Abstract
Neurological complications such as peripheral neuropathies are the most common complications among patients with morbid obesity following bariatric surgery. Reduction in nutrient intake especially thiamin may develop polyneuropathy, while neuropathic symptoms improved in patients with diabetes independent of glycemic control after bariatric surgery. The aim of the present review is to investigate the effect of bariatric surgery on peripheral neuropathy. Electronic literature search was done via scientific search engines. After the removal of duplicates and selection of articles of interest, 4 studies were included. A random effects model was applied in this meta-analysis. Considering the pooled analysis, bariatric surgery was significantly associated with Neuropathy Symptoms Score (NSS) (ES = − 3.393, 95% CI (− 4.507, − 2.278), and P value < 0.0001). Reduction in NSS for patients with type 2 diabetes and BMI < 35 kg/m2 who were insulin-dependent was more than patients with morbid obesity without diabetes. Furthermore, neuropathy disability score (NDS) significantly decreased in patients having bariatric surgery (ES = − 0.626, 95% CI (− 1.120, − 0.132), and P value < 0.013). The NDS significantly decreased in patients with type 2 diabetes and BMI < 35 kg/m2 treated with insulin as well as patients with morbid obesity and type 2 diabetes. In subgroup of patients with follow-up of more than 6 months after surgery, a significant reduction in NDS was detected while this reduction was not significant in patients with a follow-up of 6 months or less. Bariatric surgery had a positive effect on peripheral neuropathy, though many studies showed neuropathy as one of the complications of bariatric surgery.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The prevalence of obesity has been increasing with huge impact on society because of its associated morbidity and mortality [1]. The absence of effective treatment for obesity as well as development of laparoscopic surgery has resulted in noticeable number of bariatric surgery [2]. In bariatric surgery, restricted food intake and intestinal malabsorption lead to rapid, continuous, and remarkable weight loss [3]. Diabetes remission, reducing cardiovascular events, and mortality are other beneficial effects of bariatric surgery [4,5,6]. However, some complications are infrequently reported after surgical procedures [7]. Neurological complications are more recognized in some studies [2, 8, 9]. Peripheral neuropathies, as the most prevalent neurological complications, have been reported in about 16% of operated patients which may be due to nutritional deficiency such as thiamine deficiency [9, 10]. However, there are some other studies with improvement in neuropathic symptoms in patients with diabetes independent of glycemic control after bariatric surgery [11,12,13]. This may be due to oxidative, nitrosative, and carbonyl stress reduction following metabolic surgery, known to have an essential role in the underlying mechanisms of diabetic neuropathy [13]. Since the results of previous studies are contradictory, the present study aims to investigate the effects of bariatric surgery on peripheral neuropathy by a systematic review.
Materials and Methods
Search Strategy
An inclusive search following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [14] was carried out using the databases of PubMed, Web of Science, EMBASE, and Scopus to identify the English articles published until November 1, 2018. The following keywords were used in this search: (“bariatric surgery” OR “metabolic surgery” OR “obesity surgery” OR “sleeve OR Roux-en-Y” OR “gastric bypass” OR “duodenal switch” OR “gastric banding” OR “duodenal-jejunal bypass liner” OR “biliopancreatic diversion with duodenal switch” OR “biliopancreatic diversion” OR “adjustable gastric band” OR “duodenal switch” OR “lap band” OR “gastric balloon”) AND (“neuropathy” OR “axonal neuropathy” OR “polyneuropathy” OR “motor neuropathy” OR “sensory neuropathy” OR “autonomic neuropathy” OR “electromyography” OR “EMG” OR “nerve conduction velocity” OR “nerve conduction study”). Additionally, the references of the extracted articles were reviewed to obtain any other related articles.
Study Selection
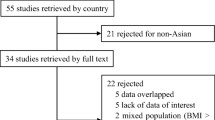
1994 articles were first isolated and the duplicates were removed; consequently, 1328 records were reviewed based on the title and abstract to see if they were eligible to be included in the project. Then, the full texts of 275 articles were reviewed and target articles were chosen based on the inclusion criteria. The inclusion criteria included the following: (1) cohort studies comparing the outcomes prior to and following the surgery; (2) studies on adult patients (18–80 years old); (3) studies on peripheral neuropathy; and (4) the studies issued in English. The exclusion criteria included the following: (1) animal studies; (2) inaccessibility to full-text articles; (3) randomized clinical trials, case reports, and review articles; and (4) studies on adolescents. Figure 1 depicts how the articles are chosen.
Data Extraction and Quality Assessment
Two authors extracted the data from each selected paper independently (A.M., and R.A.) according to the author’s name, publication year, journal name, study population, country of the study population, sex, sample size, procedure type, follow-up period, and outcomes prior to and following bariatric surgery. The same eligibility criteria were applied to the full-text articles. If the authors disagreed on the studies selection, they would solve it through discussion with another reviewer (M. M.). In addition, if some issues needed clarification, the authors were contacted. The kappa-statistics (κ) was calculated to determine the inter-reviewer agreement. Desirable agreement was achieved for the abstracts and titles (κ = 0.82), as well as for full-text screening (κ = 0.75). The nine-star Newcastle–Ottawa Scale (NOS) was used to assess each selected study regarding quality. The aforementioned scale involves 3 parts of participant selection, comparability of the study groups, and outcome assessment provided by follow-up adequacy [15].
Statistical Analysis
STATA, version 12.0 (STATA Corporation, College Station, TX, USA), was used for data analysis. The estimated effects of the outcomes of interest were determined by the mean difference (MD) and standard deviations (SDs). Cochran’s Q test (from chi-square) and the I2 statistic were used to evaluate the studies in terms of statistical heterogeneity; consequently, the inconsistency across the results of the studies was determined and the proportion of the total variations was explained according to their estimates based on the presence of heterogeneity rather than sampling errors. In detail, the I2 values of 0%, < 30%, 30–60%, and > 60% indicated no, low, moderate, and high heterogeneities, respectively [16].
The Egger’s test was used to evaluate publication bias, which was shown graphically by the funnel plots of mean difference vs. standard error. To deal with any likely small-study effects, the asymmetries of the funnel plots were inspected visually, whereas the Egger’s test was performed to address the publication bias over and above any subjective evaluations. P < 0.10 was considered statistically significant [17].
The analysis of all outcomes was done according to the surgical procedure, study population, and follow-up time. The procedures were classified into laparoscopic Roux-en-Y gastric bypass (LRGB) and vertical sleeve gastrectomy. Study population was classified as follows: insulin-dependent type 2 diabetes mellitus (T2DM) with BMI < 35 kg/m2, morbid obese patients, pre-T2DM morbid obese patients, and morbid obese patients with T2DM. The follow-up times were divided into more than 6 months and 6 months or less. For each outcome of interest, the related effect size (mean effect after the surgery minus mean effect before the surgery) was used in the analysis.
Results
Study Characteristics
After retrieving 1994 papers from the electronic databases, only 4 studies were selected in the meta-analysis following the exclusion of some because they were not consistent with the inclusion criteria (the Neuropathy Symptoms Score (NSS): n = 3, and the Neuropathy Disability Score (NDS): n = 4) (Fig. 1). Table 1 shows the key features. In the present study, the prospective human studies were only selected that were in English and their full texts were available. The shortest and longest follow-up periods were 1 month and 12 months, respectively. The quality scores of the included studies based on NOS had a range of 6–8.
Meta-analysis Results of the Outcomes of Interest
NSS (Overall Changes)
Three appropriate studies were covered in the analysis. To show the effect size prior to and following in each study and the pooled effect sizes, a forest plot was used (Fig. 2). A random effects model was applied to combine the results of the studies due to their heterogeneity (Q = 68.72, P < 0.0001, and I2 = 95.6%). According to the pooled analysis, there was an association between bariatric surgery and a significant decrease in the NSS (ES = − 3.393, 95% CI (− 4.507, − 2.278), and P value < 0.0001). Finally, there was no publication bias in the funnel plot (Fig. 3).
NSS (According to Population)
From among the included studies, 3 studies reported alterations in NSS prior to and following bariatric surgery. The pooled results of 4 sub-groups according to population demonstrated a significant decrease after bariatric surgery. The reduction in NSS for patients with type 2 diabetes and BMI < 35 kg/m2 who were insulin-dependent was 3.830 (95% CI = − 4.234, − 3.426 and P < 0.001). Moreover, a decrease of 2.904 (95% CI = − 4.864, − 0.944 and P value = 0.004) was detected in patients with morbid obesity without diabetes (Fig. 4). Significant heterogeneity was not found between the studies (I2 = 0.0%, P value = 0.585) performed in patients with type 2 diabetes and BMI < 35 kg/m2 treated with insulin, but high heterogeneity was observed in group of patients with morbid obesity without diabetes (I2 = 98.3%, P < 0.001).
NSS (According to the Procedure Type)
Considering the only one surgical procedure, the results of this subgroup were exactly the same as the results of overall change in this variable.
NSS (According to the Follow-up Time)
We found a significant reduction in NSS in both sub-groups of follow-up time (ES = − 3.864, 95% CI (− 4.114, − 3.615), P value < 0.0001, for sub-group of more than 6 months, and ES = − 2.949, 95% CI (− 5.239, − 0.659), P value = 0.012, for sub-group of 6 months or less). Test of heterogeneity showed that there was no significant heterogeneity in the sub-group of more than 6 months (I2 = 0.0%, P value = 0.707) whereas among studies with follow-up time of 6 months or less, significant heterogeneity was detected (I2 = 87.8%, P value = 0.004) (Fig. 5).
NDS (Overall Changes)
NDS was significantly reduced in patients having a bariatric surgery (ES = − 0.626, 95% CI (− 1.120, − 0.132), and P value < 0.013) (Fig. 6). Significant heterogeneity was observed between the studies (Q = 532.43, P value < 000.1) using the chi-square test before the analysis and pooling the results. To have the pooled estimates of the studies, the random effects model was used. Publication bias was found based on the funnel plot (Fig. 7).
NDS (According to Population)
Figure 8 illustrates the pooled results of 4 studies in the sub-group of population. NDS significantly decreased in patients with type 2 diabetes and BMI < 35 kg/m2 treated with insulin as well as patients with morbid obesity and type 2 diabetes (ES = − 2.174, 95% CI (− 3.093, − 1.255), and P value < 0.0001; ES = − 1.188, 95% CI (− 1.651, − 0.726), and P value < 0.0001, respectively). There was no heterogeneity among these studies for sub-group analysis (I2 = 62.4%, P value = 0.103 for patients with type 2 diabetes and BMI < 35 kg/m2 treated with insulin; and I2 = 51.8%, P value = 0.126 for patients with morbid obesity and type 2 diabetes).
NDS (According to the Procedure Type)
Four studies had reported NDS in the patients undergoing bariatric surgeries. Between two procedure types (LRYGB and vertical sleeve gastrectomy), the NDS decrease was only significant in vertical sleeve gastrectomy; however, NDS also declined in patients underwent LRYGB (ES = − 0.463, 95% CI (− 0.807, − 0.119), and P value = 0.008; ES = − 0.959, 95% CI (− 2.491, 0.573), and P value = 0.220, respectively) (Fig. 9). The results indicated a heterogeneity between the studies in both sub-groups (I2 = 99.1%, P value < 0.001 for LRYGB group and I2 = 93.3%, P value < 0.001 for vertical sleeve gastrectomy).
NDS (According to the Follow-up Time)
A significant reduction in NDS was seen in subgroup of patients with follow-up of more than 6 months after surgery (ES = − 1.570, 95% CI (− 3.015, − 0.126), and P value = 0.033), though slight and non-significant decrease was also observed in patients with follow-up of 6 months or less (ES = − 0.341, 95% CI (− 0.708, − 0.025), and P value = 0.068). A significant heterogeneity in both sub-groups were detected according to time of follow-up (I2 = 98.4%, P value < 0.001 for more than 6 months sub-group; I2 = 94.7%, P value < 0.001 for 6 months or less sub-group) (Fig. 10).
Discussion
Although we found that bariatric surgery had a positive effect on NSS and NDS overall as well as in all subgroups, several studies showed that neuropathy was a significant complication that may happen after bariatric surgery due to nutritional deficiencies [18, 19]. It can be described by the fact that serious deficiencies in a wide range of micronutrients are present in morbid obesity. Therefore, it seems that the excessive dietary intakes do not include nourishing food items [20,21,22]. Furthermore, nutrient deficiencies may also happen after bariatric surgery due to incorrect supplementation. Thus, morbid obese patients should have close nutritional monitoring before and after bariatric surgery.
Considering all mentioned above, improvement in neuropathy in this study may be indebted to multifactorial effects. It seems that oxidative, nitrosative, and carbonyl stress, which may cause diabetic neuropathy, improves after bariatric surgery [13]. The above mentioned findings are supported by other experimental evidence, as well. In a study that was conducted by Obrosova et al., it was found that targeting peroxy nitrite formation in an animal model of T1DM induced diabetic neuropathy; therefore, peroxynitrite decomposition reduces neuropathy [23].
Dyslipidemia may be another factor. Dyslipidemia results in high levels of oxidized low-density lipoprotein (oxLDLs) that may injure dorsal root ganglion (DRG) neurons via lectin-like oxLDL receptor-1 leading to the development and progression of diabetic neuropathy [24]. As different bariatric surgeries improve dyslipidemia independent of weight loss [25, 26], it can also affect neuropathy.
Hyperglycemia also induces mitochondrial oxidative stress as well as acute injury in DRG neurons [24]. Therefore, diabetes remission after bariatric surgery may also result in decreased risk of microvascular complications such as neuropathy even if type 2 diabetes relapses. This supports the “legacy effect” of bariatric surgery. Up to now, nonsurgical treatment for poor glycemic control had shown the “legacy effect” on microvascular complications [27].
Weight loss may be another factor leading to improvement in peripheral nerve function and nerve regeneration after bariatric surgery [28]. As obesity affects small fiber integrity, it can significantly increase risk for peripheral neuropathy, independent of glucose control because glucose control is specifically related with large myelinated fiber function [29].
There are some limitations in this meta-analysis. Because of small sample sizes and number of articles in each subgroup, it was not possible to investigate the publication bias. Moreover, significant heterogeneity was observed among studies; nevertheless, the data were analyzed in subgroups in the present study. Consequently, to correct them partly, a random effects model was applied.
Conclusion
In conclusion, current studies provide consistent evidence that bariatric surgery leads to neuropathy improvement. However, many other studies showed neuropathy as one of the complications following bariatric surgery.
References
Khosravi-Largani M, Nojomi M, Aghili R, et al. Evaluation of all types of metabolic bariatric surgery and its consequences: a systematic review and meta-analysis. Obes Surg. 2019;29(2):651–90.
Lin I-C, Lin Y-L. Peripheral polyneuropathy after bariatric surgery for morbid obesity. J Fam Community Med. 2011;18(3):162–4.
Buchwald H, Avidor Y, Braunwald E, et al. Bariatric surgery: a systematic review and meta-analysis. Jama. 2004;292(14):1724–37.
Carlsson LM, Peltonen M, Ahlin S, et al. Bariatric surgery and prevention of type 2 diabetes in Swedish obese subjects. N Engl J Med. 2012;367(8):695–704.
Sjöström L, Peltonen M, Jacobson P, et al. Bariatric surgery and long-term cardiovascular events. JAMA. 2012;307(1):56–65.
Adams TD, Davidson LE, Litwin SE, et al. Health benefits of gastric bypass surgery after 6 years. JAMA. 2012;308(11):1122–31.
Gasteyger C, Suter M, Gaillard RC, et al. Nutritional deficiencies after Roux-en-Y gastric bypass for morbid obesity often cannot be prevented by standard multivitamin supplementation. Am J Clin Nutr. 2008;87(5):1128–33.
Machado FCN, Valério BCO, Morgulis RNF, et al. Acute axonal polyneuropathy with predominant proximal involvement: an uncommon neurological complication of bariatric surgery. Arq Neuropsiquiatr. 2006;64(3A):609–12.
Thaisetthawatkul P, Collazo-Clavell M, Sarr M, et al. A controlled study of peripheral neuropathy after bariatric surgery. Neurology. 2004;63(8):1462–70.
Chaves LCL, Faintuch J, Kahwage S, et al. A cluster of polyneuropathy and Wernicke-Korsakoff syndrome in a bariatric unit. Obes Surg. 2002;12(3):328–34.
Azmi S, Alam U, Ferdousi M, et al. Bariatric surgery improves neuropathic symptoms, deficits, and corneal nerve morphology in obese patients with diabetes. Diabetes. 2017;66:A550. English
Casellini CM, Parson HK, Hodges K, et al. Bariatric surgery restores cardiac and sudomotor autonomic C-fiber dysfunction towards normal in obese subjects with type 2 diabetes. PLoS One. 2016;11(5):e0154211.
Müller-Stich BP, Billeter AT, Fleming T, et al. Nitrosative stress but not glycemic parameters correlate with improved neuropathy in nonseverely obese diabetic patients after roux-Y gastric bypass. Surg Obes Relat Dis. 2015;11(4):847–54.
Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med. 2009;6(7):e1000100.
Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25(9):603–5.
Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ Br Med J. 2003;327(7414):557–60.
Sterne JA, Egger M, Smith GD. Systematic reviews in health care: investigating and dealing with publication and other biases in meta-analysis. BMJ. 2001;323(7304):101–5.
Alsabah A, Al Sabah S, Al-Sabah S, et al. Investigating factors involved in post laparoscopic sleeve gastrectomy (LSG) neuropathy. Obes Surg. 2017;27(5):1271–6.
Malek M, Yousefi R, Safari S, et al. Dietary intakes and biochemical parameters of morbidly obese patients prior to bariatric surgery. Obes Surg. 2019;29(6):1816–22.
Al-Mutawa A, Anderson A, Alsabah S, et al. Nutritional status of bariatric surgery candidates. Nutrients. 2018;10(1):67.
Bordalo LA, Teixeira TFS, Bressan J, et al. Bariatric surgery: how and why to supplement. Rev Assoc Méd Bras (Engl Ed). 2011;57(1):111–8.
Frame-Peterson LA, Megill RD, Carobrese S, et al. Nutrient deficiencies are common prior to bariatric surgery. Nutr Clin Pract. 2017;32(4):463–9.
Obrosova IG, Mabley JG, Zsengellér Z, et al. Role for nitrosative stress in diabetic neuropathy: evidence from studies with a peroxynitrite decomposition catalyst. FASEB J. 2005;19(3):401–3.
Vincent AM, Hayes JM, McLean LL, et al. Dyslipidemia-induced neuropathy in mice: the role of oxLDL/LOX-1. Diabetes. 2009;58(10):2376–85.
Al Khalifa K, Al Ansari A, Alsayed AR, et al. The impact of sleeve gastrectomy on hyperlipidemia: a systematic review. J Obes. 2013;2013:1–7.
Spivak H, Sakran N, Dicker D, et al. Different effects of bariatric surgical procedures on dyslipidemia: a registry-based analysis. Surg Obes Relat Dis. 2017;13(7):1189–94.
Coleman KJ, Haneuse S, Johnson E, et al. Long-term microvascular disease outcomes in patients with type 2 diabetes after bariatric surgery: evidence for the legacy effect of surgery. Diabetes Care. 2016;39(8):1400–7.
Smith AG, Graham T, Volckmann E, et al. Bariatric surgery improves peripheral nerve function and intraepidermal nerve fiber density in obese patients without symptomatic neuropathy (P1. 144). AAN Enterprises. 2016;86(16 Supplement):P1.144.
Smith AG, Singleton JR. Obesity and hyperlipidemia are risk factors for early diabetic neuropathy. J Diabetes Complicat. 2013;27(5):436–42.
Funding
This study was funded (grant No. 97-4-75-12930) and supported by Iran University of Medical Sciences.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Ethical Approval Statement
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed Consent Statement
Does not apply.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Aghili, R., Malek, M., Tanha, K. et al. The Effect of Bariatric Surgery on Peripheral Polyneuropathy: a Systematic Review and Meta-analysis. OBES SURG 29, 3010–3020 (2019). https://doi.org/10.1007/s11695-019-04004-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11695-019-04004-1