Abstract
Background
Bariatric surgery (BS) may help transplant patients by improving their comorbidities and graft function and reducing the recurrence of the disease that led to the transplant. Different timings for BS have been proposed. This study aims to describe the outcomes of BS before, during, and after solid organ transplantation.
Methods
We identified patients with history of solid organ transplantation that underwent BS between January 1, 2012, and April 31, 2022, at our hospital site. We analyzed patients’ demographics, obesity-related comorbidities, and transplant history. Measured outcomes included post-operative morbidity; readmission; comorbidity management; weight loss at 6-, 12-, and 24-month follow-up; and survival.
Results
Seventy-eight patients were included in our analysis, with a median age of 57 (28–75) years and a median BMI of 40.91 (28.9–61) kg/m2. The most transplanted organ was the liver (53.6%), followed by the kidney (31.9%). Ten patients underwent BS before the transplant, 11 had simultaneous BS and liver transplant, and 57 underwent BS after the transplant. The median operative time, ICU requirement, length of hospital stay, and early post-operative complications were significantly higher in the simultaneous group. The median EBWL% at 6-, 12-, and 24-month follow-up was 47.51%, 57.89%, and 64.22%, respectively, with no significant difference between the three groups. Thirty-four (44.3%) and 40 (50.8%) patients reduced their HTN and DM medication dosage, respectively. One- and five-year survival rates were 98.2% and 87.4%.
Conclusion
BS before, during, or after solid organ transplant is safe, leads to a significant weight loss and improvement of obesity-related comorbidities, and improves patient’s survival.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Obesity has become a global epidemic in the last few years. According to the World Health Organization, worldwide obesity has tripled since 1975, with 13% of the world’s adult population being obese [1]. Numbers are even worse in the USA, where obesity prevalence is 41.9% and is predicted to reach 51.1% by 2030 [2, 3].
Many conditions associated with obesity are major risk factors for solid organ transplantation: non-alcoholic fatty liver disease (NAFLD) is becoming the most common cause of liver transplant, diabetes and hypertension are the leading cause of kidney transplantation, and heart failure due to obesity-related comorbidities is one of the most common causes of heart transplant [4]. At the same time, patients who undergo solid organ transplant are more prone to obesity, with up to 40% developing obesity 3 years later [5].
Obesity also increases the risk of post-transplant complications and decreases overall patient survival. One study showed that obesity increased the risk of mortality and graft failure by approximately 40% at 1 year post-transplant [6]. So, it should not be surprising that obese patients are less likely to be placed on waitlists and undergo organ transplantation [7, 8].
It has been demonstrated that bariatric surgery (BS) is the most effective long-term treatment for severe obesity, as it not only leads to a significant weight loss but also decreases obesity-related comorbidities such as hypertension, diabetes, and NAFLD [9, 10]. BS may help transplant patients by improving their comorbidities and graft function, improving immunosuppressive medication stability, and reducing the recurrence of the disease that led to the transplant [4].
Different timings for BS in transplant patients have been proposed: BS before, at the same time, or after the transplant. Each timing has its benefits and downfalls. This study aims to describe the outcomes of BS before, during, and after solid organ transplantation.
Methods
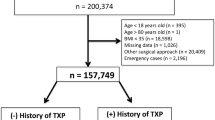
We queried our electronic medical records service for patients that underwent BS between January 1, 2012, and April 31, 2022, at our hospital site. Only patients that had a history of solid organ transplantation were included.
We analyzed patients’ demographics, obesity-related comorbidities, and transplant history, including transplanted organ, history of organ rejection or previous transplant, immunosuppressive medication daily dosage, and time from transplant to BS. Patients were later divided into three groups depending on the timing of the BS in relation to the transplant: BS pre-transplant, simultaneous BS and transplant, and BS post-transplant.
All the patients in our study underwent BS after meeting the eligibility criteria based on the National Institute of Health Guidelines on obesity [11]. Sleeve gastrectomy (SG) was the technique of choice in the patients undergoing simultaneous bariatric surgery and liver transplant, as previously described [12]. The surgical procedure in the other two groups was either laparoscopic/robotic sleeve gastrectomy (SG) or Roux-en-Y gastric bypass (RYGB), depending on the patient’s basal condition. SG was preferred for patients with liver transplantation to maintain endoscopic access to the biliary tree and avoided in patients with lung transplant due to the reported high incidence of reflux and risk of aspiration [13, 14]. SG and RYGB were performed as previously described [15].
Measured outcomes include intervention details; intra- and post-operative complications; readmission; comorbidity management; weight loss at 6-, 12-, and 24-month follow-up; mortality; and survival at 1 and 5 years. Changes in immunosuppressive drug dosage were also reviewed in patients that underwent BS after transplant. Readmission within 30 days from the surgery was considered early readmission. Comorbidity management was assessed by medication usage and changes in dosage. At the respective follow-up appointment, weight loss was evaluated using BMI (kg/m2) and the percentage of excess body weight loss (EBWL%).
Categorical variables were summarized as counts (percentages), and continuous variables were reported as medians (range). Comparisons of continuous variables between the three groups were made using the Kruskal–Wallis test, and categorical variables were compared using the chi-square test. The Kaplan–Meier method was used to estimate overall survival rates at 1 and 5 years and draw the corresponding survival curve. The log-rank test was used to compare the survival rates between the three groups. All tests were two-sided, with p-value < 0.05 considered statistically significant. The analysis was done using BlueSky and SPPS Statistics software.
Results
A total of 78 patients were included in our analysis, with a median age of 57 (28–75) years and a median BMI of 40.91 (28.9–61) kg/m2. Thirty-eight (49.4%) of the patients were female. The most transplanted organ was the liver (53.6%), followed by the kidney (31.9%). Ten patients underwent BS before the transplant, 11 had simultaneous BS and liver transplant, and 57 underwent BS after the transplant (Table 1) (Fig. 1). Median time from BS to transplant was 719 (209–1882) days or 1.9 years, and median time from transplant to BS was 1337 (306–7700) days or 3.7 years.
Demographic and baseline comorbidities were not significantly different between the three groups except for chronic corticosteroid use and history of organ rejection, which were more prevalent in the group that underwent BS after the transplant (Table 1).
Open SG was the technique of choice in patients undergoing simultaneous BS and liver transplant. There was no statistically significant difference in the surgical approach used in the other two groups, either a robotic/laparoscopic SG or RYGB (Table 2). Only one patient in the BS after transplant group required a conversion to open surgery due to dense adhesions and difficult anatomy.
The median operative time for the whole cohort was 117 (42–540) minutes. The median operative time for simultaneous BS and liver transplant group was the longest, naturally, as two surgeries were performed simultaneously. There was no statistically significant difference in the operative time between the other two groups (p = 0.393) (Table 2). ICU requirement was also significantly higher in the simultaneous BS and liver transplant group. Only 1 patient from the BS after transplant group required ICU.
The median length of hospital stay was 2 (1–30) days for the whole cohort. Again, patients undergoing simultaneous BS and transplant had a statistically significant longer length of stay. There was no statistically significant difference in the length of stay between the other two groups (p = 0.293) (Table 2).
Early post-operative complications were reported using the Clavien-Dindo classification as listed in Table 2. None of the patients in our study had post-operative leaks. Additionally, none of the patients required reintervention during their post-operative hospital stay. However, we had 5 patients that required surgical reintervention after discharge. Two of them were from the simultaneous group: one underwent reintervention for an intra-abdominal hemorrhage due to the rupture of splenic varix 6 months after the surgery, and the other for an incarcerated incisional hernia 2 months after the surgery. Similarly, there were 3 patients in the BS after transplant group that required reintervention: the first patient had an intra-abdominal abscess as a complication from percutaneous endoscopic gastrostomy tube insertion for a prolonged history of nausea and vomiting; the second one underwent a magnetic sphincter augmentation 1 year after the BS for worsening gastroesophageal reflux; the third patient developed a hernia from previous liver transplant incision and required repair.
We also had 7 patients that required early readmissions without surgical intervention: five from the BS after transplant group that were readmitted for persistent nausea and vomiting, wound erythema (at first believed to be an infection), acute kidney injury (AKI), diabetic ketoacidosis, and atrial fibrillation with hyponatremia, respectively; one from the simultaneous group due right hepatic artery stenosis; 1 from the BS before transplant group due to transient transaminitis. There was no statistically significant difference in the readmission rate between the three groups.
At the 6-, 12-, and 24- month follow-up, the median EBWL% was 47.51%, 57.89%, and 64.22%, respectively. There was no statistically significant difference in weight loss between the three groups in none of the follow-up time points (Table 3). Moreover, 34 (44.3%) patients reduced their HTN medication dosage and 40 (50.8%) their DM medication dosage. HTN resolved in 7 (8.6%) patients, whereas DM resolved in 10 (12.1%) patients. Again, there was no statistically significant difference between the three groups (Table 3).
During a median follow-up of 33.5 months (2.8 years), 4 patients died. All of them were from the BS after transplant group: one died 6 months after the BS surgery due to cardiogenic shock secondary to myocardial infarction, another one 3 years after the BS due to sepsis, the third one died 5 years after the BS because of a gastrointestinal hemorrhage associated with oral anticoagulant use, and the last one died 9 years after the BS due to a metastatic renal cell carcinoma (Table 3).
One- and five-year survival rates for the whole cohort were 98.2% and 87.4%, respectively (Fig. 2). There was no statistically significant difference in survival between the three groups (p = 0.554).
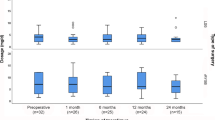
Finally, in the BS after the transplant group, we analyzed Tacrolimus dosage (mg/day) before and after BS. We found that the median dosage did not change 6 and 12 months after the surgery. The median dosage was slightly lower at the 24-month follow-up, although it was not statistically significant (Fig. 3). We also compared the median dosage changes by type of surgery (RYGB or LSG) in this same group and found no statistically significant difference between pre- and post-operative dosage (Fig. 4).
Discussion
The best timing to perform BS in transplant patients has not yet been defined. Since obese patients are less likely to be placed on waitlists and undergo transplantation, some advocate performing BS before transplant as a “getaway to transplant” [16]. Others argue that before the transplant, patients may be too weak to undergo BS and therefore suggest performing the weight loss surgery after the transplant [17]. Finally, recent studies report that simultaneous liver transplant and SG is feasible and safe, but larger cohorts of patients are needed to confirm those findings [12].
When comparing the three groups, we found that the simultaneous group’s operative time, length of hospital stay, and ICU requirement were significantly higher. This was an expected finding as two surgeries, including a transplant, are being performed simultaneously. Previous reports have pointed out that BS after transplant may be associated with longer operative times and hospital stay due to a more complex surgical field and higher morbidity [10]. Nevertheless, we found no statistically significant difference in these parameters between BS before and after transplant, and the median length of stay for both timings (2 days) was similar to the reported for LSG and RYGB without transplant history (2 days and 1 day, respectively) [18].
We also found that simultaneous BS and liver transplant were associated with a significant higher incidence of early post-operative complications. However, most of these adverse events were mild and required no intervention. Only two patients required intervention: one had hepatic artery stenosis, which occurs in 3.1–7.4% of liver transplantations [19], and the other had AKI over CKD due to hepatorenal syndrome, which was diagnosed before the transplant. On the other hand, the 30-day adverse event rate for the other two groups was also higher than the reported for BS alone, which ranges between 0.6 and 10.3% [20]. This correlates to what other studies have reported [4, 17, 21], and it is probably because transplant patients have a higher prevalence of underlying comorbidities. Although chronic steroid use was significantly more prevalent in the BS after the transplant group, the incidence of postop complications in this group was the same as in the transplant after BS group and lower than in the simultaneous group.
Similarly, we had 3 (3.8%) patients with abdominal wall complications: two incisional hernias and 1 intra-abdominal abscess. One of the incisional hernias was in a patient who underwent simultaneous open sleeve gastrectomy and liver transplant. According to literature, approximately 5–15% of open bariatric procedures are complicated by incisional hernias [22], and in our cohort, out of 12 open BS, only 1 (8.3%) patient developed this complication. The other patient that developed a hernia had the BS after a liver transplant in which incisional hernias occur in 5–46% of the cases [23]. Finally, we had 1 patient that developed an intra-abdominal abscess after a gastrostomy tube insertion. This procedure has a complication rate of 5.8%, with 1.2% major complications (including intra-abdominal abscess) [24].
Our cohort’s readmission rate was higher than the reported in primary BS (8.6% vs 5%, respectively) [25]. Similarly, Verhoeff et al. reported a readmission rate of 10.5% for patients undergoing BS with previous transplant history vs 3.5% for patients without transplant history (p ≤ 0.001) [4]. Nevertheless, there was no statistically significant difference in readmission between the three groups. Most of the readmissions in the BS after transplant group were because of decompensated baseline comorbidities and resolved without needing intervention. The two patients in the simultaneous group were readmitted for complications likely related to the hepatic transplant alone: hepatic artery stenosis and transaminitis, which is very common after liver transplantation.
Five of our patients underwent late reinterventions. Again, the two from the simultaneous group were likely due to liver transplant-related complications. Of the 3 patients in the BS after transplantation, two of the reinterventions were for complications directly related to BS: PEG tube insertion due to malnutrition and magnetic sphincter augmentation due to severe reflux. Gastroesophageal reflux is a known side effect of BS, affecting around 19–23% of patients [14]. Also, about 3.9% of the patients require feeding tube placement after BS [26].
Despite the higher risk of post-operative complications, considerable weight loss and comorbidity improvement were noted in most of our patients. At 12-month follow-up, all the groups had lost more than 50% excess body weight (EBW). Improvement in comorbidity control was also noted in a great part of the cohort, with no difference between the three groups. As other studies have suggested, BS leads to a significant weight loss and comorbidity improvement in transplant patients [21, 27], and the timing of BS in relation to the transplant seems not to affect these outcomes.
There is a marked concern regarding the malabsorptive effect of BS in patients with history of organ transplants. It is debatable that patients that undergo BS after transplant may require higher doses of immunosuppressive medications [27, 28]. We found no statistically significant difference in Tacrolimus dosage before and after BS similarly to what Cheng and Elli reported [21]. As malabsorption has been more commonly associated with RYGB, we analyzed these patients separately and found no significant difference in Tacrolimus doses before and after the bypass.
Only 4 (4.3%) patients died in our study. Even though all the deceased patients belonged to the BS after the transplant group, they all died long after the BS due to unrelated causes. Moreover, the 5-year survival rate after BS was 87.4%, while the 5-year survival rate for liver and kidney transplants without BS are 74.6% and 79.9%, respectively [29, 30]. So, regardless of the timing, BS seems to improve survival in patients with transplanted organs.
To our knowledge, this is the first cohort study to compare the outcomes of BS before, during, and after solid organ transplantation. Yet, this was a retrospective study, subject to inherent error and bias. We had the limitation that some groups (BS before transplant and simultaneous BS and transplant) had a small number of patients and that not all the included subjects had a complete post-operative follow-up, as some of the surgeries were performed recently. Nevertheless, we were still able to manage a significant and representative cohort.
Conclusion
Bariatric surgery before, during, or after solid organ transplant is safe, leads to a significant weight loss and improvement of obesity-related comorbidities, and improves patient’s survival. However, it may increase the incidence of post-operative adverse events, especially when performed simultaneously with the transplant.
References
Obesity and overweight. World Health Organization. [Online]. 2021. Available from: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight. Accessed 4 Aug 2022.
Adult obesity facts. Centers for Disease Control and Prevention. [Online]. 2022. Available from: https://www.cdc.gov/obesity/data/adult.html#. Accessed 4 Aug 2022.
Wang Y, Beydoun MA, Liang L, Caballero B, Kumanyika SK. Will all Americans become overweight or obese? Estimating the progression and cost of the US obesity epidemic. Obesity (Silver Spring). 2008;16(10):2323–30.
Verhoeff K, Dang JT, Modasi A, Switzer N, Birch DW, Karmali S. Bariatric surgery outcomes in patients with previous organ transplant: scoping review and analysis of the MBSAQIP. Obes Surg. 2021;31(2):508–16.
Beckmann S, Nikolic N, Denhaerynck K, Binet I, Koller M, Boely E, et al. Evolution of body weight parameters up to 3 years after solid organ transplantation: the prospective Swiss Transplant Cohort Study. Clin Transplant. 2017;31(3):e12896.
Hoogeveen EK, Aalten J, Rothman KJ, Roodnat JI, Mallat MJ, Borm G, et al. Effect of obesity on the outcome of kidney transplantation: a 20-year follow-up. Transplantation. 2011;91(8):869–74.
Meier-Kriesche H-U, Arndorfer JA, Kaplan B. The impact of body mass index on renal transplant outcomes: a significant independent risk factor for graft failure and patient death. Transplantation. 2002;73(1):70–4.
Hoogeveen EK, Aalten J, Rothman KJ, Roodnat JI, Mallat MJK, Borm G, et al. Effect of obesity on the outcome of kidney transplantation: a 20-year follow-up. Transplantation. 2011;91(8):869–74.
Christou NV, Sampalis JS, Liberman M, Look D, Auger S, McLean APH, et al. Surgery decreases long-term mortality, morbidity, and health care use in morbidly obese patients. Ann Surg. 2004;240(3):416–24.
Lazzati A, Iannelli A, Schneck A-S, Nelson AC, Katsahian S, Gugenheim J, et al. Bariatric surgery and liver transplantation: a systematic review a new frontier for bariatric surgery. Obes Surg. 2015;25(1):134–42.
Clinical Guidelines on the Identification. Evaluation, and Treatment of Overweight and Obesity in Adults-The Evidence Report. National Institutes of Health. Obes Res. 1998;6(Suppl 2):51s–209s.
Heimbach JK, Watt KDS, Poterucha JJ, Ziller NF, Cecco SD, Charlton MR, et al. Combined liver transplantation and gastric sleeve resection for patients with medically complicated obesity and end-stage liver disease. Am J Transplant. 2013;13(2):363–8.
Mandeville Y, Van Looveren R, Vancoillie P-J, Verbeke X, Vandendriessche K, Vuylsteke P, et al. Moderating the enthusiasm of sleeve gastrectomy: up to fifty percent of reflux symptoms after ten years in a consecutive series of one hundred laparoscopic sleeve gastrectomies. Obes Surg. 2017;27(7):1797–803.
Yeung KTD, Penney N, Ashrafian L, Darzi A, Ashrafian H. Does sleeve gastrectomy expose the distal esophagus to severe reflux? A systematic review and meta-analysis. Ann Surg. 2020;271(2):257–65.
Tsamalaidze L, Elli EF. Bariatric surgery is gaining ground as treatment of obesity after heart transplantation: report of two cases. Obes Surg. 2017;27(11):3064–7.
Tsamalaidze L, Elli EF. Solid organ transplantation and bariatric surgery. In: Reavis KM, Barrett AM, Kroh MD, editors. The SAGES manual of bariatric surgery. Cham: Springer International Publishing; 2018. p. 615–33.
Diwan TS, Rice TC, Heimbach JK, Schauer DP. Liver transplantation and bariatric surgery: timing and outcomes. Liver Transpl. 2018;24(9):1280–7.
Mahmood F, Sharples AJ, Rotundo A, Balaji N, Rao VSR. Factors predicting length of stay following bariatric surgery: retrospective review of a single UK tertiary centre experience. Obes Surg. 2018;28(7):1924–30.
Molvar C, Ogilvie R, Aggarwal D, Borge M. Transplant hepatic artery stenosis: endovascular treatment and complications. Semin Intervent Radiol. 2019;36(2):84–90.
Ibrahim AM, Ghaferi AA, Thumma JR, Dimick JB. Variation in outcomes at bariatric surgery centers of excellence. JAMA Surg. 2017;152(7):629–36.
Cheng YL, Elli EF. Outcomes of bariatric surgery after solid organ transplantation. Obes Surg. 2020;30(12):4899–904.
Abo-Ryia MH, El-Khadrawy OH, Abd-Allah HS. Prophylactic preperitoneal mesh placement in open bariatric surgery: a guard against incisional hernia development. Obes Surg. 2013;23(10):1571–4.
Kniepeiss D, Waha JE, Auer T, Berghold A, Schemmer P. PRevention of INCisional hernia after liver transplantation (PRINC trial): study protocol for a randomized controlled trial. Trials. 2019;20(1):371.
Zener R, Istl AC, Wanis KN, Hocking D, Kachura J, Alshehri S, et al. Thirty-day complication rate of percutaneous gastrojejunostomy and gastrostomy tube insertion using a single-puncture, dual-anchor technique. Clin Imaging. 2018;50:104–8.
Lazzati A, Chatellier G, Katsahian S. Readmissions after bariatric surgery in France, 2013–2016: a nationwide study on administrative data. Obes Surg. 2019;29(11):3680–9.
Charles EJ, Mehaffey JH, Hawkins RB, Safavian D, Schirmer BD, Hallowell PT. Benefit of feeding tube placement for refractory malnutrition after bariatric surgery. Surg Obes Relat Dis. 2018;14(2):162–7.
Yemini R, Nesher E, Winkler J, Carmeli I, Azran C, Ben David M, et al. Bariatric surgery in solid organ transplant patients: Long-term follow-up results of outcome, safety, and effect on immunosuppression. Am J Transplant. 2018;18(11):2772–80.
Rogers CC, Alloway RR, Alexander JW, Cardi M, Trofe J, Vinks AA. Pharmacokinetics of mycophenolic acid, tacrolimus and sirolimus after gastric bypass surgery in end-stage renal disease and transplant patients: a pilot study. Clin Transplant. 2008;22(3):281–91.
Kim WR, Lake JR, Smith JM, Schladt DP, Skeans MA, Harper AM, et al. OPTN/SRTR 2016 Annual Data Report: Liver. Am J Transplant. 2018;18(S1):172–253.
Ghelichi-Ghojogh M, Ghaem H, Mohammadizadeh F, Vali M, Ahmed F, Hassanipour S, et al. Graft and patient survival rates in kidney transplantation, and their associated factors: a systematic review and meta-analysis. Iran J Public Health. 2021;50(8):1555–63.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical Approval
For this type of study, formal consent is not required.
Informed Consent
Informed consent does not apply.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Key points
• Bariatric surgery may help transplant patients by improving graft function.
• Bariatric surgery before, during, or after solid organ transplant is safe and beneficial.
• It also leads to significant weight loss improving patient’s survival.
• However, it may increase the incidence of post-operative adverse events.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Castillo-Larios, R., Gunturu, N.S. & Elli, E.F. Outcomes of Bariatric Surgery Before, During, and After Solid Organ Transplantation. OBES SURG 32, 3821–3829 (2022). https://doi.org/10.1007/s11695-022-06334-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11695-022-06334-z