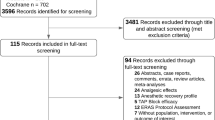
Abstract
Background
Postoperative nausea and vomiting (PONV) is problematic in bariatric surgery patients and has negative impacts on perioperative outcome. Antiemetic prophylaxis may reduce PONV. Perioperative antiemetic prophylaxis or therapy is crucial and may enhance fast-track bariatric surgery. This study examined the impact of intraoperative multimodal antiemetic prophylaxis on fast-track bariatric surgery.
Methods
This prospective observational clinical study explored the perioperative data of 400 consecutive laparoscopic bariatric surgery patients, over a 6-year period. Perioperative outcomes and variables were analyzed and compared between different intraoperative antiemetic modes.
Results
The mean BMI was 49, mean age was 42, and male:female ratio was 1:4. About 70% of patients received intraoperative multimodal antiemetic, comprising combinations of prochlorperazine, dexamethasone, ondansetron, or cyclizine. PONV occurred in 19.5% of patients. Intraoperative multimodal antiemetic was associated with significantly less PONV, shorter post-anesthesia care unit duration, earlier postoperative drinking, and shorter hospital stay (p = 0.001). Compared to other multimodal antiemetic modes, dexamethasone + cyclizine + prochlorperazine provided the best prophylaxis and outcome: p = 0.002.
Conclusion
PONV is a common and peculiar problem in bariatric surgery patients. However, intraoperative multimodal antiemetic prophylaxis effectively minimizes PONV. Intraoperative multimodal antiemetic enhances fast-track bariatric surgical care, patient satisfaction, and perioperative outcomes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Fast-track laparoscopic bariatric surgery is a popular and efficacious treatment for morbid or complex obesity [1,2,3,4]. Bariatric surgery patients present major perioperative challenges and have many factors that potentially hinder their postoperative recovery or outcome [5,6,7,8]. Postoperative nausea and vomiting (PONV) may be significant; with adverse impacts on recovery, analgesia, gastrointestinal function, ambulation, activity, patient satisfaction, and length of hospital stay [6, 9,10,11]. Antiemetic prophylaxis may minimize PONV. Therefore, optimal perioperative antiemetic prophylaxis is crucial and may facilitate fast-track bariatric surgery. There is a dearth of studies regarding intraoperative antiemetic prophylaxis in bariatric surgery, and the impact on perioperative outcome. This study examined the overall impact of intraoperative multimodal antiemetic prophylaxis on fast-track bariatric surgery.
Methods
This large prospective observational clinical study was registered and authorized by the research unit of Central Manchester University Hospital, Manchester, England. The perioperative outcome data of consecutive bariatric surgery patients were recorded prospectively from March 2007 to January 2013. The patients underwent laparoscopic Roux-en-Y gastric bypass (LRYGB) under general anesthesia. LRYGB was performed by two surgeons, using similar methods, including five trocar sites (two 12 mm and three 5 mm), hand-sewn gastrojejunal anastomosis and trocar site suturing. No concurrent surgery was performed. Operative time was relatively similar for the patients, with mean duration of 104 min and median duration of 97 min.
Balanced general anesthesia, including modified rapid sequence induction, was provided by three anesthesiologists. Nitrous oxide was avoided in all cases. Patients received surgical site infiltration with bupivacaine + epinephrine, at start and completion of surgery. They also received intraoperative multimodal analgesia, comprising combinations of intravenous (IV) acetaminophen, non-steroidal anti-inflammatory drug (NSAID), and morphine or tramadol. Intraoperative antiemetic prophylaxis was administered based on the clinical judgment, knowledge, and experience of the anesthesiologist, comprising combinations of prochlorperazine 12.5 mg, dexamethasone 8 mg, ondansetron 4 mg, or cyclizine 50 mg. The 1st anesthesiologist routinely administered dexamethasone + cyclizine + ondansetron. The 2nd anesthesiologist routinely administered dexamethasone + cyclizine + prochlorperazine. The 3rd anesthesiologist administered dexamethasone + ondansetron to non-diabetic patients, but administered only ondansetron to diabetic patients, to avoid the possible hyperglycemic effect of dexamethasone.
In the post-anesthesia care unit (PACU) and surgical ward, antiemetic therapy included intramuscular (IM) prochlorperazine 12.5 mg 8 hourly, IV/IM cyclizine 50 mg 6 hourly, and IV/IM ondansetron 4 mg 6 hourly, as required. Postoperative analgesia comprised IV acetaminophen 1 g 6hourly and morphine patient-controlled analgesia (PCA). Postoperative drinking of water was encouraged, from the day of surgery.
Data collection included patient’s age, gender, body mass index (BMI), American Society of Anesthesiologists (ASA) physical status, comorbidities, gastro-esophageal reflux disease (GERD), surgery duration, perioperative monitoring, complications, PACU duration, and length of hospital stay. Additional data collection included intraoperative antiemetic, intraoperative analgesic, PONV score at PACU and 24 h, antiemetic requirement at PACU and 24 h, numeric pain score at PACU and 24 h, postoperative analgesic, and time to sustained postoperative drinking. Postoperative antiemetic requirement was measured by frequency and dose administered. Obesity was categorized per BMI as severe obesity = 36–39.9, morbid obesity = 40–49.9, and super obesity is BMI ≥ 50.
Data were analyzed with SPSS® version 20 (IBM Corp, Armonk, NY, USA). Descriptive statistics was performed using Student’s t test for continuous variables, and Pearson Chi-square test or Fisher’s Exact test for categorical variables. p value < 0.05 was considered statistically significant.
Results
Study Population Characteristics
A total of 400 patients were studied over the 6-year period. Age distribution is shown in Table 1, with age range of 21–64 years, mean of 42 (± 8) years, and median of 42 years. BMI and gender distribution is shown in Table 2, with BMI range of 39–80, mean of 49 (±7), median of 47, and male:female ratio of 1:4. The patients were a homogeneous adult population.
Intraoperative Antiemetic Prophylaxis
Intraoperative multimodal antiemetic prophylaxis was administered to 70% of patients: 25% received dexamethasone + cyclizine + ondansetron; 24% received dexamethasone + ondansetron; and 21% received dexamethasone + cyclizine + prochlorperazine. Intraoperative unimodal antiemetic prophylaxis comprising ondansetron was administered to 30% of patients. Table 3 shows the antiemetic distribution.
Intraoperative Antiemetic and PONV in PACU
In PACU, 19.5% of patients had PONV (Table 4). PONV was significantly less in patients who received multimodal antiemetic compared to patients who received unimodal ondansetron antiemesis: p = 0.001. Similarly, PONV was less in patients who received multimodal triple antiemetic compared to others: p = 0.001. Compared to other multimodal antiemetic modes, prophylaxis with dexamethasone + cyclizine + prochlorperazine provided the best outcome, and no patient in this group had PONV: p = 0.001.
Intraoperative Antiemetic and PACU Duration
PACU duration was categorized as the following: short ≤ 50 min, medium = 51–100 min, long = 101–150 min, and longest ≥ 151 min. PACU duration varied with intraoperative antiemetic mode (Table 5). Approximately 68.7% of patients had short PACU stay: this group mainly comprised patients who received multimodal antiemetic, and they had shorter PACU stay than patients who received unimodal antiemetic (p = 0.001). Multimodal prophylaxis with dexamethasone + cyclizine + prochlorperazine provided shorter PACU stay compared to other multimodal modes: p = 0.001.
Intraoperative Antiemetic and PONV at 24 h
PONV prevalence was less at 24 h compared to PACU: p = 0.02. PONV occurred in 9% of patients at 24 h (Table 6). Patients who received multimodal antiemetic had less PONV at 24 h, compared to patients who received unimodal antiemetic: p = 0.01. Compared to other multimodal antiemetic modes, prophylaxis with dexamethasone + cyclizine + prochlorperazine provided the best outcome, and no patient in this group had PONV at 24 h: p = 0.002.
Intraoperative Analgesia and PONV within 24 h
PONV occurred in 9% of patients within 24 h and varied with intraoperative analgesia mode (Table 7). Multimodal intraoperative analgesia was associated with lower PONV rates compared to unimodal morphine analgesia, and this was significant in patients who received multimodal antiemetic (p = 0.001). Compared to other multimodal analgesia modes, tramadol + acetaminophen + diclofenac provided the best outcome and least rate of PONV: p = 0.001.
Intraoperative Antiemetic and Sustained Postoperative Drinking
Approximately 87.3% of patients had sustained postoperative drinking on the day of surgery (day 0), and this varied with intraoperative antiemetic mode (Table 8). Patients who received multimodal antiemetic had better and earlier sustained postoperative drinking: p = 0.005. Compared to other multimodal antiemetic modes, prophylaxis with dexamethasone + cyclizine + prochlorperazine provided the best clinical outcome, and all the patients in this group had sustained postoperative drinking on day 0: p = 0.001.
Intraoperative Antiemetic and PONV in Patients with Gastro-esophageal Reflux Disease
Approximately 49% of patients had chronic gastro-esophageal reflux disease (GERD). Multimodal intraoperative antiemetic minimized PONV in patients with GERD: p = 0.007 (Table 9). Compared to other multimodal modes, prophylaxis with dexamethasone + cyclizine + prochlorperazine provided the best outcome and all the GERD patients in this antiemetic group did not have PONV: p = 0.007.
Length of Hospital Stay
Majority of patients (59%) were discharged home by 24 h. The length of hospital stay was associated with intraoperative antiemetic mode (Table 10). Multimodal intraoperative antiemetic comprising dexamethasone + cyclizine + prochlorperazine was associated with earlier postoperative drinking, ambulation, and discharge, compared to other antiemetic modes (p = 0.002).
Discussion
PONV is a major challenge in bariatric surgery patients, despite antiemetic prophylaxis [6, 9,10,11]. Despite intraoperative antiemetic, 19.5% of patients in our study experienced PONV in the PACU. However, this is a lower and better PONV rate than in previous bariatric surgery studies [9, 10, 12]. Multimodal antiemetic therapy has better efficacy and less side effects [10, 12, 13]. About 70% of patients in our study received multimodal intraoperative antiemetic, which enabled their favorable clinical outcome. Our study showed that multimodal antiemetic is associated with less PONV, shorter PACU stay, earlier postoperative drinking, and shorter hospital stay, and these are essential clinical and economic factors for fast-track bariatric surgical care [6, 11]. This confirms that multimodal antiemetic prophylaxis is advantageous and essential in bariatric surgery patients and corroborates other studies [10, 12, 13].
Multimodal antiemetic therapy involves the simultaneous use of different antiemetics that act via different physiologic pathways, to produce effective synergistic antiemesis, with less side effects [14]. In our study, it involved combinations of prochlorperazine, dexamethasone, ondansetron, or cyclizine. Cyclizine acts as an antihistamine and anticholinergic; prochlorperazine is a phenothiazine which acts as a dopamine receptor antagonist; and ondansetron acts as a 5-hydroxytryptamine-3 receptor antagonist [14]. Dexamethasone is a steroid which acts as a prostaglandin synthesis inhibitor, but may be associated with the transient or minor side effect of hyperglycemia [14].
Multimodal therapy may involve dual or triple antiemetics. Dual therapy comprising dexamethasone and ondansetron was used in 24% of our patients: it was better than unimodal ondansetron therapy, but less effective than triple therapy, as shown in other studies [9, 10, 12]. Triple therapy is the best approach and is more effective than unimodal or dual therapy because it involves more physiologic pathways [10, 13, 14]. Triple therapy was used in 46% of our patients and was greatly effective. Our study confirms that triple therapy is the best approach in bariatric patients and corroborates other studies [10, 13]. Our study also reveals that triple therapy comprising dexamethasone + cyclizine + prochlorperazine provide the best outcome in bariatric patients, and this is the first study to highlight this modality, which will be beneficial for fast-track bariatric surgical care.
GERD is common in bariatric surgery patients and may complicate PONV in affected patients. Our study shows a significant GERD prevalence rate of 49%; but it also shows that intraoperative multimodal antiemetic prophylaxis is effective in reducing PONV in bariatric patients with GERD. This is the first study to highlight this great benefit, which is an important factor in fast-track bariatric surgical care.
Intraoperative multimodal analgesia is associated with lower PONV rates compared to unimodal morphine analgesia [14, 15]. Our study confirms that multimodal intraoperative analgesia, especially triple therapy comprising tramadol + acetaminophen + diclofenac, significantly reduces PONV. Multimodal analgesia reduces postoperative pain, autonomic activity, and opioid requirement, with consequent reduction in PONV.
Our study is a reliable prospective clinical research, with significant population size and valid results. However, it may be relatively limited by the prospective observational methodology and time-consuming consecutive sample of patients. Larger randomized controlled studies of multimodal antiemetic therapy involving newer antiemetics, such as intravenous fosaprepitant, will be useful in bariatric perioperative care.
Conclusion
PONV is a common and major challenge in bariatric surgery patients. However, intraoperative multimodal antiemetic prophylaxis effectively minimizes PONV and associated complications. Intraoperative multimodal antiemetic enables optimal patient satisfaction, aids better perioperative outcomes, and facilitates fast-track bariatric surgical care.
References
Bamgbade OA, Adeogun BO, Abbas K. Fast-track laparoscopic gastric bypass surgery: outcomes and lessons from a bariatric surgery service in the United Kingdom. Obes Surg. 2012;22(3):398–402.
Dogan K, Kraaij L, Aarts EO, et al. Fast-track bariatric surgery improves perioperative care and logistics compared to conventional care. Obes Surg. 2015;25(1):28–35.
Bamgbade OA. Advantages of doxapram for post-anaesthesia recovery and outcomes in bariatric surgery patients with obstructive sleep apnoea. Eur J Anaesthesiol. 2011;28(5):387–8.
Lemanu DP, Srinivasa S, Singh PP, et al. Optimizing perioperative care in bariatric surgery patients. Obes Surg. 2012;22(6):979–90.
Bamgbade OA, Rutter TW, Nafiu OO, et al. Postoperative complications in obese and non-obese patients. World J Surg. 2007;31(3):556–60.
Weingarten TN, Hawkins NM, Beam WB, et al. Factors associated with prolonged anesthesia recovery following laparoscopic bariatric surgery: a retrospective analysis. Obes Surg. 2015;25(6):1024–30.
Bamgbade OA, Alfa JA. Dexmedetomidine anaesthesia for patients with obstructive sleep apnoea undergoing bariatric surgery. Eur J Anaesthesiol. 2009;26(2):176–7.
Bamgbade OA, Chung AS, Khalaf WM, et al. Survey of perioperative care of adults with obstructive sleep apnoea. Eur J Anaesthesiol. 2009;26(8):706–8.
Bataille A, Letourneulx JF, Charmeau A, et al. Impact of a prophylactic combination of dexamethasone-ondansetron on postoperative nausea and vomiting in obese adult patients undergoing laparoscopic sleeve gastrectomy during closed-loop propofol-remifentanil anaesthesia. Eur J Anaesthesiol. 2016;33(12):898–905.
Benevides ML, Oliveira SS, de Aguilar-Nascimento JE. The combination of haloperidol, dexamethasone, and ondansetron for prevention of postoperative nausea and vomiting in laparoscopic sleeve gastrectomy: a randomized double-blind trial. Obes Surg. 2013;23(9):1389–96.
Chen J, Mackenzie J, Zhai Y, et al. Preventing returns to the emergency department following bariatric surgery. Obes Surg. 2017;27(8):1986–92.
Mendes MN, Monteiro RS, Martins FA. Prophylaxis of postoperative nausea and vomiting in morbidly obese patients undergoing laparoscopic gastroplasties: a comparative study among three methods. Rev Bras Anestesiol. 2009;59(5):570–6.
Therneau IW, Martin EE, Sprung J, et al. The role of aprepitant in prevention of postoperative nausea and vomiting after bariatric surgery. Obes Surg. 2017; https://doi.org/10.1007/s11695-017-2797-0.
Ho KY, Chiu JW. Multimodal antiemetic therapy and emetic risk profiling. Ann Acad Med Singap. 2005;34(2):196–205.
Bamgbade OA, Oluwole O, Khaw RR. Perioperative analgesia for fast-track laparoscopic bariatric surgery. Obes Surg. 2017;27(7):1828–34.
Acknowledgements
This study was approved and registered by the research department of Central Manchester University Hospital, Manchester, England. Institutional support is acknowledged, but there was no conflict of interest or financial involvement regarding any of the authors.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Informed Consent
Informed consent was obtained from all individual participants in the study.
Statement of Human Rights
All procedures performed in this study were in accordance with the ethical standards of the institutional and national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Rights and permissions
About this article
Cite this article
Bamgbade, O.A., Oluwole, O. & Khaw, R.R. Perioperative Antiemetic Therapy for Fast-Track Laparoscopic Bariatric Surgery. OBES SURG 28, 1296–1301 (2018). https://doi.org/10.1007/s11695-017-3009-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11695-017-3009-7