M, pH = 7.4), the chemosensor participated in fluorescence enhancement process upon contact with Zn2+ among other metal ions of interest. The remaining cations induced no dramatic effect on the fluorescence spectra of 42. A 1:1 binding mode of compound 42 with Zn2+ was given.
Tamanini et al. showed that variant compounds of the same parental block could behave as homogeneous and heterogeneous chemosensors for Zn2+ detection. The reported PET compounds 43 and 44 (Fig. 21) (Tamanini et al. 2010) are typical examples of such compounds. Compounds 43 and 44 exhibited fluorescence enhancement towards Zn2+; the latter compound displays twofold fluorescence property than the former. In H2O/CH3CN (7:3) buffer solution (50 mM HEPES buffer; pH 7), large fluorescence increment in the fluorescence signal of 44 (10 μM) was observed upon the addition of 1 equiv. of Zn2+ (a 12.7-fold amplification) amidst other divalent cations. The fluorescence of 44 was slightly suppressed by Cu2+ and Hg2+. The calculated dissociation constant of 44 was 107 M−1. Ligands 43 and 44 were employed for the fabrication of nanostructured zinc chemosensors via the sol–gel process.
Ag+ ion-selective chemosensors
The novel mono- and di-substituted N–n-butyl-1, 8-naphthalimide derivative 45, efficient as a fluorescent chemosensor for Ag+ tracking was reported by Fu et al. (Figure 22) (Fu et al. 2016). Results showed that the addition of foreign cations to 45 in ethanol–water solution (4:1, v/v, 10 mM HEPES buffer, pH = 7.06) did not result in any change in the fluorescence intensity of 45 except for Ag+ that lowered the chemosensor’s fluorescence intensity at the emission wavelength of 535 nm. Job’s plot analysis indicated the formation of a 2:1 complex between 45 and Ag+.
The lab of Zhou and co-workers made a breakthrough in the fabrication of the 4-amino-1, 8-naphthalimide-based compound 46, which anchors Schiff base and vanillin units (Zhou et al. 2012). In its detection mode, compound 46 acted as a fluorescent chemosensor with strict singularity for Ag+ over other various cations under the same experimental condition. The fluorescence intensity of 46 was dampened upon the incremental addition of Ag+ at 682 nm. The estimated detection limit was low, down to the level of 3.0 × 10−6 mol L−1. Job’s method of continuous variation gave the binding ratio of 46 and Ag+ in 46-Ag+ complex to be 2:1 (Fig. 22).
Xu and co-workers designed two structurally similar naphthalimide derivatives 47 and 48 (Fig. 22) as fluorescent chemosensors for Ag+ monitoring (Xu et al. 2010e). In aqueous solution (CH3CN: HEPES = 50:50, v/v; 0.5 M HEPES buffer at pH 7.4), compound 47 detects Ag+ effectively with an approximate 14-time enhancement of its fluorescence. Large association constant of 1.24 × 105 M−1 was determined for 47 and low detection limit of 1.0 × 10−8 M was calculated for 48. The reference compound 48, devoid of carbonyl group, did not strongly bind with Ag+ owing to that the carbonyl group between 1, 8-naphthalimide and [15]aneNO2S2 played an active role in the increment of the fluorescence intensity.
Pd2+-selective chemosensors
Liu and teammates developed the novel water-soluble fluorescent chemosensor 49 (Fig. 23) for Pd2+ monitoring in phosphate-buffered saline (PBS) solution (10 μM, pH 7.4), which operated within the linear range of 0–6 μM (R2 = 0.995) and with the low detection limit of 25 nM or 2.7 μg/L calculated (Liu et al. 2014). The incremental addition of 5.0 equiv. of 21 different metal ions to solution of compound 49 impressed a rather insignificant effect on the fluorescence intensity of 49. Meanwhile, only Pd2+ left a massive increment (sevenfold) in the fluorescence intensity of 49. The efficacy of the reported compound was delineated by its ability for the intracellular fluorescence imaging of Pd2+ in Hep G2 and HL60 living cells.
Wang et al. evaluated the sensing properties of the fluorescent chemosensor 50 towards Pd2+ (Fig. 23) (Wang et al. 2012). The optimal pH range for Pd2+ detection as revealed by experimental analysis was between 6 and 9. In phosphate-buffered saline (PBS) (10 mM, pH 7.4) solution, the designated compound only had affinity for Pd2+ amongst others metal ions (i.e. Ca2+, Mg2+, Zn2+, Cd2+, Ni2+, Li+, Mn2+, Cu2+, Na+, K+, Co2+, Ag+, Pb2+, and Hg2+) in coexistence under the same analytical condition. The fluorescence intensity of 50 was enlarged by Pd2+ in the order of 7.2-fold enlargement.
Jiang and his team members constructed the colourimetric, ratiometric fluorescent chemosensor 51 (Fig. 23) for Pd2+ sensing (Jiang et al. 2011). In 10 mM acetonitrile–water solution (CH3CN:H2O = 4:1, NaBH4-PPh3), there was a decrement in the fluorescence intensity upon treatment of compound 51 with Pd2+ (Fig. 23) in the presence of other tested metal ions. The detection of Pd2+ with 51 was linear within the concentration range of 0–1 μM. The detection limit of 51 for Pd2+ monitoring was calculated to be 6.1 nM. The practical utility of the synthesised compound was realised by its effective monitoring of Pd2+ concentrations in real-world pool and tap water samples.
Al3+-selective chemosensor
Wang et al. synthesised the 1, 8-naphthalimide derivative 52 as a PET fluorescent chemosensor for Al3+ detection (Fig. 24) (Wang et al. 2017). When the fluorescence sensing property of the compound was tested in HEPES (PH = 7.4)/DMF (v/v, 1:1) solution that contained several cations including Al3+, only Al3+ induced significant fluorescence enhancement on 52. It was portrayed by the fluorescence plot that the compound exhibited good linearity within the concentration range of 3–11 μM. The detection limit and association constant of 52 interaction with Al3+ were calculated to be 3.4 × 10−8 M and 1 × 104 M−1, respectively. Experimental results of Job’s plot and other titration analyses indicated the formation of a 1:1 stoichiometric complex between 52 and Al3+. Desirably, a reversible complexation mode was achieved between the designed compound and Al3+ and the compound was successfully employed for Al3+ tracking in real water samples.
Recently, Li’s research group reported the novel naphthalimide-appended chemosensor 53 whose fluorescence behaviour was taken advantage of to selectively and sensitively detect Al3+ amongst several other cations present under the same standard testing conditions (Li et al. 2017). In methanol (2.0 × 10−5 mol L−1) and at 590 nm emission wavelength, 20-fold fluorescence amplification was observed by 53 when Al3+ was added. Contrastingly, this observation was not exhibited by other investigated competitive cations. High binding constant of 2.55 × 105 mol−1 L of 53 (Fig. 24) towards Al3+ was obtained and low detection limit of 1.75 × 10−7 mol L−1 of 53 for Al3+ was estimated. What is more important, the reported compound enjoyed interesting application in the construction of logic circuit.
The effective 1, 8-naphthalimide-based chemosensor 54 that utilised both ICT and CHEF sensing mechanisms for Al3+ detection was reported by Kang and co-workers (Fig. 24) (Kang et al. 2016). The fluorescence titration experiment of the chemosensor for Al3+ monitoring in an array of other co-cations was conducted in CH3OH solvent system. No significant fluorescence modulation was observed for ions investigated, except for 31.4-fold fluorescence enhancement noticed in the case of Al3+. The competition experiment yielded the same trend of observation, demonstrating the remarkable ability of the chemosensor for singular sensing of Al3+ amongst other metal ions. The linear range of response was between 8 and 13 μM, with high association constant of 7.6 × 104 M−1 and low detection limit of 6.9 μM calculated. Compound 54 was reversible in its detection mechanism and was consequently applied to sequester Al3+ from other cations in actual environmental system of some water samples.
Au3+ ion-selective chemosensors
Li et al. in 2016 designed two structurally similar 1, 8-naphthalimide-based derivatives, 57 and 58 (Fig. 25), bearing 4-N, N-dimethyl unit, as fluorescent chemosensors for Au3+ monitoring (Li et al. 2016). The reported compounds were well selective for Au3+ detection in H2O–ethanol solution, displaying enhanced fluorescence responses towards Au3+ in the presence of 24 other metal ions tested under similar standard conditions. Compounds 57 and 58 exhibited 145-fold and 14-fold enhancements, respectively, in the magnitudes of their fluorescence intensities. The detection limit of 57 for Al3+ monitoring was 0.050 μM while that of 58 was one-third of that of 57. Results showed that chemosensor 57 exhibited better sensing properties than its counterpart, chemosensor 58, but only 58 could be employed for Au3+ imaging in living cells.
Ba2+ ion-selective chemosensors
Panchenko et al. explored Ba2+-sensing characteristics of the two naphthalimide derivatives 55 (that bears an N-phenyl-4-amino- unit) and 56 (which anchors an N-phenyl-4-acetamido- unit), both being appended with N-benzocrown ether fragment (Fig. 26) (Panchenko et al. 2010). In acetonitrile solution, gradual addition of Ba2+ solution to chemosensor 55 first led to initial fluorescence enlargement, then seconded by sudden reversal of the fluorescence intensity of 55 as the amount of Ba2+ was further increased. For 56, there was significant amplification of its fluorescence intensity upon the addition of Ba2+ without any reversal observed as in the case of 55.
Bi3+-selective chemosensor
Kavitha and co-workers recently reported the novel compound 59 designed as a PET chemosensor for Bi3+ monitoring (Ramasamy and Thambusamy 2017). The best working pH of 59 for tracking Bi3+ was within the range 5–9. There was massive upward shift in the fluorescence intensity of chemosensor 59 upon interaction with Bi3+ in aqueous medium (5 × 10−5 M) in the concurrent presence of other metal ions. From the analysis of Job’s plot, the binding stoichiometry of 59 with Bi3+ (Fig. 27) was established to be 1:1. The association constant and detection limit were obtained as 311 M−1 and 0.58 μg mL−1, respectively.
Multi-ion-selective chemosensors
Liu and teammates reported the novel naphthalimide-based ratiometric, fluorescent chemosensor 60 for the selective and sensitive detection of Fe3+ and Hg2+ (Fig. 28) (Liu and Qian 2017). The best working pH of compound 60 was confined to the narrow range of 2.92–4.5. When each of Fe3+ and Hg2+ cations was added to the chemosensor in acetonitrile/H2O (v/v, 7:3) solution, the fluorescence intensity of 60 experienced sharp increase at the expense of those of other metal ions. Job’s plot analysis yielded a 2:3 binding ratio of compound 60 with each of Fe3+ and Hg2+ ions. The calculated detection limits of 60 for Fe3+ and Hg2+ sensing are 2.72 × 10−8 and 9.08 × 10−8 M, respectively, while the calculated dissociation constants of the binding of 60 with Fe3+ and Hg2+ are 4.95 × 10−7 M3/2 and 6.68 × 10−8 M3/2, respectively. It was reported that compound 60 displayed excellent reversibility in its sensing of Fe3+ and Hg2+.
Georgiev’s research team reported the PET, FRET and ICT chemosensor 61 that bears 1, 8-naphthalimide fluorophore, which is sensitive and selective first for H+ detection, and second for Cu2+ and Hg2+ monitoring in water/acetonitrile (v/v, 4:1) (Fig. 28) (Georgiev et al. 2015). There was upward rise of the fluorescence intensity of 61 upon addition of Cu2+ to solution of the compound in coexistence with other metal ions. The fluorescence response of the chemosensor with Cu2+ fell within the linear range of 2–10 µM while the limit of detection was obtained to be 0.5 µM. The binding stoichiometry as provided by Job’s plot analysis was 1:1. The fluorescence emission of the compound was grossly reduced upon gradual addition of Hg2+ in the presence of other metal ions in water/acetonitrile (4:1, v/v), which was accrued to the ‘switching on’ of the fluorescence resonance energy transfer (FRET) process. The linear range of fluorescence response of 61 to Hg2+ was 2–20 µM while the detection limit was obtained to be 0.09 µM.
Janakipriya’s group synthesised the fluorescence ‘turn-on’ PET-induced naphthalimide-based chemosensor 62 for the sensing of three trivalent metal ions, specifically, Fe3+, Al3+ and Cr3+ (Fig. 28) (Janakipriya et al. 2016). Results of the Job’s plot analysis revealed that Fe3+, Al3+ and Cr3+ ions existed in a 1:1 binding ratio in complexes 62-Fe3+, 62-Al3+ and 62-Cr3+, respectively. The detection limits of 62 were estimated to be 3.5 × 10−7, 3.6 × 10−7 and 3.8 × 10−7 M, respectively, for Fe3+, Al3+ and Cr3+ ions. The association constants were calculated as 3.8 × 104, 3.5 × 104 and 2.0 × 104 M−1, respectively, for 62-Fe3+, 62-Al3+ and 62-Cr3+ complexes. In aqueous medium of H2O:CH3CN (9:1, v/v), addition of several other metal ions impacted no marked influence on the fluorescence emission intensity of 62, except for Fe3+, Al3+ and Cr3+ that induced significant fluorescence enhancement. The effectiveness of the compound was appraised in biological monitoring of the three metal ions, i.e. Fe3+, Al3+ and Cr3+ in human keratinocyte (HaCaT) cells within the pH range of 6.0–9.2.
Zhang et al. came up with the stable 1, 8-naphthalimide-thiourea conjugate 63 used first for colourimetric detection of Fe3+ and Pb2+ and second for fluorescent recognition of Hg2+ (Fig. 28) (Zhang et al. 2014b). The colourimetric detections of Fe3+ and Pb2+ by 63 were carried out in MeCN/H2O (99:1, v/v) while the fluorescent detection of Hg2+ was conducted in MeCN/H2O (v/v, 85:15). In each of the two solvent systems utilised, the chemosensor selectively and sensitively tracked Fe3+, Pb2+, and Hg2+ in cohabitation with other rival cations, yielding ‘turn on’ fluorescence effects in both cases. The linear ranges of colourimetric and fluorescence responses for Fe3+, Pb2+, and Hg2+ were 0–150, 0–80, and 0–90 µM, respectively. The calculated detection limits of the designed compound for Fe3+, Pb2+, and Hg2+ were 6.86 µM, 5.09 µM, and 82.1 nM, respectively, while the obtained association constants of interaction of the reported compound with Fe3+, Pb2+, and Hg2+ were 1.854 × 103, 4.961 × 103, and 6.33 × 103 M−1, respectively. The monitoring of these three ions, i.e. Fe3+, Pb2+, and Hg2+ was reversible as demonstrated by the freeing of the chemosensor upon the addition of EDTA solution to the metal complexes. Job’s plot analysis indicated a 1:1 binding ratio of 63 for the three cations. Ultimately, the chemosensor was demonstrated for its practical effectiveness for Hg2+ tracking in pond and tap water samples and intracellular Hg2+ imaging in living cells.
Chemosensors that exhibit simultaneous fluorescence ‘turn on’ response for one metal ion and fluorescence ‘turn off’ response for another allow for the possibility of multi-cation detection. Compound 64 developed by Huang et al. (Fig. 29) is a typical example of such chemosensors (Huang et al. 2014b). 64 displayed fluorescence enhancement towards Hg2+ but fluorescence quenching towards Cu2+ in aqueous solution (10 mM HEPES, pH 7.5) in the presence of other cations. The strength of binding between the documented compound and investigated ions was justified by their high association constants of 6.06 × 106 and 3.51 × 106 M−1, respectively. Job’s plot revealed the formation of 1:1 complexes between 64 and each of the two metal ions. The binding mode was based on PET and CHEF mechanisms.
Sharma and co-workers synthesised the naphthalimide derivative 65, commendable as chemosensor for Co2+ and Cu2+ detection (Fig. 29) (Sharma et al. 2012). In HEPES-buffered DMF/H2O (8:2, v/v) solution, only Co2+ and Cu2+ induced massive lowering of the fluorescence intensity of the reported compound in the presence of other cations. Job’s method of continuous variation corroborated a 1:1 binding ratio of 65 with Cu2+ and Co2+. The calculated association constant of 65-Co2+ and 65-Cu2+ complexes were obtained to be 1.69 (± 0.1) × 102 and 2.9 (± 0.1) × 102 M−1, respectively. It was shown that the reported compound 65 could be efficiently employed for detecting Cu2+ and Co2+ in solutions where they are present in a 1:2 ratio. The designated compound 65 proved applicable for the detection of Cu2+ and Co2+ in Osteosarcoma cells.
The two naphthalimide derivatives 66 and 67 were developed by Mahato et al. (Fig. 30) (Mahato et al. 2012). The compounds were selective and sensitive for monitoring Hg2+ or Cr3+ in the presence of several other competing metal ions. In CH3CN-1.0 mM aq. HEPES buffer (pH = 7.2; 1:1, v/v), only Hg2+ and Cr3+ induced marked increase in the fluorescence intensities of 66 and 67. This phenomenon was not observed in the case of other investigated ions. Reversible binding properties of 66 and 67 were successfully demonstrated for both Hg2+ and Cr3+. A binding stoichiometry of 1:1 was given by Job’s plot for the interaction of Hg2+ or Cr3+ with 66 or 67. The respective emission binding constants of the reported compound 66 for the detections of Hg2+ and Cr3+ ions are (3.07 ± 0.3) × 105 and (1.28 ± 0.08) × 105 M−1. Meanwhile, the emission binding constants determined for Hg2+ and Cr3+ by the reported compound 67 are (1.12 ± 0.01) × 105 and (1.09 ± 0.02) × 105 M−1, respectively. Furthermore, the calculated detection limits of 66 for Hg2+ and Cr3+ were 0.35 and 0.14 ppb, respectively. Only chemosensor 67, which worked optimally under physiological conditions, could be used for the imaging of Hg2+ and Cr3+ ions in living human epidermoid A431 cells.
Dong et al. brought to research limelight the 1, 8-naphthalimide-appended derivative that acted as an effective chemosensor for Hg2+ and Au3+ tracking in HEPES buffer (0.01 M; pH 7.4; 0.05% DMSO, v/v) (Fig. 30) (Dong et al. 2010). The fluorescence emission intensity of 68 plummeted upon the addition of Hg2+ to solution of compound 68. Contrastingly, the addition of other coexisting ions impinged no great influence on the fluorescence intensity of compound 68, which lend weight to the great selectivity of 68 for Hg2+. The detection limit of 68 for Hg2+ sensing was determined to be 0.05 µM (10 ppb). The reported compound was successfully envisaged as an excellent chemosensor for Au3+ monitoring amongst several other metal ions tested.
Although there is no room for detailed discussion, additional published works to the use of 1, 8-naphthalimide for the constructions of fluorescent and colourimetric chemosensors are cited (Saini et al. 2014; Zhang et al. 2017; Un et al. 2014b; Aderinto et al. 2016; Wu et al. 2013; Hou et al. 2011; Chen et al. 2012; Liu et al. 2012b; Duan et al. 2008; Zhang et al. 2010; Chinapang et al. 2015; Zhang et al. 2012; Chen et al. 2013a; Hu et al. 2014; Zhang et al. 2011a; Yu et al. 2012; Yu and Zhang 2014; Choi et al. 2013).
Conclusions
The successfulness of the robust fluorophore, 1, 8-naphthalimide, which exists often as 4-amino-1, 8-naphthalimide for the constructions of diverse fluorescent chemosensors of interesting applications in environmental- and biological systems, is intriguing. In this review, various representative 1, 8-naphthalimide-based fluorescent chemosensors for selected cations (i.e. Cu2+, Hg2+, Cr3+, Fe3+, Zn2+, Ag+, Pd2+, Al3+, Ba2+, Au3+, and Bi2+), and/or a combination of these metal ions, have been summarised. All reported chemosensors contained three essential parts: fluorophore, spacer, and receptor. Different mechanisms such as Photoinduced Electron Transfer (PET), Internal Charge Transfer (ICT), and Fluorescence Resonance Energy Transfer (FRET) were employed in the constructions of these naphthalimide derivatives, although details about these mechanisms have not been elucidated. For a summary of a few key parameters about chemosensors 1–68, readers are referred to Table 1.
While a significant great success has been achieved in the developments of fluorescent and colourimetric 1, 8-naphthalimide-based chemosensors of interesting sensing parameters and great environmental- and biological application significances, much work still remains to be done. If researchers in this field continue to exert more efforts, it is foreseeable that chemosensors of improved sensing properties and practical application values that would meet future demands of metal ion tracking would be generated, thereby revolutionising the field of sensing science.
References
Aderinto SO, Xu Y, Peng H, Wang F, Wu H, Fan X (2016) A highly selective fluorescent sensor for monitoring Cu2+ ion: synthesis, characterization and photophysical properties. J Fluoresc 27:79–87. https://doi.org/10.1007/s10895-016-1936-7
Anikin VF, Fedko NF (2006) Synthesis of ion-active naphthalimide derivatives. Russ J Org Chem 42:73–76. https://doi.org/10.1134/S107042800601012X
Bicker KL, Wiskur SL, Lavigne JJ (2011) Chemosensors: principles, strategies, and applications, 1st edn. Wiley, New York, pp 275–295
Bojinov V, Grabchev I (2003) Synthesis of new polymerizable 1,8-naphthalimide dyes containing a 2-hydroxyphenylbenzotriazole fragment. Dyes Pigm 59:277–283. https://doi.org/10.1016/S0143-7208(03)00113-X
Bojinov VB, Panova IP (2009) Novel 4-(2, 2, 6, 6-tetramethylpiperidin-4-ylamino)-1, 8- naphthalimide based yellow-green emitting fluorescence sensors for transition metal ions and protons. Dyes Pigm 80:61–66. https://doi.org/10.1016/j.dyepig.2008.05.007
Bojinov VB, Panova IP, Chovelon J-M (2008) Novel blue emitting tetra-and pentamethylpiperidin-4-yloxy-1, 8-naphthalimides as photoinduced electron transfer based sensors for transition metal ions and protons. Sens Actuators B: Chem 135:172–180. https://doi.org/10.1016/j.snb.2008.08.016
Bojinov VB, Georgiev NI, Bosch P (2009) Design and synthesis of highly photostable yellow-green emitting 1, 8-naphthalimides as fluorescent sensors for metal cations and protons. J Fluoresc 19:127–139. https://doi.org/10.1007/s10895-008-0394-2
Bricks JL, Kovalchuk A, Trieflinger C, Nofz M, Büschel M, Tolmachev AI, Daub J, Rurack K (2005) On the development of sensor molecules that display FeIII-amplified fluorescence. J Am Chem Soc 127:13522–13529. https://doi.org/10.1021/ja050652t
Bryan AJ, de Silva AP, de Silva SA, Rupasinghe RADD, Sandanayake KRAS (1989) Photo-induced electron transfer as a general design logic for fluorescent molecular sensors for cations. Biosensors 4:169–179. https://doi.org/10.1016/0265-928X(89)80018-5
Burdette SC, Walkup GK, Spingler B, Tsien RY, Lippard SJJ (2001) Fluorescent sensors for Zn2+ based on a fluorescein platform: synthesis, properties and intracellular distribution. J Am Chem Soc 123:7831–7841
Carter KP, Young AM, Palmer AE (2014) Fluorescent sensors for measuring metal ions in living systems. Chem Rev 114(8):4564–4601. https://doi.org/10.1021/cr400546e
Chen Z, Wang L, Zou G, Teng M, Yu J (2012) Highly selective fluorescence turn-on chemosensor based on naphthalimide derivatives for detection of trivalent chromium ions. Chin J Chem 30:2844–2848. https://doi.org/10.1002/cjoc.201201070
Chen S, Hou P, Foley JW, Song X (2013a) A colorimetric and ratiometric fluorescent probe for Cu2+ with a large red shift and its imaging in living cells. RSC Advances 3:5591–5596. https://doi.org/10.1039/C3RA23057K
Chen Z, Wang L, Zou G, Tang J, Cai X, Teng M, Chen L (2013b) Highly selective fluorescence turn-on chemosensor based on naphthalimide derivatives for detection of copper(II) ions. Spectrochim Acta Mol Biomol Spectrosc 105:57–61. https://doi.org/10.1016/j.saa.2012.12.005
Chen J, Su W, Wang E, Liu Y (2016) 1,8-Naphthalimide-based turn-on fluorescent chemosensor for Cu2+ and its application in bioimaging. J Lumin 180:301–305. https://doi.org/10.1016/j.jlumin.2016.08.040
Chereddy NR, Raju MVN, Nagaraju P, Krishnaswamy VR, Korrapati PS, Bangal PR, Rao VJ (2014) A naphthalimide based PET probe with Fe3+ selective detection ability: theoretical and experimental study. Analyst 139:6352–6356. https://doi.org/10.1039/C4AN01528B
Chinapang P, Ruangpornvisuti V, Sukwattanasinitt M, Rashatasakhon P (2015) Ferro derivative of 1, 8-naphthalimide as a new turn-on fluorescent sensor for Au(III) ion. Dyes Pigm 112:236–238. https://doi.org/10.1016/j.dyepig.2014.07.013
Choi JY, Kim G-H, Guo Z, Lee HY, Swamy KMK, Pai J, Shin S, Shin I, Yoon J (2013) Highly selective ratiometric fluorescent probe for Au3+ and its application to bioimaging. Biosens Bioelectron 49:438–441. https://doi.org/10.1016/j.bios.2013.05.033
Chovelon J-M, Grabchev I (2007) A novel fluorescent sensor for metal cations and protons based of bis-1,8-naphthalimide. Spectrochimica Acta A 67:87–91. https://doi.org/10.1016/j.saa.2006.06.037
Cosnard F, Wintgens V (1998) A new fluoroionophore derived from 4-amino-N-methyl-1, 8-naphthalimide. Tetrahedron Lett 39:2751–2754. https://doi.org/10.1016/S00404039(98)00302-5
de Silva AP, Gunaratne HON, Lynch PLM (1995a) Luminescence and charge transfer. Part 4. ‘On–off’ fluorescent PET (photoinduced electron transfer) sensors with pyridine receptors: 1, 3-diaryl-5-pyridyl-4,5-dihydropyrazoles. J Chem Soc Perkin Trans 2:685–690. https://doi.org/10.1039/P29950000685
de Silva AP, Gunaratne HQN, Habib-Jiwan J-L, McCoy CP, Rice TE, Soumillion J-P (1995b) New fluorescent model compounds for the study of photoinduced electron transfer: the influence of a molecular electric field in the excited state. Angew Chem Int Edit Engl 34:1728–1731. https://doi.org/10.1002/anie.199517281
de Silva AP, Gunaratne HQN, Gunnlaugsson T, Lynch PLM (1996) Molecular photoionic switches with an internal reference channel for fluorescent pH sensing applications. New J Chem 20:871–880
de Silva AP, Gunaratne HQN, Gunnlaugsson T, Huxley AJM, McCoy CP, Rademacher JT, Rice TE (1997a) Novel 4-(2,2,6,6-tetramethylpiperidin-4-ylamino)-1,8-naphthalimide based yellow-green emitting fluorescence sensors for transition metal ions and protons. Chem Rev 97:1515–1566. https://doi.org/10.1016/j.dyepig.2008.05.007
de Silva AP, Gunaratne HQN, McCoy CP (1997b) Molecular photoionic AND logic gates with bright fluorescence and “off − on” digital action. J Am Chem Soc 119:7891–7892. https://doi.org/10.1021/ja9712229
de Souza MM, Correa R, Filho VC, Grabchev I, Bojinov V (2002) 4-Nitro-1,8-naphthalimides exhibit antinociceptive properties. Pharmazie 57:430–431
Deng M, Gong D, Han SC, Zhu X, Iqbal A, Liu W, Qin W, Guo H (2017) BODIPY based phenylthiourea derivatives as highly selective MeHg+ and Hg2+ ions fluorescent chemodosimeter and its application to bioimaging. Sens Actuators B: Chem 243:195–202. https://doi.org/10.1016/j.snb.2016.11.13
Dey S, Sarkar S, Maity D, Roy P (2017) Rhodamine based chemosensor for trivalent cations: synthesis, spectral properties, secondary complex as sensor for arsenate and molecular logic gates. Sens Actuators B: Chem 246:518–534. https://doi.org/10.1016/j.snb.2017.02.094
Dong M, Wang Y-W, Peng Y (2010) Highly selective ratiometric fluorescent sensing for Hg2+ and Au3+, respectively, in aqueous media. Org Lett 12:5310–5313. https://doi.org/10.1021/ol1024585
Du J, Hu M, Fan J, Peng X (2012) Fluorescent chemodosimeters using “mild” chemical events for the detection of small anions and cations in biological and environmental media. Chem Soc Rev 41:4511–4535. https://doi.org/10.1039/C2CS00004K
Duan L, Xu Y, Qian X (2008) Highly sensitive and selective Pd2+ sensor of naphthalimide derivative based on complexation with alkynes and thio-heterocycle. Chem Commun 0:6339–6341. https://doi.org/10.1039/B815298E
Duke RM, Veale EB, Pfeffer FM, Kruger PE, Gunnlaugsson T (2010) Colorimetric and fluorescent anion sensors: an overview of recent developments in the use of 1,8- naphthalimide-based chemosensors. Chem Soc Rev 39:3936–3953. https://doi.org/10.1039/B910560N
Erdemir S, Kocyigit O (2017) A novel dye based on phenolphthalein-fluorescein as a fluorescent probe for the dual-channel detection of Hg2+ and Zn2+. Dyes Pigm 145:72–79. https://doi.org/10.1016/j.dyepig.2017.05.053
Fan J, Peng X, Wu Y, Lu E, Hou J, Zhang H, Zhang R, Fu X (2005) A new PET fluorescent sensor for Zn2+. J Lumin 114:125–130. https://doi.org/10.1016/j.jlumin.2004.12.008
Fu Y, Li P, Kang J-X, Liu X-Y, Li G-Y, Ye F (2016) A novel 1, 8-naphthalimide derivative as an efficient silver (I) fluorescent sensor. J Lumin 178:156–162. https://doi.org/10.1016/j.jlumin.2016.05.023
Fu ZH, Han X, Shao Y, Fang J, Zhang Z, Wang Y-W, Peng Y (2017a) Fluorescein-based chromogenic and ratiometric fluorescence probe for highly selective detection of cysteine and its application in bioimaging. Anal Chem 89:1937–1944. https://doi.org/10.1021/acs.analchem.6b04431
Fu Y, Fan C, Liu G, Pu S (2017b) A colorimetric and fluorescent sensor for Cu2+ and F− based on a diarylethene with a 1, 8-naphthalimide Schiff base unit. Sens Actuators B: Chem 239:295–303. https://doi.org/10.1016/j.snb.2016.08.020
Gao Y-G, Tang Q, Shi Y-D, Zhang Y, Lu Z-L (2016) 1, 8-Naphthalimide modified [12]aneN3 compounds as selective and sensitive probes for Cu2+ ions and ATP in aqueous solution and living cells. Talanta 152:438–446. https://doi.org/10.1016/j.talanta.2016.02.040
Georgiev NI, Bojinov VB (2012) Design, synthesis and sensor activity of a highly photostable blue emitting 1, 8-naphthalimide. J Lumin 132:2235–2241. https://doi.org/10.1016/j.jlumin.2012.04.023
Georgiev NI, Bojinov VB, Nikolov PS (2011) The design, synthesis and photophysical properties of two novel 1, 8-naphthalimide fluorescent pH sensors based on PET and ICT. Dyes Pigm 88:350–357. https://doi.org/10.1016/j.dyepig.2010.08.004
Georgiev NI, Dimitrova MD, Asiri AM, Alamry KA, Bojinov VB (2015) Synthesis, sensor activity and logic behaviour of a novel bichromophoric system based on rhodamine 6G and 1,8-naphthalimide. Dyes Pigm 115:172–180. https://doi.org/10.1016/j.dyepig.2015.01.001
Goncalves AC, Capelo JL, Lodeiro C, Santos AAD (2017) A seleno-pyrene selective probe for Hg2+ detection in either aqueous or aprotic systems. Sens Actuators B: Chem 239:311–318. https://doi.org/10.1016/j.snb.2016.08.014
Gonçalves AC, Martinez JLC, Lodeiro C, Santos AAD (2017) A selective emissive chromogenic and fluorogenic seleno-coumarin probe for Cu2+ detection in aprotic media. Photochem Photobiol Sci 16:1174–1181. https://doi.org/10.1039/C7PP00036G
Grabchev I, Chovelon JM (2003) Synthesis and functional properties of green fluorescent poly (methylmethacrylate) for use in liquid crystal systems. Polym Adv Technol 14:601–608. https://doi.org/10.1002/pat.376
Grabchev I, Chovelon J-M (2008) New blue fluorescent sensors for metal cations and protons based on 1, 8-naphthalimide. Dyes Pigm 77:1–6. https://doi.org/10.1016/j.dyepig.2007.02.012
Grabchev I, Meallier P, Konstantinova T, Popova M (1995) Synthesis of some unsaturated 1, 8-naphthalimide dyes. Dyes Pigm 28:41–46. https://doi.org/10.1016/0143-7208(94)00078-G
Grabchev I, Chovelon J-M, Qian X (2003) A copolymer of 4-N, N-dimethylaminoethylene-N-allyl-1,8-naphthalimide with methylmethacrylate as a selective fluorescent chemosensor in homogeneous systems for metal cations. J Photochem Photobiol A Chem 158:37–43. https://doi.org/10.1016/S1010-6030(03)00100-X
Grabchev I, Soumillion JP, Muls B, vanova G (2004) Poly (amidoamine) dendrimer peripherally modified with 4-N, N-dimethylaminoethyleneamino-1, 8-naphthalimide as a sensor of metal cations and protons. Photochem Photobiol Sci 3:1032–1037. https://doi.org/10.1039/B412384K
Gruzinskii VV, Kukhto AV, Shakkah GKh (1998) Spectra of lasing efficiency in lasers with solutions of complex organic compounds. J Appl Spectrosc 65:463–465
Gunnlaugsson T, Lee TC, Parkesh R (2003) A highly selective and sensitive fluorescent PET (photoinducedelectron transfer) chemosensor for Zn(II). Org Biomol Chem 1:3265–3267. https://doi.org/10.1039/B309569J
Gunnlaugsson T, Glynn M, Tocci GM, Kruger PE, Pfeffer FM (2006) Anion recognition and sensing in organic and aqueous media using luminescent and colorimetric sensors. Coord Chem Rev 250:3094–3117. https://doi.org/10.1016/j.ccr.2006.08.017
Gupta RC, Razi SS, Ali R, Diwivedi SK, Srivastava P, Singh P, Koch B, Mishra H, Misra A (2017) An efficient Hg2+ ensemble based on a triazole bridged anthracene and quinoline system for selective detection of cyanide through fluorescence turn-off–on response in solution and live cell. Sens Actuators B: Chem 251:729–738. https://doi.org/10.1016/j.snb.2017.04.096
Hanaoka K, Muramatsu Y, Urano Y, Terai T, Nagano T (2010) Design and synthesis of a highly sensitive off–on fluorescent chemosensor for zinc ions utilizing internal charge transfer. Chemistry 16:568–572. https://doi.org/10.1002/chem.200901591
He H, Mortellaro MA, Leiner MJP, Fraatz RJ, Tusa JK (2003) A fluorescent sensor with high selectivity and sensitivity for potassium in water. J Am Chem Soc 125:1468–1469. https://doi.org/10.1021/ja0284761
He G, Meng Q, Zhao X, He C, Zhou P, Duan C (2015) A new copper (II) selective fluorescence probe based on naphthalimide: synthesis, mechanism and application in living cells. Inorg Chem Commun 65:28–31. https://doi.org/10.1016/j.inoche.2015.10.022
Hou C, Urbanec AM, Cao H (2011) A rapid Hg2+ sensor based on aza-15-crown-5 ether functionalized 1, 8-naphthalimide. Tetrahedron Lett 52:4903–4905. https://doi.org/10.1016/j.tetlet.2011.07.056
Hou L, Feng J, Wang Y, Dong C, Shuang S, Wang Y (2017) Single fluorescein-based probe for selective colorimetric and fluorometric dual sensing of Al3+ and Cu2+. Sens Actuators B: Chem 247:451–460. https://doi.org/10.1016/j.snb.2017.03.027
Hu F, Zheng B, Wang D, Liu M, Du J, Xiao D (2014) A novel dual-switch fluorescent probe for Cr(III) ion based on PET–FRET processes. Analyst 139:3607–3613. https://doi.org/10.1039/C4AN00303A
Hu X-X, Zheng X-L, Fan X-X, Su Y-T, Zhan X-Q, Zheng H (2015) Semicarbazide-based naphthalimide as a highly selective and sensitive colorimetric and “turn-on” fluorescent chemodosimeter for Cu2+. Sens Actuators B: Chem 227:191–197. https://doi.org/10.1016/j.snb.2015.12.037
Huang C, Jia T, Tang M, Yin Q, Zhu W, Zhang C, Yang Y, Jia N, Xu Y, Qian X (2014a) Selective and ratiometric fluorescent trapping and quantification of protein vicinal dithiols and in situ dynamic tracing in living cells. J Am Chem Soc 136:14237–14244. https://doi.org/10.1021/ja5079656
Huang C-B, Li H-R, Luo Y, Xu L (2014b) A naphthalimide-based bifunctional fluorescent probe for the differential detection of Hg2+ and Cu2+ in aqueous solution. Dalton Trans 43:8102–8108. https://doi.org/10.1039/C4DT00014E
Huang K, Jiao X, Liu C, Wang Q, Qiu X, Zheng D, He S, Zhao L, Zeng X (2017) Highly selective and sensitive fluorescent probe for mercury ions based on a novel rhodol-coumarin hybrid dye. Dyes Pigm 142:437–446. https://doi.org/10.1016/j.dyepig.2017.04.005
Janakipriya S, Chereddy NR, Korrapati P, Thennarasu S, Mandal AB (2016) Selective interactions of trivalent cations Fe3+, Al3+ and Cr3+ turn on fluorescence in a naphthalimide based single molecular probe. Spectrochima Acta Mol Biomol Spectrosc. https://doi.org/10.1016/j.saa.2015.08.044
Jiang J, Jiang H, Liu W, Tang X, Zhou X, Liu W, Liu R (2011) A colorimetric and ratiometric fluorescent probe for palladium. Org Lett 13:4922–4925. https://doi.org/10.1021/ol202003j
Jiang C, Wang M, Wang Y, Tang X, Zhang Y, Zhang H, Ma L, Wang J (2017) Synthesis and evaluation of two novel rhodamine-based fluorescence probes for specific recognition of Fec+ ion. Tetrahedron Lett 58:2560–2565. https://doi.org/10.1016/j.tetlet.2017.05.052
Kang L, Xing Z-Y, Ma X-Y, Liu Y-T, Zhang Y (2016) A highly selective colorimetric and fluorescent turn-on chemosensor for Al3+ based on naphthalimide derivative. Spectrochima Acta Mol Biomol Spectrosc 167:59–65. https://doi.org/10.1016/j.saa.2016.05.030
Kim HN, Lee MH, Kim HJ, Kim JS, Yoon J (2008) A new trend in rhodamine-based chemosensors: application of spirolactam ring-opening to sensing ions. Chem Soc Rev 37:1465–1472. https://doi.org/10.1039/b802497a
Kim HN, Ren WX, Kim JS, Yoon J (2012) Fluorescent and colorimetric sensors for detection of lead, cadmium, and mercury ions. Chem Soc Rev 41:3210–3244. https://doi.org/10.1039/C1CS15245A
Kim KT, Yoon SA, Ahn J, Choi Y, Lee MH, Jung JH, Park J (2017) Synthesis of fluorescent naphthalimide-functionalized Fe3O4 nanoparticles and their application for the selective detection of Zn2+ present in contaminated soil. Sens Actuators B: Chem 243:1034–1041. https://doi.org/10.1016/j.snb.2016.11.131
Kumar M, Kumar N, Bhalla V, Singh H, Sharma PR, Kaur T (2011) Naphthalimide appended rhodamine derivative: through bond energy transfer for sensing of Hg2+ ions. Org Lett 13:1422–1425. https://doi.org/10.1021/ol2001073
La YK, Hong JA, Jeong YJ, Lee J (2016) A 1,8-naphthalimide-based chemosensor for dual-mode sensing: colorimetric and fluorometric detection of multiple analytes. RSC Advances 6:84098–84105. https://doi.org/10.1039/c6ra20100h
Lakowicz JR (2006) Principles of fluorescent spectroscopy, 3rd edn. Springer, New york
Lan H, Liu B, Lv G, Li Z, Yu X, Liu K, Cao X, Yang H, Yang S, Yi T (2012) Dual-channel fluorescence “turn on” probe for Cu2+. Sens Actuators B: Chem 173:811–816. https://doi.org/10.1016/j.snb.2012.07.102
Law KY (1993) Organic photoconductive materials: recent trends and developments. Chem Rev 93:449–486. https://doi.org/10.1021/cr00017a020
Lee MH, Yoon B, Kim JS, Sessler JL (2013) Naphthalimide trifluoroacetyl acetonate: a hydrazine-selective chemodosimetric sensor. Chem Sci 4:4121–4126. https://doi.org/10.1039/C3SC51813B
Lee MH, Jeon HM, Han JH, Park N, Kang C, Sesser JL, Kim JS (2014) Toward a chemical marker for inflammatory disease: a fluorescent probe for membrane-localized thioredoxin. J Am Chem Soc 136(23):8430–8437. https://doi.org/10.1021/ja503356q
Lee MH, Kim JS, Sessler JL (2015) Small molecule-based ratiometric fluorescence probes for cations, anions, and biomolecules. Chem Soc Rev 44:4185–4191. https://doi.org/10.1039/C4CS00280F
Li CY, Zhang XB, Qiao L, Zhao Y, He CM, Huan SY, Lu LM, Jian LX, Shen GL, Yu RQ (2009) Naphthalimide-porphyrin hybrid based ratiometric bioimaging probe for Hg2+: well-resolved emission spectra and unique specificity. Anal Chem 81:9993–10001. https://doi.org/10.1021/ac9018445
Li C-Y, Xu F, Li Y-F, Zhou K, Zhou Y (2012a) A fluorescent chemosensor for Hg2+ based on naphthalimide derivative by fluorescence enhancement in aqueous solution. Anal Chim Acta 717:122–126. https://doi.org/10.1016/j.aca.2011.12.018
Li Q, Peng M, Li H, Zhong C, Zhang L, Cheng X, Peng X, Wang Q, Qin J, Li Z (2012b) A new, “Turn-on” naphthalenedimide-based chemosensor for mercury ions with high selectivity: successful utilization of the mechanism of twisted intramolecular charge transfer, near-ir fluorescence, and cell images. Org Lett 14:2094–2097. https://doi.org/10.1021/ol300607m
Li Z, Zhou Y, Yin K, Yu Z, Li Y, Ren J (2014) A new fluorescence “turn-on” type chemosensor for Fe3+ based on naphthalimide and coumarin. Dyes Pigm 105:7–11. https://doi.org/10.1016/j.dyepig.2013.12.032
Li G, Gao G, Cheng J, Chen X, Zhao Y, Ye Y (2016a) Two new reversible naphthalimide-based fluorescent chemosensors for Hg2+. Luminescence 31:992–996. https://doi.org/10.1002/bio.3063
Li Y, Qiu Y, Zhang J, Zhu X, Zhu B, Liu X, Zhang X, Zhang H (2016b) Naphthalimide derived fluorescent probes with turn-on response for Au3+ and the application for biological visualization. Biosens Bioelectron 83:334–338. https://doi.org/10.1016/j.bios.2016.04.034
Li L, Li H, Liu G, Pu S (2017) A novel fluorescent sensor for Al3+ based on a new diarylethene with a naphthalimide unit. J Photochem Photobiol A 338:192–200. https://doi.org/10.1016/j.jphotochem.2017.02.011
Lippert AR, New EJ, Chang CJ (2011) Reaction-based fluorescent probes for selective imaging of hydrogen sulfide in living cells. J Am Chem Soc 133:10078–10080. https://doi.org/10.1021/ja203661j
Liu J, Qian Y (2017) A novel pyridylvinyl naphthalimide-rhodamine dye: synthesis, naked-eye visible and ratiometric chemodosimeter for Hg2+/Fe3+. J Lumin 187:33–39. https://doi.org/10.1016/j.jlumin.2017.02.058
Liu B, Tian H (2005) A selective fluorescent ratiometric chemodosimeter for mercury ion. Chem Commun 0:3156–3158. https://doi.org/10.1039/B501913C
Liu J, Tu G, Zhou Q, Cheng Y, Geng Y, Wang L, Ma D, Jing X, Wang F (2006) Highly efficient green light emitting polyfluorene incorporated with 4-diphenylamino-1, 8-naphthalimide as green dopant. J Mater Chem 16:1431–1438. https://doi.org/10.1039/b514359d
Liu Y, Lv X, Zhao Y, Chen M, Liu J, Wang P, Guo W (2012a) A naphthalimide–rhodamine ratiometric fluorescent probe for Hg2+ based on fluorescence resonance energy transfer. Dyes Pigm 92:909–915. https://doi.org/10.1016/j.dyepig.2011.07.020
Liu Z, Zhang C, Wang X, He W, Guo Z (2012b) Design and synthesis of a ratiometric fluorescent chemosensor for Cu(II) with a fluorophore hybridization approach. Org Lett 14:4378–4381. https://doi.org/10.1021/ol301849z
Liu D-Y, Qi J, Liu X-Y, He H-R, Chen J-T, Yang G-M (2014) 4-Amino-1,8- naphthalimide-based fluorescent sensor with high selectivity and sensitivity for Zn2+ imaging in living cells. Inorg Chem Commun 43:173–178. https://doi.org/10.1016/j.inoche.2014.02.035
Liu W, Jiang J, Chen C, Tang X, Shi J, Zhang P, Zhang K, Li Z, Dou W, Yang L, Liu W (2014) Water-soluble colorimetric and ratiometric fluorescent probe for selective imaging of palladium species in living cells. Inorg Chem 53:12590–12594. https://doi.org/10.1021/ic502223n
Liu F, Tang P, Ding R, Liao L, Wang L, Wang M, Wang J (2017a) A glycosylation strategy to develop a low toxic naphthalimide fluorescent probe for the detection of Fe3+ in aqueous medium. Dalton Trans 46:7515–7522. https://doi.org/10.1039/c7dt01099k
Liu X, Niu LY, Chen YZ, Yang Y, Yang QZ (2017b) A multi-emissive fluorescent probe for the discrimination of glutathione and cysteine. Biosens Bioelectron 90:403–409. https://doi.org/10.1016/j.bios.2016.06.076
Lodeiro C, Pina F (2009) Luminescent and chromogenic molecular probes based on polyamines and related compounds. Coord Chem Rev 253:1353–1383. https://doi.org/10.1016/j.ccr.2008.09.008
Lu C, Xu Z, Cui J, Zhang R, Qian X (2007) Ratiometric and highly selective fluorescent sensor for cadmium under physiological pH range: a new strategy to discriminate cadmium from zinc. J Org Chem 72:3554–3557. https://doi.org/10.1021/jo070033y
Mahato P, Saha S, Suresh E, Liddo RD, Parnigotto PP, Conconi MT, Kesharwani MK, Ganguly B, Das A (2012) Ratiometric detection of Cr3+ and Hg2+ by a naphthalimide-rhodamine based fluorescent probe. Inorg Chem 51:1769–1777. https://doi.org/10.1021/ic202073q
Marinova NV, Georgiev NI, Bojinov VB (2011) Design, synthesis and pH sensing properties of novel 1,8-naphtalimide-based bichromophoric system. J Photochem Photobiol A 222:132–140. https://doi.org/10.1016/j.jphotochem.2011.05.012
Mason WT (1999) Fluorescent and luminescent chemosensors for biological activity, 2nd edn. Academic Press, Cambridge
Mironenko AY, Tutov MV, Sergeev AA, Voznesenskiy, Bratskaya SY (2017) On/off rhodamine based fluorescent probe for detection of Au and Pd in aqueous solutions. Sens Actuators B: Chem 246:389–394. https://doi.org/10.1016/j.snb.2017.02.092
Moon JO, Choi MG, Sun T, Choe J-I, Chang S-K (2013) Synthesis of thionaphthalimides and their dual Hg2+-selective signaling by desulfurization of thioimides. Dyes Pigm 96:170–175. https://doi.org/10.1016/j.dyepig.2012.08.007
Mu H, Gong R, Ma Q, Sun Y, Fu E (2007) A novel colorimetric and fluorescent chemosensor: synthesis and selective detection for Cu2+ and Hg2+. Tetrahedron Lett 48:5525–5529. https://doi.org/10.1016/j.tetlet.2007.05.155
Nandhikonda P, Begaye MP, Heagy MD (2009) Highly water-soluble, OFF–ON, dual fluorescent probes for sodium and potassium ions. Tetrahedron Lett 50:2459–2461. https://doi.org/10.1016/j.tetlet.2009.02.197
Ott I, Xu Y, Liu J, Kokoschka M, Harlos M, Sheldrick W, Qian X (2008) Sulfur-substituted naphthalimides as photoactivatable anticancer agents: DNA interaction, fluorescence imaging, and phototoxic effects in cultured tumor cells. Bioorg Med Chem 16:7107–7116. https://doi.org/10.1016/j.bmc.2008.06.052
Panchenko PA, Fedorov YV, Perevalov VP, Jonusauskas G, Fedorova OA (2010) Cation-dependent fluorescent properties of naphthalimide derivatives with N-benzocrown ether fragment. J Phys Chem A 114:4118–4122. https://doi.org/10.1021/jp9103728
Papalia T, Barattucci A, Barreca D, Bellocco E, Bonaccorsi P, Minuti L, Nicolo MS, Temperini A, Foti C (2017) Sequestering ability to Cu2+ of a new bodipy-based dye and its behavior as in vitro fluorescent sensor. J Inorg Biochem 167:116–123. https://doi.org/10.1016/j.jinorgbio.2016.11.030
Parkesh R, Lee TC, Gunnlaugsson T (2007) Highly selective 4-amino-1, 8-naphthalimide based fluorescent photoinduced electron transfer (PET) chemosensors for Zn(II) under physiological pH conditions. Org Biomol Chem 5:310–317. https://doi.org/10.1039/B614529A
Parkesh R, Veale EB, Gunnlaugsson T (2011) Fluorescent Detection Principles and Strategies. In: Wang B, Anslyn EV (eds) Chemosensors principles, strategies, and applications, Chap 12. Wiley, Hoboken, New Jersey, pp 229–252
Que EL, Domaille DW, Chang CJ (2008) Metals in neurobiology: probing their chemistry and biology with molecular imaging. Chem Rev 108:1517–1549. https://doi.org/10.1021/cr078203u
Ramasamy K, Thambusamy S (2017) Dual emission and pH based naphthalimide derivative fluorescent sensor for the detection of Bi3+. Sens Actuators B: Chem 247:632–640. https://doi.org/10.1016/j.snb.2017.03.043
Rurack K, Resch-Genger U, Bricks JL, Spieles M (2000) Cation-triggered ‘switching on’ of the red/near infra-red (NIR) fluorescence of rigid fluorophore–spacer–receptor ionophores. Chem Commun 0:2103–2104. https://doi.org/10.1039/B006430K
Saini A, Singh J, Kaur R, Singh N, Kaur N (2014) Naphthalimide-based organic nanoparticles for aluminium recognition in acidic soil and aqueous media. New J Chem 38:4580–4586. https://doi.org/10.1039/C4NJ00473F
Saura AV, Burguete MI, Galindo F, Luis SV (2017) Novel fluorescent anthracene–bodipy dyads displaying sensitivity to pH and turn-on behaviour towards Cu(II) ions. Org Biomol Chem 15:3013–3024. https://doi.org/10.1039/C7OB00274B
Shaki H, Gharanjiga K, Rouhani S, Khosravi A (2010) Synthesis and photophysical properties of some novel fluorescent dyes based on naphthalimide derivatives. J Photochem Photobiol, A 216:44–50. https://doi.org/10.1016/j.jphotochem.2010.09.004
Shao X, Kang R, Zhang Y, Huang Z, Peng F, Zhang J, Wang Y, Pan F, Zhang W, Zhao W (2015) Highly selective and sensitive 1-amino BODIPY-based red fluorescent probe for thiophenols with high off-to-on contrast ratio. Anal Chem 87:399–405. https://doi.org/10.1021/ac5028947
Sharma H, Kaur N, Singh N (2012) Imine linked 1, 8-naphthalimide: chromogenic recognition of metal ions, density function theory and cytotoxic activity. Inorg Chimica Acta 391:83–87. https://doi.org/10.1016/j.ica.2012.05.003
Staneva D, Grabchev I, Soumillion J-P, Bojinov V (2007) Pteridine-based fluorescent pH sensors designed for physiological applications. J Photochem Photobiol A: Chem 189:192–197. https://doi.org/10.1016/j.jphotochem.2012.08.002
Staneva D, Bosch P, Grabchev I (2012) Ultrasonic synthesis and spectral characterization of a new blue fluorescent dendrimer as highly selective chemosensor for Fe3+ cations. J Mol Struct 1015:1–5. https://doi.org/10.1016/j.molstruc.2012.02.010
Stewart WW (1981) Synthesis of 3, 6-disulfonated 4-aminonaphthalimides. J Am Chem Soc 103:7615–7620. https://doi.org/10.1021/ja00415a033
Sun S, Hu W, Gao H, Qi H, Ding L (2017) Luminescence of ferrocene-modified pyrene derivatives for turn-on sensing of Cu2+ and anions. Spectrochima Acta Mol Biomol Spectrosc 184:30–37. https://doi.org/10.1016/j.saa.2017.04.073
Tamanini E, Katewa A, Sedger LM, Todd MH, Watkinson M (2009) A synthetically simple, click-generated cyclam-based zinc (II) sensor. Inorg Chem 48:319–324. https://doi.org/10.1021/ic8017634
Tamanini E, Flavin K, Motevalli M, Piperno S, Gheber LA, Todd MH, Watkinson M (2010) Cyclam-based “clickates”: homogeneous and heterogeneous fluorescent sensors for Zn(II). Inorg Chem 49:3789–3800. https://doi.org/10.1021/ic901939x
Un H-I, Huang C-B, Huang C, Jia T, Zhao X-L, Wang C-H, Xu L, Yang H-B (2014a) A versatile fluorescent dye based on naphthalimide: highly selective detection of Hg2+ in aqueous solution and living cells and its aggregation-induced emission. Org Chemistry Frontiers 1:1083–1090. https://doi.org/10.1039/C4QO00185K
Un HI, Huang CB, Huang J, Huang C, Jia T, Xu L (2014b) A Naphthalimide-based fluorescence “Turn-On” probe for the detection of Pb2 + in aqueous solution and living cells. Chemistry 9:3397–3402. https://doi.org/10.1002/asia.201402946
Valeur B, Leray I (2001) PCT (Photoinduced Charge Transfer) fluorescent molecular sensors for cation recognition. In: Valeur B, Brochon JC (eds) New Trends in Fluorescence Spectroscopy, vol 1. Springer, Berlin, pp 187–207
Vonlanthen M, Connelly CM, Deiters A, Linden A, Finney NS (2014) Thiourea-based fluorescent chemosensors for aqueous metal ion detection and cellular imaging. J Org Chem 79:6054–6060. https://doi.org/10.1021/jo500710g
Wang J, Xiao Y, Zhang Z, Qian X, Yang Y, Xu Q (2005) A pH-resistant Zn (II) sensor derived from 4-aminonaphthalimide: design, synthesis and intracellular applications. J Mater Chem 15:2836–2839. https://doi.org/10.1039/B500766F
Wang C, Zheng X, Huang R, Yan S, Xie X, Tian T, Huang S, Weng X, Zhou X (2012) A 4-Amino-1, 8-naphthalimide derivative for selective fluorescent detection of palladium (ii) ions. Asian J Org Chem 1:259–263. https://doi.org/10.1002/ajoc.201200061
Wang F, Xu Y, Aderinto SO, Peng H, Zhang H, Wu H (2017) A new highly effective fluorescent probe for Al3+ ions and its application in practical samples. J Photochem Photobiol A 332:273–282. https://doi.org/10.1016/j.jphotochem.2016.09.004
Wasielewski MR (1992) Photoinduced electron transfer in supramolecular systems for artificial photosynthesis. Chem Rev 92(3):435–461. https://doi.org/10.1021/cr00011a005
Wei T, Wang J, Chen Y, Han Y (2015) Combining the PeT and ICT mechanisms into one chemosensor for the highly sensitive and selective detection of zinc. RSC Adv 5:57141–57146. https://doi.org/10.1039/C5RA11194C
Wu X-F, Ma Q-J, Wei X-J, Hou Y-M, Zhu X (2013) A selective fluorescent sensor for Hg2+ based on covalently immobilized naphthalimide derivative. Sens Actuators B: Chem 183:565–573. https://doi.org/10.1016/j.snb.2013.04.024
Wu S, Zhang K, Wang Y, Mao D, Liu X, Yu J, Wang L (2014) A novel Cr3+ turn-on probebased on naphthalimide and BINOL framework. Tetrahedron Lett 55:351–353. https://doi.org/10.1016/j.tetlet.2013.11.024
Xiao Y, Rowe AA, Plaxco KW (2007) Electrochemical detection of parts-per-billion lead via an electrode-bound DNAzyme assembly. J Am Chem Soc 129:262–263. https://doi.org/10.1021/ja067278x
Xu Z, Qian X, Cui J (2005) Colorimetric and ratiometric fluorescent chemosensor with a large red-shift in emission: Cu(II)-only sensing by deprotonation of secondary amines as receptor conjugated to naphthalimide fluorophore. Org Lett 7:3029–3032. https://doi.org/10.1021/ol051131d
Xu Z, Qian X, Cui J, Zhang R (2006) Exploiting the deprotonation mechanism for the design of ratiometric and colorimetric Zn2+ fluorescent chemosensor with a large red-shift in emission. Tetrahedron 62:10117–10122. https://doi.org/10.1016/j.tet.2006.08.050
Xu Z, Han SJ, Lee C, Yoon J, Spring DR (2010a) Development of off–on fluorescent probes for heavy and transition metal ions. Chem Commun 46:1679–1681. https://doi.org/10.1039/B924503K
Xu Z, Pan J, Spring DR, Cui J, Yoon J (2010b) Ratiometric fluorescent and colorimetric sensors for Cu2+ based on 4,5-disubstituted-1,8-naphthalimide and sensing cyanide via Cu2+ displacement approach. Tetrahedron 66:1678–1683. https://doi.org/10.1016/j.tet.2010.01.008
Xu Z, Yoon J, Spring DR (2010c) Fluorescent chemosensors for Zn2+. Chem Soc Rev 39:1996–2006. https://doi.org/10.1039/B916287A
Xu Z, Yoon J, Spring DR (2010d) A selective and ratiometric Cu2+ fluorescent probe based on naphthalimide excimer–monomer switching. Chem Commun 46:2563–2565. https://doi.org/10.1039/C000441C
Xu Z, Zheng S, Yoon J, Spring DR (2010e) Discovery of a highly selective turn-on fluorescent probe for Ag+. Analyst 135:2554–2559. https://doi.org/10.1039/C0AN00405G
Xu J-H, Hou Y-M, Ma Q-J, Wu X-F, Wei X-J (2013) A highly selective fluorescent sensor for Fe3+ based on covalently immobilized derivative of naphthalimide. Spectrochima Acta Mol Biomol Spectrosc 112:116–124. https://doi.org/10.1016/j.saa.2013.04.044
Xu Y, Aderinto SO, Wu H, Peng H, Zhang H, Zhang J, Fan X (2017) A highly selective fluorescent chemosensor based on naphthalimide and schiff base units for Cu2+ detection in aqueous medium. Z Naturforsch B Chemical Science 72:35–43. https://doi.org/10.1515/znb-2016-0138
Xue D, Zheng C, Fan C, Liu G, Pu S (2015) A colorimetric fluorescent sensor for Cr3+ based on a novel diarylethene with a naphthalimide-rhodamine B group. J Photochem Photobiol A Chem 303–304:59–66. https://doi.org/10.1016/j.jphotochem.2015.02.006
Yang R, Guo X, Wang W, Zhang Y, Jia L (2012) Highly selective and sensitive chemosensor for Hg2+ based on the naphthalimide fluorophore. J Fluoresc 22:1065–1071. https://doi.org/10.1007/s10895-012-1044-2
Yang L, Yang W, Xu D, Zhang Z, Liu A (2013) A highly selective and sensitive Fe3+ fluorescent sensor by assembling three 1, 8-naphthalimide fluorophores with a tris (aminoethylamine) ligand. Dyes Pigm 97:168–174. https://doi.org/10.1016/j.dyepig.2012.12.016
Yu C, Zhang J (2014) Copper (II)-Responsive “Off-On” chemosensor based on a naphthalimide derivative. Asian J Org Chem 3:1312–1316. https://doi.org/10.1002/ajoc.201402160
Yu C, Chen L, Zhang J, Li J, Liu P, Wang W, Yan B (2011) “Off-On” based fluorescent chemosensor for Cu2+ in aqueous media and living cells. Talanta 85:1627–1633. https://doi.org/10.1016/j.talanta.2011.06.057
Yu C, Zhang J, Ding M, Chen L (2012) Silver (I) ion detection in aqueous media based on “off-on” fluorescent probe. Anal Methods 4:342–344. https://doi.org/10.1039/C2AY05714J
Yu C, Wen Y, Qin X, Zhang J (2014) A fluorescent ratiometric Cu2+ probe based on FRET by naphthalimide-appended rhodamine derivatives. Anal Methods 6:9825–9830. https://doi.org/10.1039/C4AY01863J
Yu M, Du W, Zhou W, Liu C, Wei L, Zhang H (2016) A 1,8-naphthalimide-based chemosensor with an off-on fluorescence and lifetime imaging response for intracellular Cr3+ and further for S2−. Dyes Pigm 126:279–285. https://doi.org/10.1016/j.dyepig.2015.12.001
Zhang JF, Zhou Y, Yoon J, Kim Y, Kim SJ, Kim JS (2010) Naphthalimide modified rhodamine derivative: ratiometric and selective fluorescent sensor for Cu2+ based on two different approaches. Org Lett 12:3852–3855. https://doi.org/10.1021/ol101535s
Zhang JF, Kim S, Han JH, Lee S-J, Pradhan T, Cao QY, Lee SJ, Kang C, Kim SJ (2011a) Pyrophosphate-selective fluorescent chemosensor based on 1, 8-naphthalimide–DPA–Zn(II) complex and its application for cell imaging. Org Lett 13:5294–5297. https://doi.org/10.1021/ol202159x
Zhang JF, Zhou Y, Yoon J, Kim JS (2011b) Recent progress in fluorescent and colorimetric chemosensors for detection of precious metal ions (silver, gold and platinum ions). Chem Soc Rev 40:3416–3429. https://doi.org/10.1039/C1CS15028F
Zhang J, Yu C, Lu G, Fu Q, Li N, Ji Y (2012) A Ag+-selective “off–on” probe based on a naphthalimide derivative. New J Chem 36:819–822. https://doi.org/10.1039/C2NJ20974H
Zhang C, Liu Z, Li Y, He W, Gao X, Guo Z (2013a) In vitro and in vivo imaging application of a 1, 8-naphthalimide-derived Zn2+ fluorescent sensor with nuclear envelope penetrability. Chem Commun 49:11430–11432. https://doi.org/10.1039/c3cc46862c
Zhang Z, Chen Y, Xu D, Yang L, Liu A (2013b) A new 1, 8-naphthalimide-based colorimetric and “turn-on” fluorescent Hg2+ sensor. Spectrochima Acta Mol Biomol Spectrosc 105:8–13. https://doi.org/10.1016/j.saa.2012.11.113
Zhang X, Yin J, Yoon J (2014a) Recent advances in development of chiral fluorescent and colorimetric sensors. Chem Rev 114(9):4918–4959. https://doi.org/10.1021/cr400568b
Zhang Z, Lu S, Sha C, Xu D (2014b) A single thiourea-appended 1,8-naphthalimide chemosensor for three heavy metal ions: Fe3+, Pb2+, and Hg2+. Sens Actuators B: Chem 208:258–266. https://doi.org/10.1016/j.snb.2014.10.136
Zhang Y-M, Zhong K-P, Su J-X, Chen X-P, Yao H, Wei T-B, Lin Q (2017) A novel histidine-functionalized 1, 8-naphthalimide-based fluorescent chemosensor for the selective and sensitive detection of Hg2+ in water. New J Chem 41:3303–3307. https://doi.org/10.1039/C6NJ03930H
Zhao LY, Mi QL, Wang GK, Chen JH, Zhang JF, Zhao QH, Zhou Y (2013) 1, 8-Naphthalimide-based ‘turn-on’fluorescent sensor for the detection of zinc ion in aqueous media and its applications for bioimaging. Tetrahedron Lett 54:3353–3358. https://doi.org/10.1016/j.tetlet.2013.04.045
Zhou Y, Zhou H, Ma T, Zhang J, Niu J (2012) A new Schiff base based on vanillin and naphthalimide as a fluorescent probe for Ag+ in aqueous solution. Spectrochima Acta Mol Biomol Spectrosc 88:56–59. https://doi.org/10.1016/j.saa.2011.11.054
Zhu W, Hu M, Yao R, Tian H (2003) A novel family of twisted molecular luminescent materials containing carbazole unit for single-layer organic electroluminescent devices. J Photochem Photobiol A Chem 154:169–177. https://doi.org/10.1016/S1010-6030(02)00325-8
Acknowledgements
The work reported in this review would not have been a possibility without the scholarship supports benefitted from the China Scholarship Council (CSC Nos. 2014BSZ528 and 2017BSZ012726) which are not taken lightly.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Aderinto, S.O., Imhanria, S. Fluorescent and colourimetric 1, 8-naphthalimide-appended chemosensors for the tracking of metal ions: selected examples from the year 2010 to 2017. Chem. Pap. 72, 1823–1851 (2018). https://doi.org/10.1007/s11696-018-0411-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11696-018-0411-0