Abstract
This systematic review and meta-analysis intend to evaluate the efficacy of metabolic/bariatric surgeries (MBS) in patients with type-1 diabetes mellitus. A systematic literature search and meta-analysis were performed in electronic databases up to July 2021. In total, 27 primary studies comprising 648 subjects were included in this systematic review and meta-analysis. Patients had a mean age of 38.0 ± 7.3 years. Preoperative mean BMI was 42.6 ± 4.7 kg/m2 and 29.4 ± 4.7 kg/m2 after surgery, respectively. Following bariatric surgeries in patients with type 1 diabetes mellitus, insulin (unit/day) decreased by a weighted mean difference (WMD) of − 10.59. Also, insulin (unit/kg/day) decreased by a WMD of − 0.2, and HbA1C decreased by a WMD of − 0.71, showing MBS acceptable and durable effects of bariatric surgical procedures.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Nowadays, metabolic/bariatric surgery (MBS) is a well-established treatment for type 2 diabetes (T2DM) in severely obese patients, proven to have a greater substantial effect in the long-term remission of T2DM and durable weight loss compared to conventional medical treatment [1, 2]. Nevertheless, the role of MBS in type 1 diabetes (T1DM) remains debated, and with limited existing data in the current literature.
T1DM is a complex autoimmune disease resulting from cytotoxic T-cells attack to insulin-secreting beta cells [3, 4]. The genetic, epigenetic, and environmental factors and immune effects have a role in T1DM pathogenesis and can lead to its presentation at any age (even advanced ages). However, it mainly occurs in children and adolescents under 20 years old [4, 5]. The most common form of T1DM in adults is latent autoimmune diabetes (LADA), a subset that may have clinical features of T2DM with absolute insulin deficiency [6]. The incidence of T1DM has increased 3–4% in the last three decades. An increased emphasis on the achievement of glycemic goals and intensive insulin regimens has contributed to the propensity for weight gain. Thus, approximately 50% of patients with T1DM are currently obese or overweight [5].
The greatest challenge in translating the benefits of MBS to individuals with T1DM is the lack of well-designed clinical trials. Despite the positive relationship between childhood obesity and T1DM [7], due to lack of enough beta cells reserve, the decreased peripheral resistance after MBS may not be so effective compared to results in T2DM, and the role of MBS in T1DM is still contradictory [8,9,10,11,12]. This systematic review and meta-analysis intend to evaluate the efficacy of MBS in patients with T1DM.
Methods
Data Sources
A comprehensive search of electronic databases including PubMed, EMBASE, and SCOPUS up to July 2021 was completed. Title searching was restricted to include “type 1 diabetes mellitus” in conjunction with the following keywords/terms: bariatric, gastric bypass, gastric band, and sleeve gastrectomy. To find any additional related research, a manual search of the reference lists of relevant publications was conducted.
Selection Criteria
Abstracts were examined by two independent reviewers and selected based on the following inclusion criteria: human studies, primary bariatric surgery performed diagnosis of T1DM at baseline confirmed by the presence of pancreatic autoantibodies (islet cell or glutamic acid decarboxylase), documented history of diabetic ketoacidosis (DKA), and/or insulin therapy required from the time of diagnosis. Articles were excluded if they were letters, comments, and conference abstracts or published in abstract form only. Using the same filtering criteria, full papers for all selected abstracts were screened more carefully.
Data Extraction
The relevant data were collected from full-text articles for all selected abstracts by two independent reviewers and differences were resolved by consensus. Although there was more than one publication from the same study population, only the most recent findings were included in the final analysis. Preoperative and postoperative HbA1c and insulin requirements were the primary outcomes of interest.
Statistical Analysis
Descriptive categorical data were expressed as percentage, and continuous data were expressed as weighted mean difference (WMD). In this meta-analysis, continuous variables including HbA1C and insulin need to be measured before and after bariatric surgery, where the mean, standard deviation, and sample size in each group were known. The weight given to each study (how much influence each study has on the overall results of the meta-analysis) was determined by the precision of its estimate of effect and was equal to the inverse of the variance. This method assumes that all of the studies have measured the outcome on the same scale for each meta-analysis. The weighted mean difference was calculated for groups before and after bariatric surgery, and it was the difference between start and finish values. Meta-analysis was used to compare glycemic control indicators (HbA1c and total daily insulin requirement) where the relevant data were available. The estimated effects have been calculated using STAT version 14 software. The random-effects method was used in our analysis. All included studies were evaluated for heterogeneity.
Results
Study Selection and Characteristics
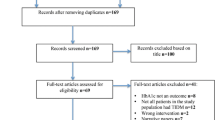
Preliminary searches of electronic databases identified 478 potentially relevant articles. All re-publication studies were excluded. After screening abstracts against the selection criteria, 27 full-text articles were selected and evaluated for eligibility. In total, 27 primary studies comprising 648 subjects were included in this systematic review and meta-analysis (Fig. 1). Included studies were published between February 1981 and July 2021; 2 were case series and 3 were case reports (Table 1).
Baseline Patient Demographics
In this review, 648 patients were included in this analysis. Subjects had a mean age of 38.0 ± 7.3 years. Preoperative mean BMI was 42.6 ± 4.7 kg/m2 and 29.4 ± 4.7 kg/m2 after surgery, respectively. Follow-up time had a mean of 32.6 ± 23.2 months. The most common procedure for MBS was Roux-en-Y gastric bypass (RYGB) accounting for 72.5% of procedures (n = 470).
Complications
Complications were reported for 41 patients. These included multiple hypoglycemic episodes, DKA, deep vein thrombosis (DVT), gastro-jejunal ulcer, esophageal motility disorder, persistent nausea, gastric fistula, incisional hernia, stenosis, leak, intestinal obstruction, and one vitreous hemorrhage. No mortality was reported in any of the studies.
Meta-analysis of Insulin Requirement Outcomes (unit/kg/day or unit/kg/day)
A random-effect model was also used to measure the effectiveness of the WMD. Based on the results of the included studies, bariatric surgeries decrease insulin doses (unit/day) by a WMD of − 10.59 (Fig. 2).
Figure 3 shows that bariatric surgeries decrease insulin doses (unit/kg/d) by a WMD of − 0.21 (Fig. 3).
Meta-Analysis of Hb1Ac Outcomes
Figure 4 shows how bariatric surgeries have been effective in reducing HbA1C. Nonetheless, HbA1C decreased by a WMD of − 0.71, showing the acceptable and durable effects of bariatric surgeries (Fig. 4).
Forest plot showing the effect of bariatric surgery on HbA1c [32]
Publication Bias
The analysis results showed that biased publication did not influence the creation of negative results, which is shown as symmetry in the funnel plot. Meanwhile, no evidence of publication bias was detected using Egger’s test (Egger’s test t = − 0.37, P = 0.722, 95% CI: − 5.132 to 3.739).
Discussion
Severe obesity is rapidly increasing worldwide; accordingly, obesity is now considered an epidemic. Additionally, numerous significant comorbidities may accompany severe obesity, such as diabetes, metabolic syndrome, and hypertension, which, as evidence would suggest, significantly improve after bariatric surgery [35]. It has been shown that MBS facilitates and improves the management of T2DM [1, 36]. However, it was only until recently that T1DM was associated with obesity [37]. The increased prevalence of severe obesity and metabolic syndrome among T1DM patients, with the cardiovascular disease becoming their leading cause of death, has led clinicians to consider MBS for severely obese T1DM patients [38]. Indeed, MBS is recognized to be effective for both metabolic syndrome and cardiovascular disease [39, 40]. Therefore, since MBS seems to improve the lives of obese T1DM patients, it is of great importance to analyze and interpret the results of several studies performed on this subject. Consequently, significant focus has been given during the last years by the medical and surgical community on the potential beneficial effect of MBS for patients suffering from T1DM.
This systematic review and meta-analysis found 27 eligible studies, including data from 648 T1DM patients undergoing bariatric surgery for severe obesity. Firstly, it demonstrates that MBS is safe and effective for T1DM severely obese patients, resulting in significant weight loss and without causing major postoperative metabolic complications. Secondly, it demonstrates that MBS is associated with a significant reduction in daily insulin requirement sustained in the long term. Insulin doses dropped dramatically (both absolute values and those indexed to weight), and heterogeneity was minimal in the included studies (lowest quality studies were excluded at initial selection). Glycosylated hemoglobin (HbA1c) levels also significantly decreased by 66%. Some of the potential factors responsible for these results, regarding insulin requirements and HbA1c levels after bariatric surgery, could be a reduction in insulin resistance, improvement in the function of the remaining beta cells, preservation of beta-cell mass, and/or increased hepatic insulin sensitivity. Furthermore, studies suggest that MBS in T1DM results in improved comorbidities related to obesity, including hypertension and dyslipidemia. Therefore, in patients with T1DM, it seems that through the metabolic changes achieved by MBS, the effect of a sustainable and lasting caloric reduction is enabled, leading ultimately to weight loss. This is entirely the opposite of the currently common “overtherapy” with insulin in T1DM.
RYGB and biliopancreatic diversion with duodenal switch (BPD) obtain the most impressive results. This can be partially attributed to the secretion of hormones improving glucose disposal, like incretins, mainly glucagon-like peptide 1 (GLP-1) and peptide YY (PYY). The highly-secreted gut hormones deliver the gastric content to the distal part of the small intestine [41]. Dirksen et al. [16] noticed a significant improvement in insulin requirement within a week after RYGB, which was associated with marked increases in postprandial GLP-1 and PYY secretions. The most potent of these anorexigenic hormones is GLP-1, a peptide secreted from L cells in the distal ileum and from neurons in the nucleus tractus solitarius of the caudal brain stem. GLP-1 affects the brain indirectly through vagal nerve fibers in the intestine, whereby it transmits various signals to the nucleus of the solitary tract (NTS), an area in the brain which plays a crucial role in regulating feeding patterns [42]. GLP-1 seems to induce satiety through slow gastric emptying and the nervous system’s action, resulting in significant weight loss. It also increases glucogenesis in hepatic and skeletal muscle cells. It further participates in a redistribution of overall body fat mass, while the visceral and intramuscular depot is also reduced [43]. GLP-1 has also been linked to increased insulin secretion by acting directly on the beta cell membrane receptors. Finally, it accelerates lipogenesis in adipocytes, which may result in improved insulin sensitivity [44]. However, one should not forget that diabetes improvement after MBS is also linked to numerous other mechanisms, such as intestinal microbiota changes, bile acids increase, and brain plasticity changes.
A thorough examination to uncover the most suitable T1DM patient group remains. We believe that MBS ought not routinely to be recommended to all T1DM patients. It seems that patients with LADA are the most appropriate candidates for surgery since the secretory burden on beta cells elicited by insulin resistance leads to their subsequent apoptosis if the burden is prolonged. In LADA cases, when MBS is conducted at the early stages of the disease, subsequent weight loss and metabolic effects preserve beta-cell mass and delay total insulin deficiency. Indeed, cases of remission of slowly progressive T1DM or insulin weaning in T1DM severely obese patients have been reported in the literature [22, 29], providing elements of support for the use of MBS for T1DM patients with LADA. For all other T1DM patients, a careful and multidisciplinary approach is necessary to define which patient is a good candidate for MBS.
The most common surgical procedure reported in this systematic review is RYGB, but data is insufficient to recommend any particular procedure. The spectacular results of RYGB in T2DM control have undoubtedly played a role in this procedural choice in T1DM patients. However, it is still unclear whether this is the most appropriate surgical procedure for patients with T1DM. BPD has demonstrated having had a more significant effect on weight reduction, insulin requirements, and resolution of dyslipidemia when compared to sleeve gastrectomy and that BPD compared to RYGB could lower the risk of postprandial glycemia and hyperinsulinemic hypoglycemia [22]. Hypoglycemia is a relatively frequent complication in T1DM patients undergoing bariatric surgery, reaching an incidence of even 70%. The majority of cases occur within the first few days to months after surgery. The stress of the surgical operation, dietary changes, food intolerance, and improvement in insulin sensitivity may result in an incorrect adjustment of insulin doses, and thus hypoglycemic episodes following surgery. Some authors suggest that sleeve gastrectomy may be a more attractive procedure for patients with T1DM because of more predictable carbohydrate absorption [19], even if hypoglycemic episodes are also observed with intensive insulin management of T1DM. Thus, a trend towards laparoscopic RYGB as a procedure of choice in patients in T1DM seems to exist, offering the best risk-to-benefit ratio. However, further randomized prospective studies have to be conducted in order to draw safe conclusions on this issue.
Diabetic ketoacidosis is a life-threatening complication of T1DM. In a study by Aminian et al. [45], the incidence of DKA in the early post-operative period in T1DM patients was reported to be as high as 25%. In insulinopenic T1DM patients, continuous exogenous insulin is required for the prevention of fatty acid breakdown into keto-acids. If insulin is held or severely reduced, for example, when oral intake is low in the postoperative period, life-threatening DKA may develop. In this setting, i.e., fasting state or low carbohydrate ingestion, DKA may occur even in the absence of significantly elevated blood glucose values. Therefore, special attention to insulin requirements and fluid-electrolyte status may be warranted. Input from endocrinologists during pre-and postoperative phases is necessary.
This study has several limitations, mainly due to the design of the analyzed studies. First, the level of evidence is low, considering only a small number of T1DM patients undergoing MBS have been recorded in current literature. Second, different bariatric surgical procedures are described for T1DM patients. Third, heterogeneity in the results and variability in follow-up may represent reporting biases leading to unsafe conclusions. Fourth, data on glycemic variability in T1DM patients undergoing MBS are not available in the current literature. Finally, the lack of comparative or prospective studies in the subject represents a significant interpretive limitation. Therefore, randomized, prospective trials are required to conclusively evaluate the effects of surgery in T1DM both in the short and long term and define the most suitable candidates who will benefit the most out of it.
Conclusion
The current study shows that MBS reduces postoperative insulin requirement and improves HbA1c in obese and severely obese T1DM patients. A careful multidisciplinary approach is necessary for each of these patients to determine whether weight reduction and benefits of obesity comorbidities outweigh the overall operation risks. High-volume randomized, prospective trials are necessary to elucidate the role of MBS in the treatment of severely obese patients with T1DM.
References
Ribaric G, Buchwald JN, McGlennon TW. Diabetes and weight in comparative studies of bariatric surgery vs conventional medical therapy: a systematic review and meta-analysis. Obes Surg. 2014;24(3):437–55.
Mingrone G, Panunzi S, De Gaetano A, et al. Metabolic surgery versus conventional medical therapy in patients with type 2 diabetes: 10-year follow-up of an open-label, single-centre, randomised controlled trial. Lancet (London, England). 2021;397(10271):293–304.
DiMeglio LA, Evans-Molina C, Oram RA. Type 1 diabetes. Lancet (London, England). 2018;391(10138):2449–62.
Cabrera SM, Chen YG, Hagopian WA, et al. Blood-based signatures in type 1 diabetes. Diabetologia. 2016;59(3):414–25.
Norris JM, Johnson RK, Stene LC. Type 1 diabetes-early life origins and changing epidemiology. Lancet Diabetes Endocrinol. 2020;8(3):226–38.
Liu B, Xiang Y, Liu Z, et al. Past, present and future of latent autoimmune diabetes in adults. Diabetes Metab Res Rev. 2020;36(1):e3205.
Verbeeten KC, Elks CE, Daneman D, et al. Association between childhood obesity and subsequent Type 1 diabetes: a systematic review and meta-analysis. Diabet Med. 2011;28(1):10–8.
Garciacaballero M, Martínez-Moreno JM, Toval JA, et al. Improvement of C peptide zero BMI 24–34 diabetic patients after tailored one anastomosis gastric bypass (BAGUA). Nutr Hosp. 2013;28(Suppl 2):35–46.
Blanco J, Jiménez A, Casamitjana R, et al. Relevance of beta-cell function for improved glycemic control after gastric bypass surgery. Surg Obes Relat Dis. 2014;10(1):9–13 (quiz 189-90).
Al Sabah S, Al Haddad E, Muzaffar TH, et al. Laparoscopic sleeve gastrectomy for the management of type 1 diabetes mellitus. Obes Surg. 2017;27(12):3187–93.
Czupryniak L, Wiszniewski M, Szymański D, et al. Long-term results of gastric bypass surgery in morbidly obese type 1 diabetes patients. Obes Surg. 2010;20(4):506–8.
Brethauer SA, Aminian A, Rosenthal RJ, et al. Bariatric surgery improves the metabolic profile of morbidly obese patients with type 1 diabetes. Diabetes Care. 2014;37(3):e51–2.
Mendez CE, Tanenberg RJ, Pories W. Outcomes of Roux-en-Y gastric bypass surgery for severely obese patients with type 1 diabetes: a case series report. Diabetes Metab Syndr Obes. 2010;3:281–3.
Reyes Garcia R, Romero Muñoz M, Galbis VH. Bariatric surgery in type 1 diabetes. Endocrinol Nutr. 2013;60(1):46–7.
Fuertes-Zamorano N, Sánchez-Pernaute A, Torres García AJ, et al. Bariatric surgery in type 1 diabetes mellitus; long-term experience in two cases. Nutr Hosp. 2013;28(4):1333–6.
Dirksen C, Jacobsen SH, Bojsen-Møller KN, et al. Reduction in cardiovascular risk factors and insulin dose, but no beta-cell regeneration 1 year after Roux-en-Y gastric bypass in an obese patient with type 1 diabetes: a case report. Obes Res Clin Pract. 2013;7(4):e269–74.
Chuang J, Zeller MH, Inge T, et al. Bariatric surgery for severe obesity in two adolescents with type 1 diabetes. Pediatrics. 2013;132(4):e1031–4.
Raab H, Weiner RA, Frenken M, et al. Obesity and metabolic surgery in type 1 diabetes mellitus. Nutr Hosp. 2013;28(Suppl 2):31–4.
Lannoo M, Dillemans B, Van Nieuwenhove Y, et al. Bariatric surgery induces weight loss but does not improve glycemic control in patients with type 1 diabetes. Diabetes Care. 2014;37(8):e173–4.
Middelbeek RJ, James-Todd T, Cavallerano JD, et al. Gastric bypass surgery in severely obese women with type 1 diabetes: anthropometric and cardiometabolic effects at 1 and 5 years postsurgery. Diabetes Care. 2015;38(7):e104–5.
Maraka S, Kudva YC, Kellogg TA, et al. Bariatric surgery and diabetes: implications of type 1 versus insulin-requiring type 2. Obesity (Silver Spring, Md). 2015;23(3):552–7.
Robert M, Belanger P, Hould FS, et al. Should metabolic surgery be offered in morbidly obese patients with type I diabetes? Surg Obes Relat Dis. 2015;11(4):798–805.
Faucher P, Poitou C, Carette C, et al. Bariatric surgery in obese patients with type 1 diabetes: effects on weight loss and metabolic control. Obes Surg. 2016;26(10):2370–8.
Moreno-Fernandez J, Chico A. Bariatric surgery results in patients with type 1 diabetes mellitus on continuous subcutaneous insulin infusion therapy. Endocrinol Nutr. 2016;63(10):571–2.
Rottenstreich A, Keidar A, Yuval JB, et al. Outcome of bariatric surgery in patients with type 1 diabetes mellitus: our experience and review of the literature. Surg Endosc. 2016;30(12):5428–33.
Favre L, Pralong F, Suter M, et al. Treatment challenges in type 1 diabetes after roux-en-Y gastric bypass. Swiss Med Wkly. 2017;147:14420.
Vilarrasa N, Rubio MA, Miñambres I, et al. Long-term outcomes in patients with morbid obesity and type 1 diabetes undergoing bariatric surgery. Obes Surg. 2017;27(4):856–63.
Alnageeb H, Abdelgadir E, Khalifa A, et al. Efficacy of bariatric surgery in improving metabolic outcomes in patients with diabetes A 24-month follow-up study from a single center in the UAE. Diabetes Metab Syndr Obes. 2018;11:459–67.
Uno K, Seki Y, Kasama K, et al. Mid-term results of bariatric surgery in morbidly obese Japanese patients with slow progressive autoimmune diabetes. Asian J Endosc Surg. 2018;11(3):238–43.
Hironaka JY, Kitahama S, Sato H, et al. Sleeve gastrectomy induced remission of slowly progressive type 1 diabetes in a morbidly obese Japanese patient. Intern Med (Tokyo, Japan). 2019;58(5):675–8.
Landau Z, Kowen-Sandbank G, Jakubowicz D, et al. Bariatric surgery in patients with type 1 diabetes: special considerations are warranted. Ther Adv Endocrinol Metab. 2019;10:2042018818822207.
Fernandez-Ranvier G, Meknat A, Guevara DE, et al. The role of bariatric surgery in patients with obesity and type 1 diabetes mellitus. Bariatr Surg Pract Patient Care. 2020;15(1):46–50.
Vendrame F, Casu A, Pratley RE, et al. 2016-P: Bariatric surgery may improve glucose control among patients with type 1 diabetes. Diabetes. 2020;69(Supplement_1).
Höskuldsdóttir G, Ekelund J, Miftaraj M, et al. Potential benefits and harms of gastric bypass surgery in obese individuals with type 1 diabetes: a nationwide, matched, observational cohort study. Diabetes Care. 2020;43(12):3079–85.
Xu G, Song M. Recent advances in the mechanisms underlying the beneficial effects of bariatric and metabolic surgery. Surg Obes Relat Dis Off J Am Soc Bariatr Surg. 2021;17(1):231–8.
Sha Y, Huang X, Ke P, et al. Laparoscopic Roux-en-Y gastric bypass versus sleeve gastrectomy for type 2 diabetes mellitus in nonseverely obese patients: a systematic review and meta-analysis of randomized controlled trials. Obes Surg. 2020;30(5):1660–70.
Conway B, Miller RG, Costacou T, et al. Temporal patterns in overweight and obesity in type 1 diabetes. Diabet Med J Br Diabet Assoc. 2010;27(4):398–404.
Chillarón JJ, Flores Le-Roux JA, Benaiges D, et al. Type 1 diabetes, metabolic syndrome and cardiovascular risk. Metab Clin Exp. 2014;63(2):181–7.
Batsis JA, Sarr MG, Collazo-Clavell ML, et al. Cardiovascular risk after bariatric surgery for obesity. Am J Cardiol. 2008;102(7):930–7.
Shuai X, Tao K, Mori M, et al. Bariatric surgery for metabolic syndrome in obesity. Metab Syndr Relat Disord. 2015;13(4):149–60.
CastagnetoGissey L, Casella Mariolo J, Mingrone G. Intestinal peptide changes after bariatric and minimally invasive surgery: relation to diabetes remission. Peptides. 2018;100:114–22.
Rowlands J, Heng J, Newsholme P, et al. Pleiotropic effects of GLP-1 and analogs on cell signaling, metabolism, and function. Front Endocrinol. 2018;9:672.
Korakas E, Kountouri A, Raptis A, et al. Bariatric surgery and type 1 diabetes: unanswered questions. Front Endocrinol. 2020;11:525909.
Holst JJ. The physiology of glucagon-like peptide 1. Physiol Rev. 2007;87(4):1409–39.
Aminian A, Kashyap SR, Burguera B, et al. Incidence and clinical features of diabetic ketoacidosis after bariatric and metabolic surgery. Diabetes Care. 2016;39(4):e50–3.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Key Points
• MBS reduces postoperative insulin requirement in T1DM patients with severe obesity.
•MBS improves HbA1c in T1DM patients with severe obesity.
•A careful multidisciplinary approach is necessary for evaluating T1DM patients.
Rights and permissions
About this article
Cite this article
Kermansaravi, M., Valizadeh, R., Jazi, A.D. et al. Current Status of Metabolic/Bariatric Surgery in Type 1 Diabetes Mellitus: an Updated Systematic Review and Meta-analysis. OBES SURG 32, 1726–1733 (2022). https://doi.org/10.1007/s11695-022-05980-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11695-022-05980-7