Abstract
While guidelines exist for the management of postoperative nausea and vomiting (PONV) in the general surgical setting, there are no established guidelines for the prevention or treatment of PONV in bariatric patients, in whom PONV contributes significantly to perioperative morbidity and hospital resource utilization. This systematic review found that the multimodal pharmacological approach to PONV prevention recommended in current guidelines for high-risk surgical patients is appropriate for the bariatric subset. This includes multi-agent antiemetic prophylaxis with dexamethasone and one or more agents from other classes, and opioid-free total intravenous anesthesia, though the advantages of the latter need further evaluation. There remains a need for a standardized validated instrument to assess PONV in the bariatric setting.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Postoperative nausea and vomiting (PONV) is an important source of patient morbidity following bariatric surgical procedures, contributing to delays in oral intake and mobilization with subsequent prolonged hospital length of stay, unexpected hospital readmissions, and patient dissatisfaction with the perioperative experience [1,2,3].
Current strategies for the prevention and treatment of PONV include proactive risk assessment, avoidance of PONV triggers, administration of prophylactic antiemetics in the preoperative setting or rescue antiemetics postoperatively, and optimization of anesthetic protocols [4]. The risk of PONV is often estimated preoperatively using the Apfel score, which comprises female gender, history of motion sickness or PONV, non-smoking status, and postoperative use of opioids [5]. Conditions affecting the gastroesophageal junction, including hiatal hernia and obesity, blood and secretions in the stomach, choice of anesthetic technique (opioids, nitric oxide, halogenated anesthetics), and duration of surgery, may also place bariatric surgery patients at higher risk compared with other surgical subpopulations [1].
While guidelines exist for the management of PONV in the general adult and pediatric surgical setting, there are no established guidelines for the prevention or treatment of PONV in the bariatric subset in particular, in whom PONV contributes significantly to perioperative morbidity and hospital resource utilization [1, 6, 7]. To this end, the aims of this systematic review were to (1) describe instruments for PONV assessment; (2) identify effective pharmacological approaches for PONV prophylaxis in the bariatric surgical cohort; (3) compare rates of PONV with various pharmacological combinations, and (4) evaluate inhalational versus total intravenous anesthetic approaches to reduce PONV rates.
Methods
This systematic review was conducted under the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [8]. A prespecified study protocol was developed and followed accordingly; the protocol was not registered.
Data Sources and Searches
The search strategy was devised in consultation with a medical librarian at our institution. The PubMed, Cochrane Library, Ovid MEDLINE, Embase, and Institute for Scientific Information (ISI) Web of Science databases were searched from their inception dates to December 1, 2019. The “similar articles” feature of PubMed and bibliographies of related papers were reviewed to identify any remaining references meeting inclusion criteria. Only full-text original articles published in the English language on human subjects were included. Results were manually imported and de-duplicated into Microsoft Excel 2016 (Microsoft for Windows).
Medical Subject Headings (MeSH) terms pertaining to laparoscopic and open bariatric surgical procedures and PONV were as follows: Body Mass Index, Morbid Obesity, Weight Loss, Gastric Bypass, Gastrectomy, Bariatric Surgery, Gastroplasty, Jejunoileal Bypass, Lipectomy, Postoperative Nausea and Vomiting. Embase was queried with the Emtree equivalents of MeSH terms. A keyword search was also conducted to capture all possible variants of the concepts of interest; keywords included were the following: Roux-en-Y gastric bypass, RYGB, Gastric Bypass, Sleeve Gastrectomy, Gastrectomy, Gastric Sleeve, Gastric Banding, Bariatric Surgery, Metabolic Surgery, Weight Loss Surgery, Morbid Obesity, Severe Obesity, Post-operative Nausea and Vomiting, PONV, Post-operative emesis, Post-operative nausea*, Post-operative vomit*, Postoperative Nausea and Vomiting, Postoperative emesis, Postoperative nausea*, Postoperative vomit*. To ensure a comprehensive search term list, we consulted the MeSH database for synonyms and terms appearing under the explosion tree and examined keyword tags on known articles of interest.
Study Selection
Search results were screened by scanning titles and abstracts for the following exclusion criteria: animal studies; languages other than English; unpublished data, including that on clinicaltrials.gov; publications of abstracts only, case reports, errata, letters, comments, reviews, or meta-analyses; duplicate entries; not pertaining to the population or outcome of interest. As the scope of the paper is limited to pharmaceutical management of PONV in the bariatric surgery setting, publications relating to the impact of surgical technique (e.g., omentopexy), non-surgical bariatric approaches (e.g., intragastric balloon placement), and non-pharmacological techniques (e.g., acupuncture and hypnosis) on PONV were excluded.
Full-text files of the remaining articles were obtained and reviewed using the previously described exclusion criteria. Upon full-text review, we recognized the need for nuanced exclusion criteria as some studies discussed PONV as a secondary endpoint, with primary objectives only tangentially relating to the present review. The study protocol was amended with these additional exclusion criteria, which included articles assessing: recovery profiles of anesthetic regimens, of which PONV is one consideration; analgesic effects with primary endpoints including pain levels and rescue analgesia use; institution-specific Enhanced Recovery After Surgery (ERAS) protocols, of which PONV may be one component; transversus abdominis plane (TAP) block efficacy on pain control.
Studies were included if they were original research reports of adult patients aged 18 or older undergoing bariatric surgery (laparoscopic Roux-en-Y gastric bypass (LRYGB), laparoscopic sleeve gastrectomy (LSG), laparoscopic gastric banding, jejunoileal bypass, vertical banded gastroplasty, biliopancreatic diversion with duodenal switch (BPD/DS), mini-gastric bypass, or gastric plication) with PONV incidence as a primary endpoint. Two reviewers independently evaluated each publication. Any discrepancies in study inclusion were resolved with the input of a third reviewer.
Data Extraction
For each article, identifying information (PubMed ID if available, year, and country of publication) and population demographics were noted. The study design was also noted as a proxy for study quality, in line with the commonly accepted evidence pyramid: double-blind randomized controlled trial (RCT) > single-blind RCT > non-blinded RCT > prospective cohort study > retrospective cohort study. Given the heterogeneity in sample populations, treatment modalities, and reporting of outcomes between the included studies, pooling of data into a meta-analysis was not possible. The following data were derived: antiemetic prophylaxis (including number, names, and dosing of medications), PONV rates, rescue antiemetic use (including number, names, and dosing of medications), time to first administration of rescue medication, number of rescues, and type of anesthesia.
Results
Overall Findings
A flow diagram outlining the systematic review methodology is provided in Fig. 1. The initial query yielded 3596 articles. Titles and abstracts were screened for exclusion criteria, resulting in 115 articles, which then underwent full-text review. Four review articles and one meta-analysis were excluded both on the basis of their study design as well as on the scope of their study objectives, which pertained to analgesic or anesthetic recovery profiles. One article was excluded as it contained previously published data. Pertinent data from the remaining 21 studies (12 RCTs, 9 observational studies) were included in this review.
Most of the studies were published in the last decade (18 studies since 2010), primarily in the USA (n = 8), Europe (n = 5), and the Middle East (n = 4). Of the 12 RCTs, nine were double-blind, one was single-blind, and two were unblinded. Nine studies were observational in nature; five were retrospective cohort analyses. The majority of studies included patients undergoing LRYGB (n = 15), followed closely by restrictive procedures (12 LSG and 6 laparoscopic gastric banding). Study characteristics are summarized in Table 1.
A total of 3036 adults undergoing bariatric surgery were analyzed. Of note, one study compared bariatric patients with their non-bariatric counterparts; only the bariatric subset was included in our demographic analysis [9]. The mean age of participants was 41 years; 75.73% were female. The mean body mass index (BMI) was 45 kg/m2. Six studies also reported total body weight, with a mean value of 121 kg. All patients were American Society of Anesthesiologists (ASA) class I to III.
Definitions and Assessment Instruments for PONV
Nine studies (5 RCTs) explicitly defined PONV [7, 10,11,12,13,14,15,16,17]. Five of these studies distinguished between nausea (unpleasant, subjective sensation associated with the awareness of the urge to vomit), vomiting (forceful expulsion of gastric contents through the mouth), and retching (labored, spasmodic, rhythmic contraction of the respiratory muscles without expulsion of gastric contents) [7, 10, 11, 14, 15]. PONV was recognized as a spectrum incorporating some combination of nausea, vomiting, and/or retching in the postoperative period. Three studies further graded nausea on a scale of severity, with severe nausea interfering with one’s ability to eat, drink, or socialize [14, 16, 17]. Six RCTs and two prospective observational studies conducted pre-operative PONV risk assessments using the Apfel scoring system [2, 7, 11, 13, 14, 16, 18, 19]. In the postoperative period, PONV was assessed at designated time points, ranging from 0 to 72 h, using a variety of methods, including the visual analog scale (VAS) for nausea (validated by Boogaerts et al.) or the verbal rating scale (VRS), each rating nausea intensity on a numerical scale (n = 6 studies) [3, 7, 11, 13, 19,20,21]; number or doses of antiemetics administered during the recovery period (n = 4) [3, 9, 16, 22]; direct questioning (n = 4) [1, 10, 18, 23] or documentation of PONV (n = 3) [9, 15, 17] by a trained medical professional; the PONV Impact Scale devised by Myles et al. (n = 3) [2, 14, 16, 24]; self-reported 4-point ordinal scale: none, mild, moderate, severe (n = 3) [12, 18, 25]; Quality of Life – Functioning Living Index Emesis (QoL-FLIE) Questionnaire devised by Lindley 1992 (n = 1) [12, 26]; and spontaneous complaint by the patient (n = 1) [10]. Nineteen studies used at least one of these methods; two did not specify a mode of assessment.
The Effect of Combination Pharmacotherapy
A complete listing of studies included in this review can be found in Table 2. Ondansetron, metoclopramide, and droperidol were the most frequently prescribed rescue antiemetics in the postoperative period (Fig. 2). Eleven studies (8 RCTs, 3 observational studies) analyzed antiemetic prophylaxis regimens, largely administered alongside inhalational anesthetics and opioids. The studies overwhelmingly found better efficacy in minimizing PONV with a combination antiemetic prophylactic approach compared with a single agent approach (n = 5 of the 6 studies making this comparison) [1, 7, 10,11,12, 22]. Of the approaches consisting of two drugs, the most commonly utilized agents were dexamethasone in conjunction with a serotonin receptor antagonist (n = 5), dopamine receptor antagonist (n = 1), or phenothiazine (n = 1).
Systemic Corticosteroids
Dexamethasone 0.1 mg/kg of body weight (up to 8 mg) and ondansetron 0.1 mg/kg (up to 10 mg) significantly lowered PONV in the first 6 postoperative hours compared with placebo or either drug alone (p = 0.0002) [1]. However, the combination of dexamethasone 4 mg intravenously (IV) following tracheal intubation and ondansetron 4 mg administered during skin closure resulted in similar rates of cumulative (p = 0.5) and severe (p = 1) PONV compared with placebo [13]. One RCT evaluated dexamethasone 8 mg and granisetron 1 mg and found that the administration of these drugs intravenously 1 min prior to induction resulted in a lower incidence of PONV during the first 24 postoperative hours compared with placebo, granisetron alone, or granisetron and droperidol (p = 0.0009) [10]. Dexamethasone 8 mg combination therapy with palonosetron 0.075 mg, on the other hand, resulted in low but similar incidences of vomiting in the post-anesthesia care unit (PACU) and at 72 h versus palonosetron 0.075 mg alone (p > 0.32) [12]. Another RCT evaluated promethazine 25–50 mg intramuscular (IM) and dexamethasone 4 mg IV and found that this combination significantly reduces the mean number of PONV episodes compared with metoclopramide 10 mg IV and dexamethasone 4 mg IV (p = 0.01) [25].
Two studies examined dexamethasone in combination with two other agents [11, 22]. Dexamethasone 8 mg, haloperidol 2 mg, and ondansetron 8 mg resulted in significantly less nausea (p < 0.015) and vomiting (p < 0.01) during the first 36 postoperative hours compared with ondansetron alone, as well as a significantly higher time to first administration of rescue antiemetic (p = 0.006) [11]. When compared with ondansetron, ondansetron and dexamethasone, or dexamethasone, cyclizine, and ondansetron, the combination of dexamethasone, cyclizine, and prochlorperazine provided the best outcome for bariatric patients undergoing LRYGB, with no PONV in the PACU (p = 0.001) or at 24 h (p = 0.002) [22].
The Role of NK-1 Receptor Antagonists
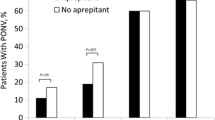
The addition of oral aprepitant 40 mg to triple antiemetic prophylaxis (0.625 mg droperidol, 4 mg dexamethasone, and 4 mg ondansetron) resulted in lower rates of PONV following PACU discharge and fewer cumulative episodes of emesis through 48 postoperative hours compared with triple prophylaxis alone [15]. The administration of aprepitant 80 mg orally 1 h prior to surgery and ondansetron 4 mg intravenously prior to the cessation of surgery was likewise effective, resulting in a significantly lower cumulative incidence of vomiting at 72 h compared with those receiving ondansetron alone [7].
Total Intravenous Anesthesia
Anesthetic approaches included inhalational anesthetics with (n = 12) or without (n = 3) opioids, total intravenous anesthesia (TIVA) with propofol-remifentanil (n = 6), and opioid-free TIVA (n = 1) (studies may be included more than once if they utilized multiple approaches). Five studies evaluated anesthetic interventions to mitigate PONV occurrence. In patients receiving triple antiemetic prophylaxis including transdermal scopolamine, dexamethasone, and ondansetron, opioid-free TIVA with dexmedetomidine, propofol, and ketamine resulted in significantly less PONV compared with general anesthesia with inhalational anesthetics and opioids (20% vs. 37.3% respectively, p = 0.04) [18]. However, another study suggested no significant difference in VAS score for PONV or antiemetic use in patients who underwent TIVA with remifentanil and propofol compared with inhalational anesthesia with opioids [21]. Two observational studies noted that in bariatric patients receiving TIVA with propofol-remifentanil and antiemetic prophylaxis with intravenous ondansetron, betamethasone, and/or droperidol, rescue antiemetics were required by 67–68% patients with PONV, with a mean time for initial rescue antiemetic of 136–142 min [14, 16].
In the setting of opioid-free general anesthesia, patients who also received an intraoperative injection of 100 mg/2 mL magnesium sulfate and 5 mL of 2% lidocaine mixture into the pyloric sphincter muscle had no PONV compared with 40% PONV among those who did not receive the mixture (p < 0.0001) and also used less than expected antiemetics based on Apfel score (p < 0.0001) [2]. Intraoperative injection of lidocaine 1 mg/kg/h IV or 2 mg/kg/h IV similarly resulted in significantly lower rates of PONV for each dose through 24 h [27]. Additionally, in bariatric patients who underwent inhalational anesthesia with opioids, those who received less total intraoperative intravenous fluids at a slower rate had a significantly higher incidence of PONV compared with those who received more fluids (p = 0.002) at a faster rate (p = 0.04) [28].
Discussion
Routine antiemetic prophylaxis is not recommended for all surgical patients because it may impose unnecessary adverse effects and contribute to increased medical costs in low-risk patients [29, 30]. Morbidly obese patients undergoing laparoscopic bariatric surgical procedures, however, are at higher risk for PONV compared with their non-bariatric counterparts and are uniquely vulnerable to its sequelae, which largely stem from elevated intraabdominal pressure [9]. These include increased suture tension, anastomotic dehiscence, and in severe cases, esophageal rupture, dehydration, electrolyte imbalance, venous hypertension, and potential aspiration of gastric contents [1, 7].
There is no single standardized validated tool to assess PONV in the postoperative period, which accounts for the variability in reporting of PONV outcomes during patients’ hospital stay and precludes pooling of data. The two most commonly used validated tools for the assessment of PONV in bariatric surgery patients in our study were the visual analog scale (VAS) and the PONV Impact Scale. The VAS, traditionally used in the setting of acute and chronic pain, was first adapted by Boogaerts et al. in 2000 for nausea assessment in the postoperative period [20, 31]. The VAS grades nausea on a horizontal line of 100 mm in length, ranging from least to most severe, and facilitates the collection of continuous data which can later be utilized for statistical and cross-cohort analyses, as opposed to descriptive scales using categorical gradations of none, mild, moderate, and severe [20]. The subjective nature of this instrument, combined with its assessment of nausea only, however, limits its use as a standalone assessment tool for PONV. Multiple studies in our review (n = 5) defined PONV as a spectrum of nausea, vomiting, and/or retching, necessitating the use of a scale which reflects these components. The PONV Impact Scale incorporates a nausea ordinal response to quantify nausea intensity or impact on the patient, as well as vomiting and/or retching count to quantify vomiting intensity, with a sum ≥ 5 indicating clinically significant PONV [24]. While this tool was validated in high-risk surgical patients at a single center in Australia, external validity testing to determine applicability to the bariatric cohort across care centers is needed. Although not reported in the studies examined herein, the Rhodes Index of Nausea and Emesis is a validated instrument for the assessment of nausea and vomiting [32]. This has been previously utilized in bariatric surgery research [33].
In line with the Society for Ambulatory Anesthesia (SAMBA) Guidelines for the management of PONV in high-risk surgical patients, this review demonstrates that a multimodal interventional approach consisting of combination antiemetic prophylaxis and opioid-free TIVA is appropriate for bariatric surgery patients [6]. Antiemetic classes include serotonin receptor antagonists, glucocorticoids, anticholinergics, neurokinin-receptor antagonists, dopamine receptor antagonists, cannabinoids, and antihistamines, among others [34]. These classes are reflective of the many chemoreceptors involved in the pathways influencing the perception and onset of nausea and vomiting. The most commonly prescribed prophylactic agent in bariatric surgery patients was the corticosteroid, dexamethasone, administered intravenously either prior to or at the induction of anesthesia at doses of 4 mg or 8 mg. While the mechanism of action by which dexamethasone prevents PONV has yet to be elucidated, its side effects are mild and include insomnia, excitation, and mood lability [34]. For dual-agent approaches, dexamethasone was effective when prescribed in conjunction with the serotonin receptor antagonist, granisetron 1 mg IV, or the phenothiazine, promethazine 25–50 mg IM [10, 25]. The SAMBA Guidelines recommend intravenous administration of granisetron at the cessation of surgery, whereas this drug was administered prior to induction in the RCT analyzed in this review, suggesting that alternative prophylaxis timing with this drug may be suitable for the bariatric cohort [6, 10]. Dexamethasone combination therapy with palonosetron 0.075 mg or metoclopramide 10 mg IV did not demonstrate superiority over a single agent or alternative dual-agent regimens [12, 25]. Interestingly, while ondansetron is often used as a first-line prophylactic and rescue agent in surgical populations due to its efficacy and low cost, the addition of ondansetron to dexamethasone in the bariatric subset yielded variable efficacy, implying that dexamethasone in combination with alternative aforementioned agents may prove more beneficial for these patients [1, 13, 35, 36].
It has been suggested that prophylactic antiemetic approaches utilizing three or more agents may be more effective than either single- or dual-agent regimens [9, 11, 22]. This is expected, as no single antiemetic has been entirely effective for PONV prevention in high-risk patients, and drugs with different mechanisms of action often have an additive effect when used in combination; the latter is especially advantageous in the setting of PONV, which has a multifactorial etiology [35, 37]. In this review of bariatric surgery patients, prophylaxis with intravenous dexamethasone 8 mg plus haloperidol 2 mg plus ondansetron 8 mg or dexamethasone 8 mg plus cyclizine 50 mg plus prochlorperazine 12.5 mg produced significant reductions in nausea and vomiting, both in the PACU and on the surgical floor [11, 22]. Two observational studies and one RCT also demonstrated efficacy with triple prophylaxis consisting of application of transdermal scopolamine (a non-polar tertiary amino compound with anticholinergic properties) patch pre-operatively, dexamethasone 4 mg IV at or after induction, and ondansetron 4 mg IV prior to the end of the case [17, 18, 23, 38].
Triple prophylaxis with 0.625 mg droperidol, 4 mg dexamethasone, and 4 mg ondansetron in conjunction with oral aprepitant 40 mg significantly reduced cumulative episodes of emesis through 48 h postoperatively compared with triple prophylaxis alone [15]. This is consistent with SAMBA Guidelines, which recommend an aprepitant dose of 40 mg per os at induction. A second RCT in this review reported that aprepitant 80 mg administered orally 1 h prior to surgery in combination with ondansetron 4 mg IV given at end of surgery was successful in reducing PONV, suggesting that the bariatric cohort may benefit from this alternate dosage/timing of aprepitant combination therapy; future guidelines should be updated to include this data [7].
Approximately half of the studies included in this review employed inhalational anesthetics with frequent use of opioids, even though volatile anesthetics, nitrous oxide, and opioids have previously been described as key contributors to PONV in a dose-dependent manner, especially in the immediate postoperative period [6, 39, 40]. In one study, intraoperative use of opioids and nitrous oxide resulted in a more than fourfold and twofold risk in PONV on logistic regression, respectively [41]. The same study reported that TIVA had a protective influence, with an odds ratio of 0.40 [41]. This holds true for bariatric patients as well, where one RCT (Ziemann-Gimmel et al.) included in this review demonstrated an absolute risk reduction of 17.3% (number needed to treat: 6) and a significantly lower incidence of PONV using opioid-free TIVA versus inhalational anesthesia with opioids [18]. The majority of the studies using inhalational anesthetics in our review were older (prior to 2015), with more recent studies using TIVA or general anesthesia without opioids, indicating that practice patterns may be moving in a specific direction, though year-to-year analysis would be needed to make conclusions about trends. Interestingly, an RCT by Aftab et al. found TIVA to be non-superior to desflurane in patients undergoing LRYGB or LSG, but the researchers posit that the lack of difference may be attributed to short operating time such that the duration of anesthesia was too small to detect a difference between the procedural groups [21]. In contrast, Ziemann-Gimmel et al. were more inclusive of the bariatric surgical landscape, including LRYGB, LSG, laparoscopic gastric banding, revision, and conversion procedures [18]. The SAMBA Guidelines promote the reduction of baseline PONV risk through the preferential use of TIVA with propofol throughout surgery, avoidance of volatile anesthetics, minimization of perioperative opioids, and via adequate hydration with intravenous fluids. Only one study in our review addressed the relationship of PONV and intravenous fluids in the bariatric cohort and found reduced PONV incidence with higher volume and rate of fluid administration, consistent with guidelines for general high-risk surgical patients [28]. The optimization of intravenous fluid dosing and rates in the bariatric cohort remains a potential area of study.
This review confirms the applicability of SAMBA Guidelines to bariatric surgery patients and the benefits of a multimodal approach to PONV prevention. Considering the innately higher PONV risk associated with bariatric surgical procedures, the relatively low cost of commonly used antiemetics, and the advantages of dual- and triple-agent approaches, especially in combination with opioid-free TIVA, providers should consider offering prophylaxis with the described drug combinations to bariatric patients at a lower threshold than they would to their non-bariatric counterparts [42].
Limitations
The present study has several limitations. Firstly, the sample size was small with 21 studies, but this is reflective of the limited literature proposing interventions targeted towards PONV reduction in bariatric surgery patients. Even with this small sample size, it was difficult to derive comparative statistics across studies due to heterogeneity in outcomes reporting. We excluded studies on ERAS protocols or analgesic interventions to ensure a focused study sample. The pain usually occurs alongside PONV, and pain control optimization may be a useful adjunct in PONV management in bariatric patients, a concept which can be explored in future reviews [43,44,45]. An evaluation of pan-institutional ERAS protocols would be similarly valuable because many of these protocols consist of PONV prevention management as one component [46,47,48]. Lastly, the strength of the systematic review is, of course, contingent on the strength of the individual studies included. Most of the studies were based at a single institution; approximately 40% of the studies were observational in nature, and a minority were underpowered for subgroup analysis. Despite these limitations, this is the first systematic review to survey PONV prevention strategies in bariatric surgery patients and, as such, provides insights which can be leveraged in developing population-specific guidelines.
Conclusions
Despite recent pharmacological advances, PONV remains a significant cause of perioperative morbidity and resource utilization for bariatric surgery patients. In this systematic review of antiemetic and anesthetic approaches to minimize PONV incidence in bariatric patients, we found the following:
-
1.
There is great heterogeneity in the tools used in PONV evaluation. There remains a need to develop a standardized validated PONV assessment tool. The PONV Impact Scale and visual analog scale, which are already being used in the bariatric literature, present two options, but external validation testing of these instruments is a prerequisite to widespread adaptation. Standardization of an assessment instrument will facilitate data comparisons and statistical analyses of PONV outcomes data.
-
2.
Although the multimodal pharmacological approach to PONV prevention proposed in the SAMBA Guidelines for high-risk surgical patients is appropriate for the bariatric cohort, there is a significant lack of evidence highlighting the single best approach. Based on the data analyzed herein, in bariatric patients, prophylactic approaches utilizing dexamethasone in combination with one or more agents from other classes (multi-agent pharmacotherapy) are likely to be effective in reducing PONV rate and rescue antiemetic use. Interestingly, the addition of ondansetron to dexamethasone in the bariatric subset yielded variable efficacy across studies.
-
3.
While relatively novel, aprepitant 80 mg PO plus ondansetron 4 mg IV demonstrated efficacy in the bariatric cohort and should be considered as a prophylactic option in future updates to SAMBA guidelines. Currently, only the 40 mg PO aprepitant dose is FDA approved for PONV prevention and included in the recommendations.
-
4.
Opioid-free TIVA was more effective than inhalational anesthetics in reducing PONV in one RCT, but the non-superiority of TIVA was demonstrated in another RCT. While the literature seems to favor the former findings, additional research specific to the bariatric cohort is needed to corroborate the value of TIVA.
References
Mendes MN, Monteiro RS, Martins FANC. Prophylaxis of postoperative nausea and vomiting in morbidly obese patients undergoing laparoscopic gastroplasties. A comparative study among three methods. Rev Bras Anestesiol. 2009;59(5):570–6.
Fathy M, Abdel-Razik MA, Elshobaky A, et al. Impact of pyloric injection of magnesium sulfate-lidocaine mixture on postoperative nausea and vomiting after laparoscopic sleeve gastrectomy: a randomized-controlled trial. Obes Surg. 2019;29(5):1614–23.
Celio A, Bayouth L, Burruss MB, et al. Prospective assessment of postoperative nausea early after bariatric surgery. Obes Surg. 2019;29(3):858–61.
Markic J, Ridge AL. Promethazine in the treatment of postoperative nausea and vomiting: a systematic review. Signa Vitae. 2011;6(2):9–16.
Apfel CC, Laara E, Koivuranta M, et al. A simplified risk score for predicting postoperative nausea and vomiting. Anesthesiology. 1999;91:693–700.
Gan TJ, Diemunsch P, Habib AS, et al. Consensus guidelines for the management of postoperative nausea and vomiting. Anesth Analg. 2014;118(1):85–113.
Sinha AC, Singh PM, Williams NW, et al. Aprepitant’s prophylactic efficacy in decreasing postoperative nausea and vomiting in morbidly obese patients undergoing bariatric surgery. Obes Surg. 2013;24(2):225–31.
Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097.
Groene P, Eisenlohr J, Zeuzem C, et al. Postoperative nausea and vomiting in bariatric surgery in comparison to non-bariatric gastric surgery. Wideochir Inne Tech Maloinwazyjne. 2019;14(1):90–5.
Moussa AA, Oregan PJ. Prevention of postoperative nausea and vomiting in patients undergoing laparosopic bariatric surgery: granisetron alone vs granisetron combined with dexamethasone/droperidol. MEJ Anesth. 2007;19(2):357–68.
Benevides ML, Oliveira SS, de Aguilar-Nascimento JE. The combination of haloperidol, dexamethasone, and ondansetron for prevention of postoperative nausea and vomiting in laparoscopic sleeve gastrectomy: a randomized double-blind trial. Obes Surg. 2013;23(9):1389–96.
Didehvar S, Viola-Blitz JD, Haile M, et al. A randomized, double blind study to evaluate the efficacy of palonosetron with dexamethasone versus palonosetron alone for prevention of post-operative nausea and vomiting in subjects undergoing bariatric surgeries with high emetogenic risk. The Open Anesthesiology Journal. 2013;7:30–6.
Bataille A, Letourneulx JF, Charmeau A, et al. Impact of a prophylactic combination of dexamethasone-ondansetron on postoperative nausea and vomiting in obese adult patients undergoing laparoscopic sleeve gastrectomy during closed-loop propofol-remifentanil anaesthesia: a randomised double-blind placebo-controlled study. Eur J Anaesthesiol. 2016;33(12):898–905.
Halliday TA, Sundqvist J, Hultin M, et al. Post-operative nausea and vomiting in bariatric surgery patients: an observational study. Acta Anaesthesiol Scand. 2017;61(5):471–9.
Therneau IW, Martin EE, Sprung J, et al. The role of aprepitant in prevention of postoperative nausea and vomiting after bariatric surgery. Obes Surg. 2017;28(1):37–43.
Zhao LP, Zou LJ, He JH. Postoperative vomiting/nausea in Chinese patients undergoing bariatric surgery. Trop J Pharm Res. 2019;18(10):2211–7.
Suh S, Helm M, Kindel TL, Goldblatt MI, Gould JC, Higgins RM. The impact of nausea on post-operative outcomes in bariatric surgery patients. Surg Endosc. 2019.
Ziemann-Gimmel P, Goldfarb AA, Koppman J, et al. Opioid-free total intravenous anaesthesia reduces postoperative nausea and vomiting in bariatric surgery beyond triple prophylaxis. Br J Anaesth. 2014;112(5):906–11.
Nordin L, Nordlund A, Lindqvist A, et al. Corticosteroids or not for postoperative nausea: a double-blinded randomized study. J Gastrointest Surg. 2016;20(8):1517–22.
Boogaerts JG, Vanacker E, Seidel L, et al. Assessment of postoperative nausea using a visual analogue scale. Acta Anaesthesiol Scand. 2000;44:470–4.
Aftab H, Fagerland MW, Gondal G, et al. Pain and nausea after bariatric surgery with total intravenous anesthesia versus desflurane anesthesia: a double blind, randomized, controlled trial. Surg Obes Relat Dis. 2019;15(9):1505–12.
Bamgbade OA, Oluwole O, Khaw RR. Perioperative antiemetic therapy for fast-track laparoscopic bariatric surgery. Obes Surg. 2018;28(5):1296–301.
Ziemann-Gimmel P, Hensel P, Koppman J, et al. Multimodal analgesia reduces narcotic requirements and antiemetic rescue medication in laparoscopic Roux-en-Y gastric bypass surgery. Surg Obes Relat Dis. 2013;9(6):975–80.
Myles PS, Wengritzky R. Simplified postoperative nausea and vomiting impact scale for audit and post-discharge review. Br J Anaesth. 2012;108(3):423–9.
Talebpour M, Ghiasnejad Omrani N, Imani F, et al. Comparison effect of promethazine/dexamethasone and metoclopramide /dexamethasone on postoperative nausea and vomiting after laparascopic gastric placation: a randomized clinical trial. Anesth Pain Med. 2017;7(4):e57810.
Lindley CM, Hirsch JD, O'Neill CV, et al. Quality of life consequences of chemotherapy-induced emesis. Qual Life Res. 1992;1(5):331–40.
Alimian M, Abedian AE, Entezari S, et al. Comparison of the effect of intraoperative 1 mg/kg/h and 2 mg/kg/h IV lidocaine infusion on postoperative pain and nausea-vomiting in laparoscopic gastric bypass surgery. Journal of Pharmaceutical Research International. 2019:1–9.
Schuster R, Alami RS, Curet MJ, et al. Intra-operative fluid volume influences postoperative nausea and vomiting after laparoscopic gastric bypass surgery. Obes Surg. 2006;16:848–51.
Hill RP, Lubarsky DA, Phillips-Bute B, et al. Cost-effectiveness of prophylactic antiemetic therapy with ondansetron, droperidol, or placebo. Anesthesiology. 2000;92:958–67.
Kim EJ, Ko JS, Kim CS, et al. Combination of antiemetics for the prevention of postoperative nausea and vomiting in high-risk patients. J Korean Med Sci. 2007;22:878–82.
Delgado DA, Lambert BS, Boutris N, et al. Validation of digital visual analog scale pain scoring with a traditional paper-based visual analog scale in adults. J Am Acad Orthop Surg Glob Res Rev. 2018;2(3):e088.
Rhodes VA, McDaniel RW. The index of nausea, vomiting, and retching: a new format of the index of nausea and vomiting. Oncol Nurs Forum. 1999;26(5):889–94.
Afaneh C, Costa R, Pomp A, et al. A prospective randomized controlled trial assessing the efficacy of omentopexy during laparoscopic sleeve gastrectomy in reducing postoperative gastrointestinal symptoms. Surg Endosc. 2015;29(1):41–7.
Hauser JM, Azzam JS, Kasi A. Antiemetic Medications. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; [Updated 2019 Nov 25].
Pierre S, Whelan R. Nausea and vomiting after surgery. Continuing Education in Anaesthesia Critical Care & Pain. 2013;13(1):28–32.
Bergese SD, Antor MA, Uribe AA, et al. Triple therapy with scopolamine, ondansetron, and dexamethasone for prevention of postoperative nausea and vomiting in moderate to high-risk patients undergoing craniotomy under general anesthesia: a pilot study. Front Med (Lausanne). 2015;2:40.
Chatterjee S, Rudra A, Sengupta S. Current concepts in the management of postoperative nausea and vomiting. Anesthesiol Res Pract. 2011;2011:748031.
Antor MA, Uribe AA, Erminy-Falcon N, et al. The effect of transdermal scopolamine for the prevention of postoperative nausea and vomiting. Front Pharmacol. 2014;5
Apfel CC, Heidrich FM, Jukar-Rao S, et al. Evidence-based analysis of risk factors for postoperative nausea and vomiting. Br J Anaesth. 2012;109(5):742–53.
Apfel CC, Stoecklein K, Lipfert P. PONV: a problem of inhalational anaesthesia? Best Pract Res Clin Anaesthesiol. 2005;19(3):485–500.
Junger A, Hartmann B Fau-Benson M, Benson M Fau-Schindler E, et al. The use of an anesthesia information management system for prediction of antiemetic rescue treatment at the postanesthesia care unit. (0003-2999 (Print)).
White PF, O'Hara JF, Roberson CR, et al. The impact of current antiemetic practices on patient outcomes: a prospective study on high-risk patients. Anesth Analg. 2008;107(2):452–8.
Alimian M, Imani F, Faiz SH, et al. Effect of oral pregabalin premedication on post-operative pain in laparoscopic gastric bypass surgery. Anesth Pain Med. 2012;2(1):12–6.
Wojcikiewicz TG, Jeans J, Karmali A, et al. The use of high-dose intrathecal diamorphine in laparoscopic bariatric surgery: a single-centre retrospective cohort study. Br J Pain. 2019;13(2):106–11.
Symons JL, Kemmeter PR, Davis AT, et al. A double-blinded, prospective randomized controlled trial of intraperitoneal bupivacaine in laparoscopic Roux-en-Y gastric bypass. J Am Coll Surg. 2007;204(3):392–8.
Ruiz-Tovar J, Garcia A, Ferrigni C, et al. Impact of implementation of an enhanced recovery after surgery (ERAS) program in laparoscopic Roux-en-Y gastric bypass: a prospective randomized clinical trial. Surg Obes Relat Dis. 2019;15(2):228–35.
Major P, Stefura TA, Malczak P, et al. Postoperative care and functional recovery after laparoscopic sleeve gastrectomy vs. laparoscopic Roux-en-Y gastric bypass among patients under ERAS protocol. Obes Surg. 2018;28(4):1031–9.
King AB, Spann MD, Jablonski P, et al. An enhanced recovery program for bariatric surgical patients significantly reduces perioperative opioid consumption and postoperative nausea. Surg Obes Relat Dis. 2018;14(6):849–56.
Acknowledgments
The authors thank Ms. Jessica Koos, MLS, MSEd, AHIP, Health Sciences Librarian at Stony Brook University, for insights on the search strategy.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Dr. Naeem and Dr. Chen report no conflicts of interest. Dr. Pryor reports personal fees from Ethicon, personal fees from Medtronic, personal fees from Stryker, personal fees from Gore, personal fees from Merck, grants from Baranova, grants from Obalon, outside the submitted work. Dr. Docimo reports personal fees from Boston Scientific, outside the submitted work. Dr. Gan reports personal fees from Acacia, personal fees from Merck, personal fees from Masimo, personal fees from Medtronic, outside the submitted work. Dr. Spaniolas reports grants from Merck, personal fees from Biom-Up, outside the submitted work.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Naeem, Z., Chen, I.L., Pryor, A.D. et al. Antiemetic Prophylaxis and Anesthetic Approaches to Reduce Postoperative Nausea and Vomiting in Bariatric Surgery Patients: a Systematic Review. OBES SURG 30, 3188–3200 (2020). https://doi.org/10.1007/s11695-020-04683-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11695-020-04683-1