Abstract
Introduction
Bariatric surgical outcomes depend heavily on proper healing of gastrointestinal anatomy, metabolic alterations, and patient lifestyle modifications which are all negatively impacted by immunosuppression and underlying inflammatory diseases. There is a lack of literature exploring how patients with diseases requiring immunosuppression respond to bariatric surgical intervention in the long term.
Methods
A retrospective analysis of chronically immunosuppressed patients who underwent primary bariatric surgeries at Mayo Clinic was conducted (2008–2020). Data collected included patient demographics, BMI, underlying disease, and immunosuppression regimen and complications at 3, 6, 12, 24, and 60 months.
Results
We identified a total of 89 (RYGB = 49, SG = 34, BPD/DS = 6) patients on chronic immunosuppression who underwent bariatric surgery at our center. RYGB (N = 49), 38.2% had a SG (N = 34) and 6.7% had a BPD/DS (N = 6). Rheumatoid arthritis and renal transplantation were the most underlying condition at 20.22% each (N = 18). There were a total of 2 (2.25%) intraoperative complications. In the immediate post-operative period, there were 15 (16.5%) minor complications. In follow-up, 6.1% of RYGB patients experienced marginal ulcerations, while no gastrointestinal leaks occurred. The mean pre-surgical BMI was 48.29 kg/m2 (SD = 18.41). Percent total weight loss (%TWL) and BMI reduction were 30.89% and 14.83 kg/m2 (SD = 9.07) at 12 months and 29.48% and 14.43 kg/m2 (SD = 13.46) at 60 months, respectively. The mean follow-up time was 30.49 months.
Conclusions
Bariatric surgery remains safe and effective therapy for chronically immunosuppressed patients with excellent long-term outcomes for patients with moderate to severe obesity.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Bariatric surgery remains the most effective therapy to reduce weight, prevent cardiovascular mortality, and decrease weight-associated comorbidities in patients with moderate to severe obesity [1,2,3]. As rates of obesity continue to increase throughout the population, there is an equal rise in obesity in patients who have inflammatory conditions or organ transplants requiring long term immunosuppression [4].
While immunosuppressed patients with moderate to severe obesity benefit from bariatric surgical intervention [5], there is still great concern about increased operative complications and uncertain long-term efficacy. Immune suppression has been associated with higher rates of perioperative infections and poor wound healing that could theoretically cause more marginal ulceration and anastomotic or staple line leaks [6, 7]. The underlying inflammatory disease or transplant may limit the patient participating in post-surgical rehabilitation, minimize activity, and present metabolic derangements that may impact long-term weight loss.
Benefits of decreasing patient weight through bariatric surgery must be balanced against the potential for surgical complications in all patients. The risk–benefit ratio changes depending on what inflammatory or transplant requiring underlying disease and degree of immunosuppressive regimen. Glucocorticoids, cytostatics, biologic modifiers, and inflammatory mediator targeted monoclonal antibodies are the most common long-term regimens. Regimen type and degree is titrated to autoimmune disease control or prevention transplant associated rejection, with varying impacts on surgical risk. Patients on chronic steroids who underwent bariatric surgery show overall low complication rates, but higher rates of anastomotic or staple line leaks and readmissions [8, 9]. Finally, bariatric surgery leads to acute decreases in oral intake, gastrointestinal absorption changes, and rapid weight loss that can impact disease and immunosuppressant [10].
Bariatric surgical alteration of the gastrointestinal tract creates long-term physiologic and metabolic changes that lead to prolonged weight loss with eventual plateau at a significantly lower body mass index [11]. As underlying inflammatory diseases and immunomodifiers can interfere with healing, adaptions, and metabolism, the impact on physiological adaption to bariatric surgery is under investigation [12]. Prolonged corticosteroid and calcineurin inhibitor use is associated with significant metabolic derangements leading to weight gain, insulin resistance, and fat redistribution [13, 14]. Data is sparse but mounting on bariatric surgical impacts on chronic steroid users, a recent study demonstrating that diabetic patients requiring long-term systemic glucocorticoids were able to achieve remission post bariatric surgery[5]. Less is known about other immunosuppressive regimens.
Clinical decisions regarding the use of bariatric surgery in chronically immunosuppressed patients with obesity can be impacted by analysis of long-term outcomes. The increasing prevalence of biologic modifiers and monoclonal antibody therapy must be reflected to represent the modern cohort of patients. While there is substantial short-term data supporting the utilization of bariatric surgery in this patient population, the field is lacking evidence regarding long-term complications and weight loss efficacy [15, 16]. We hypothesize that bariatric surgery provides stable weight loss with minimal complications in patients requiring long-term immunosuppression.
Material and Methods
This is a retrospective review of prospectively maintained bariatric surgery database. Sample inclusion criteria required patients to have undergone primary Roux-en-Y gastric bypass, sleeve gastrectomy (SG), or duodenal switch between 2008 and 2020; these patients were screened to identify prolonged use of immunosuppression (corticosteroid, monoclonal antibody, or targeted biologic) for at least 3 months in the year before surgery with continued use post-operatively. A complete list of immunosuppressive medications included in the screen is included in Table 1.
Baseline demographic and clinical information was collected for each patient, including prior organ transplantation, pulmonary, rheumatologic, or endocrine disease requiring immunosuppression and the type of immunosuppressive medication(s) taken. Obesity-related comorbidities, which included type II diabetes, hypertension, obstructive sleep apnea, and hyperlipidemia, were recorded if present preoperative.
Patient weight, height and BMI were recorded at 0, 6, 12, 60 months postoperatively and used to calculate percent total weight loss at each time point (follow-up weight subtracted from operative weight, divided by operative weight, and multiplied by 100). The presence of obesity-related comorbidities was also queried at each follow-up time point and if the patient had one of comorbidities at the time of operation. A comorbidity was considered resolved if the resolution was stated in the chart and/or the patient was taken off all medication management for at least 1 year with normal lab or vital results at follow-up (HbA1c < 6.5% or fasting blood glucose below 126 mg/dL for diabetes resolution, systolic BP < 130 mmHG for hypertension, or a normal fasting lipid panel recorded for hyperlipidemia depending on available data).
Study outcomes collected included intraoperative complications and surgery associated major adverse events including anastomotic leak, bleed, venous thromboembolism, cardiac events, and minor complications such as surgical site infections, urinary tract infections, pneumonia, acute kidney injury, or other infections. Long-term complications were assessed for the entire 60-month post-operative period by screening for small bowel obstruction, anastomotic leak or stricture, marginal ulceration, cholecystitis, dumping syndrome, and internal herniations.
Statistical analysis was conducted using GraphPad Prism 9.1 with measurements expressed as mean ± standard deviation (SD); categorical variables were expressed with counts and percentages.
Results
A total of 89 patients on chronic immunosuppression received primary bariatric surgery at our center between 2008 and 2020. The mean age of patients at the time of surgery was 52.77 years (SD = 10.7), and 71.9% (N = 64) were female. Type of weight loss surgery is identified in Fig. 1a. Underlying disease requiring immunosuppression is listed in Fig. 1b. Prednisone, taken by 65.17% (N = 58) of patients in this cohort, was the most used immunosuppressive agent, followed by methotrexate in 20.22% (N = 18) with the rest of immunosuppression regimens in Table 1.
Characteristics of immunosuppressed population in the bariatric surgery cohort. A Percentage of population undergoing each operation and total number N. B Underlying disease requiring immunosuppression as a percent of the whole population. TXP, transplant; RYGB, Roux-en-Y gastric bypass; BPD/DS, biliopancreatic diversion and duodenal switch; SLE, systemic lupus erythematous
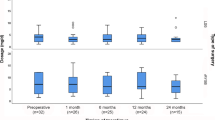
The mean pre-surgical BMI was 48.29 kg/m2 (SD = 18.41). Percent total weight loss (%TWL) and BMI reduction were 30.89% and 14.83 kg/m2 (SD = 9.07) at 12 months and 29.48% and 14.43 kg/m2 (SD = 13.46) at 60 months (Table 2, Fig. 2). The mean follow-up time was 30.49 months (SD = 24.19). Validation of the data set was conducted via subgroup analysis of patients with 60-month follow-up demonstrating similar BMI and percent total weight loss trends over the 5-year follow-up (Fig. 2D).
A Weight loss post-bariatric surgery for the entire immunosuppressed cohort, mean patient BMI (kg/m2) is plotted at time of operation and 6, 12, 24, and 60 months post-op, and percent total weight loss values are plotted starting at 6 months post-op and at 12, 24, and 60 months post-op. B Change in BMI (kg/m.2) stratified by operation type. C Percent total weight loss stratified by operation type. D Isolated weight loss outcomes in the 60-month follow-up subcohort (SG, sleeve gastrectomy; RNYGB, Roux-n-Y gastric bypass; BPD/DS, biliopancreatic diversion with duodenal switch))
Post-surgical resolution of obesity-related comorbidities included type II diabetes resolving in 60.71% of patients, hypertension in 30.99%, hyperlipidemia resolved in 36.54% of cases, and obstructive sleep apnea resolved in 37.29% of cases (Tables 3, 4 and 5, Figs.3 and 4).
Obesity-related comorbidity resolution. The base rate is the percent of total population that had the listed comorbidity at the time of bariatric operation, if the patient had the obesity-related comorbidity resolve within the 60-month follow-up period, by clinical parameters such that it was removed from problem lists in follow-ups or if they discontinued all medical management they were listed as resolved and the 60-month post-op time corresponds to patients with the comorbidities that did not resolve throughout follow-up (TIIDM, type II diabetes mellitus; HTN, hypertension; HLD, hyperlipidemia; OSA, obstructive sleep apnea)
There were a total of 2 intraoperative complications which included 1 case of splenic injury and 1 case requiring anastomosis revision. In the immediate post-operative period, there were 3 cases of UTI, 1 case of pneumonia, and 6 cases of surgical site infections which were noted. There were not any cases of anastomotic or staple line leaks. This represents a 16.5% early complication rate, occurring within 30 days of surgery (Table 3). Subgroup analysis conducted with just liver and renal transplant patients found 7.4% and 3.7% rates of early and late complications (Tables 3 and 4). In prolonged follow-up out to 60 months, 3 patients experienced marginal ulcerations corresponding to 6.1% in the RNYGB group, 2 patients developed anastomotic strictures (Table 4, Fig. 3).
Discussion
This study presents outcomes at 6 months to 5 years of a single institution cohort of chronically immunosuppressed patients who underwent bariatric surgery (short-, mid-, and long-term data). This is relevant to today’s surgeon as increasing prevalence of obesity is leading to higher rates of chronically immunosuppressed patients being evaluated for bariatric interventions. Traditionally, these patients were considered at additional surgical risk with limited insight into the differences in recovery, weight loss, and morbidity imposed by immunosuppression. Long-term complications and durable weight loss were evaluated using our cohort study with a 5-year follow-up. Despite the increased surgical risk associated with immunosuppressed patients, this cohort experienced minimal long-term complications and 29.17% total weight loss. This is the first study to assess long-term outcomes in immunosuppressed patients undergoing bariatric surgery.
Bariatric surgery is very safe, particularly when done at an experienced center, and this holds true for immunosuppressed patients in this study. Major technical intra- and perioperative complications including post-operative leaks, immediate obstruction, or bleeding were not observed in any patient, correlating to the expected rate of 1% or less in the general population [17,18,19]. There was not any perioperative mortality. A study using a large data registry (MBSAQIP), with over 7000 patients who underwent bariatric surgery and were on chronic immunosuppression, did detect a small but significant increase in perioperative major complications with a 9.6% rate of major complications in immunosuppressed patients compared to 5.0% in all patients. Major complications included leak, bleed, and reoperation in 4.1% of immunosuppressed patients; these complications were not seen in our patient cohort. They also observed pneumonia, sepsis, AKI, and deep infection in 1.4% of immunosuppressed patients compared to 5.5% of these events in our patient cohort. There was no difference noted in mortality [20]. In this smaller subset of single institution data, with experienced surgeons, it does appear that early major complications rates are not different for immunosuppressed patients, but complications are shifted toward an infectious nature.
The most obvious implications of having an immunosuppressed host would be increased risk for higher rates of and more severe infectious sequelae. Infectious complications that occurred within 30 days of the operation including surgical site infections (SSI), urinary tract infection (UTI), pneumonia (PNA), deep tissue or organ infection, or sepsis from unknown source occurred in 12 patients (13.2%) of patients in this cohort. One patient developed an acute kidney injury post-operatively. The presence of obesity in these patients increases the rate of these infections and is a known confounder in bariatric surgical patients [21]. Due to logistic and methodological challenges, there is a lack of data and randomized controlled trials investigating infectious complications of bariatric surgery [22, 23]. Additionally, the rate of obesity-related comorbidities, such as diabetes, impacts the occurrence of infectious complications. Rates of surgical site infection after bariatric surgery determined by large database analysis range from 5.1 to 12%, which the rate observed in this study is well within [24, 25]. In this cohort, post-operative management of these complications resolved the issue and did not lead to any impact on long-term morbidity or mortality.
Beyond short-term post-operative complications, it is critical to examine longer term complications that may result more frequently due to immune dysregulation impacting structural and functional healing. There were 3 observed marginal ulcers in patients in this cohort, which is within the reported ranges of the general bariatric surgery population, 55% of our cohort underwent RNYGB, so 6.1% of RNYGB patients developed a marginal ulcer. A large database study with over 44,000 patients who underwent gastric bypass was 0.35%, but they only looked at symptomatic ulcers within 30 days of the operation [26]. Marginal ulceration rates status post RNYGB vary in literature, with longer studies showing higher rates. Riberio-Parenti et al. examined 1142 patients with a mean follow-up of 48.8 months showed a 4% marginal ulceration rate, while another study showed an 11.4% marginal ulceration rate at 60 months [27, 28]. The postulated or feared risk of ulceration in patients who undergo bypass and are on steroids was not witnessed in our study as a small percentage of ulcers were noted. The observed rate of internal hernias, small bowel obstructions, and anastomotic strictures was lower in our cohort than published reports in general bariatric surgical population [29, 30].
Weight loss outcomes in this immunosuppressed cohort compares to the general population of patients at all time points. The weight loss appears durable and appropriate for the mix of procedures performed, with 94% of the patients receiving either RNYGB or SG with a %TWL of 30.89% at 12 months and 29.17% at 60 months. Similar studies in the general population receiving these procedures demonstrate 30% TWL [31, 32] over the same time. The majority, 65%, of the study patients were on long-term glucocorticoids which are known to impact metabolism and cause weight gain [13]. Methotrexate, calcineurin inhibitors, and TNFα inhibitors (20.22%, 21.35%, and 22.47% of cohort patients, respectively) are also well known to cause weight gain and metabolic disturbances [33, 34]. Patients achieved high-resolution rates of all assayed obesity-related comorbidities, with hypertension, hyperlipidemia, GERD, and type II diabetes resolution at equivalent or higher rates than general bariatric populations [35]. The metabolic derangements secondary to immune suppression are in direct opposition to the positive metabolic regulation induced post bariatric surgery [36]; however, it is clear from the weight loss outcomes in our cohort that bariatric surgery overcomes drug-induced weight gain.
Inflammatory disorders or solid organ transplantation are the primary indications for chronic immunosuppression. These underlying disorders can also impact the surgical success and long-term metabolic reregulation of bariatric surgery. Patient lifestyle modification and exercise is also critical for initial rapid loss and durability of weight reduction follow bariatric surgery [37], but patients with rheumatoid arthritis, lupus, or asthma may be less able to engage in exercise rehabilitation and have higher levels of baseline disability that impede surgical recovery and initial weight loss. However, this cohort had an appropriate immediate reduction in body weight and a durable plateau weight. Additionally, allogenic transplant patients with obesity who undergo bariatric surgery are known to have higher mortality and morbidity, but it is unclear if that is due to the suppressed immune system or underlying disease pathology [38]. A systematic review on liver transplant patients who underwent bariatric surgery demonstrated comparable weight loss to the general population at 12 and 24 months, with slightly higher morbidity and mortality [39]. Bariatric surgery has shown to improve short-term allograft function in both kidney and liver transplant recipients, supporting the need to study bariatric surgery in these populations [40, 41]. This study includes a broader patient with diverse inflammatory conditions to isolate the impacts of immunosuppression on bariatric surgery outcomes.
A retrospective analysis has inherent limitations including loss to follow-up, no standardization of patient selection, differences in procedure type, and limited information available in the follow-up documentation. These patients were on diverse immunosuppressive regimens for different underlying conditions a limitation and strength. The same applies to combining the different procedural outcomes together. We were unable to have enough patients on a single regimen or underlying condition, but this allows the data to be representative of a larger set of patients representing the common inflammatory disorders, solid organ transplants, and medication regimens. The strength in this descriptive cohort is the long-term follow-up that is enabled from single institution data with only a few surgeons, who are consistent in their techniques and follow up.
Conclusion
Bariatric surgery is a safe and effective treatment option for obesity in patients on chronic immunosuppression in the short and long term. This data supports that bariatric surgery can be performed with minimal long-term complications and excellent sustained weight loss in immunosuppressed patients.
Data Availability
The data utilized and analysed in this study are available upon reasonable request to the corresponding author.
References
Aminian A, et al. Cardiovascular outcomes in patients with type 2 diabetes and obesity: comparison of gastric bypass, sleeve gastrectomy, and usual care. Diabetes Care. 2021;44(11):2552–63.
Buchwald H, et al. Bariatric surgery: a systematic review and meta-analysis. JAMA. 2004;292(14):1724–37.
Lupoli R et al. Clinical insights into management options for recurrent type 2 diabetes and cardiovascular risk after metabolic-bariatric surgery. Nutr Metab Cardiovasc Dis 2022.
Hales CM, et al. Prevalence of obesity and severe obesity among adults: United States, 2017–2018. NCHS Data Brief. 2020;360:1–8.
Vahibe A, et al. Diabetes remission after bariatric surgery in patients on glucocorticoids: a pilot study. Surg Laparosc Endosc Percutan Tech. 2021;32(2):236–40.
Reding R, et al. Surgery in patients on long-term steroid therapy: a tentative model for risk assessment. Br J Surg. 1990;77(10):1175–8.
Chen SY, et al. Assessment of postdischarge complications after bariatric surgery: a national surgical quality improvement program analysis. Surgery. 2015;158(3):777–86.
Mazzei M, Zhao H, Edwards MA. Perioperative outcomes of bariatric surgery in the setting of chronic steroid use: an MBSAQIP database analysis. Surg Obes Relat Dis. 2019;15(6):926–34.
Abraham CR, et al. Predictors of Hospital readmission after bariatric surgery. J Am Coll Surg. 2015;221(1):220–7.
Kermansaravi M, et al. Bariatric surgery in transplant recipients: a narrative review. J Res Med Sci. 2021;26:44.
Steenackers N, et al. Adaptations in gastrointestinal physiology after sleeve gastrectomy and Roux-en-Y gastric bypass. Lancet Gastroenterol Hepatol. 2021;6(3):225–37.
Rogers CC, et al. Pharmacokinetics of mycophenolic acid, tacrolimus and sirolimus after gastric bypass surgery in end-stage renal disease and transplant patients: a pilot study. Clin Transplant. 2008;22(3):281–91.
Liu D, et al. A practical guide to the monitoring and management of the complications of systemic corticosteroid therapy. Allergy Asthma Clin Immunol. 2013;9(1):30.
Heit JJ, et al. Calcineurin/NFAT signalling regulates pancreatic beta-cell growth and function. Nature. 2006;443(7109):345–9.
Gagne DJ, et al. Effect of immunosuppression on patients undergoing bariatric surgery. Surg Obes Relat Dis. 2009;5(3):339–45.
Khaitan M, Hegde A, Rekha PD. Bariatric surgery in immunocompromised patients: outcomes from one year follow-up. Obes Surg. 2018;28(9):2811–4.
Marshall JS, et al. Roux-en-Y gastric bypass leak complications. Arch Surg. 2003;138(5):520–3 (discussion 523-4).
Sakran N, et al. Gastric leaks after sleeve gastrectomy: a multicenter experience with 2,834 patients. Surg Endosc. 2013;27(1):240–5.
D’Ugo S, et al. Comparative use of different techniques for leak and bleeding prevention during laparoscopic sleeve gastrectomy: a multicenter study. Surg Obes Relat Dis. 2014;10(3):450–4.
Hefler J, et al. Effects of chronic corticosteroid and immunosuppressant use in patients undergoing bariatric surgery. Obes Surg. 2019;29(10):3309–15.
Goto T, et al. Association of bariatric surgery with risk of infectious diseases: a self-controlled case series analysis. Clin Infect Dis. 2017;65(8):1349–55.
Hasegawa K, et al. Risk of an asthma exacerbation after bariatric surgery in adults. J Allergy Clin Immunol. 2015;136(2):288-94 e8.
Shimada YJ, et al. Bariatric surgery and emergency department visits and hospitalizations for heart failure exacerbation: population-based, self-controlled series. J Am Coll Cardiol. 2016;67(8):895–903.
Kushner BS, et al. Infection prevention plan to decrease surgical site infections in bariatric surgery patients. Surg Endosc. 2022;36(4):2582–90.
Chopra T, et al. Epidemiology and outcomes associated with surgical site infection following bariatric surgery. Am J Infect Control. 2012;40(9):815–9.
Clapp B, et al. Evaluation of the rate of marginal ulcer formation after bariatric surgery using the MBSAQIP database. Surg Endosc. 2019;33(6):1890–7.
Ribeiro-Parenti L, et al. Comparison of marginal ulcer rates between antecolic and retrocolic laparoscopic Roux-en-Y gastric bypass. Obes Surg. 2015;25(2):215–21.
Spaniolas K, et al. Association of long-term anastomotic ulceration after Roux-en-Y gastric bypass with tobacco smoking. JAMA Surg. 2018;153(9):862–4.
McCarty TR and N Kumar, Revision bariatric procedures and management of complications from bariatric surgery. Dig Dis Sci, 2022.
O’Rourke RW. Management strategies for internal hernia after gastric bypass. J Gastrointest Surg. 2011;15(6):1049–54.
Corcelles R, et al. Total weight loss as the outcome measure of choice after Roux-en-Y gastric bypass. Obes Surg. 2016;26(8):1794–8.
Salminen P, et al. Effect of laparoscopic sleeve gastrectomy vs laparoscopic Roux-en-Y gastric bypass on weight loss at 5 years among patients with morbid obesity: the SLEEVEPASS randomized clinical trial. JAMA. 2018;319(3):241–54.
Baker JF, et al. Changes in body mass related to the initiation of disease-modifying therapies in rheumatoid arthritis. Arthritis Rheumatol. 2016;68(8):1818–27.
Ersoy A, et al. Calcineurin inhibitors and post-transplant weight gain. Nephrology (Carlton). 2008;13(5):433–9.
Maroun J et al. Ten year comparative analysis of sleeve gastrectomy, Roux-en-Y gastric bypass, and biliopancreatic diversion with duodenal switch in patients with BMI >/= 50 kg/m(2). Surg Endosc, 2021.
Sinclair P, Docherty N, le Roux CW. Metabolic effects of bariatric surgery. Clin Chem. 2018;64(1):72–81.
Boppre G, et al. Can exercise promote additional benefits on body composition in patients with obesity after bariatric surgery? A systematic review and meta-analysis of randomized controlled trials. Obes Sci Pract. 2022;8(1):112–23.
Ruiz Garcia, de Gordejuela A, Ibarzabal A, Osorio J. Bariatric surgery and solid-organ transplantation. Transplant Proc. 2022;54(1):87–90.
Lazzati A, et al. Bariatric surgery and liver transplantation: a systematic review a new frontier for bariatric surgery. Obes Surg. 2015;25(1):134–42.
Adani GL, et al. Laparoscopic sleeve gastrectomy as a weight reduction strategy in obese patients after kidney transplantation. Am J Transplant. 2015;15(4):1126–7.
Osseis M, et al. Sleeve gastrectomy after liver transplantation: feasibility and outcomes. Obes Surg. 2018;28(1):242–8.
Author information
Authors and Affiliations
Contributions
All authors contributed to the planning, data analysis, and interpretation of this work.
Corresponding author
Ethics declarations
Ethics Approval
For this type of study, formal consent was not required.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Key Points
• Immunosuppressed patients have been considered higher risk surgical candidates.
• Bariatric surgery remains effective in immunosuppressed patient population.
• Surgical complications are not significantly higher in this patient cohort.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Maroun, J., Vahibe, A., Shah, M. et al. Impact of Chronic Immunosuppression on Short-, Mid-, and Long-Term Bariatric Surgery Outcomes. OBES SURG 33, 240–246 (2023). https://doi.org/10.1007/s11695-022-06372-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11695-022-06372-7