Abstract
Background
Considering conflicting results on the consequences of all types of obesity surgery, we were to summarize them via a systematic review.
Methods
Electronic literature search was done via scientific search engines. After the removal of duplicates and selection of articles of interest, 771 studies were included.
Results
Insulin resistance indicators were significantly improved after bariatric surgery. Leptin was also significantly decreased while adiponectin was significantly increased. Although the level of metabolic hormones changed after bariatric surgery, they were not statistically significant. Inflammation indicators were significantly decreased. Significant reduction was also detected in PAI-1 and sICAM-1.
Conclusions
Bariatric surgery is beneficial in morbidly obese patients. Although treating obesity in a surgical way may cause some complications, the weight loss is generally safe and effective.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Upon its annual increase in prevalence, obesity has been associated with high rates of morbidity and mortality [1, 2]. The role of low-grade inflammation in making a link between obesity and Insulin Resistance (IR) syndrome caused by endothelial dysfunction has been persistently evidenced [3]. Endothelial dysfunction represents a pathologic trait appearing in obese patients at the earliest stage of the atherogenic process [4, 5]. Atherosclerosis is pathophysiologically accompanied by the crucial stage of vascular wall inflammation inducing endothelial cells to increasingly secrete a variety of molecules; hence, measurements of the levels of these molecules can provide an assessment of abnormal endothelial cell function. The enhanced concentrations of soluble Inter-Cellular Adhesion Molecule-1 (sICAM-1) and Plasminogen Activator Inhibitor-1 (PAI-1) have been observed in obese patients [6, 7], thus serving as the earliest indicators of endothelial dysfunction [8, 9]. Adipose tissue has a role in energy storage and body health, while producing adipokines, which serve as pro-inflammatory and anti-inflammatory proteins contributing to metabolic control and regulations of appetite and energy expenditure. Pro-inflammatory and anti-inflammatory agents have been shown to be respectively decreased and increased through serum pattern re-arrangements of circulating adipokines induced by the marked loss of adipose tissue following bariatric surgery. Thus, metabolic conditions can be improved after the operation. Nevertheless, investigations have failed to document any significantly modified concentrations of some serum adipokines after the surgery resulting in a weight loss [10]. Various gut and metabolic hormones have been documented in some research to play significant roles in hunger, satiation, and energy expenditure [11]. Yet, conflicting results on the association between these hormones and weight loss have been reported in the studies investigating post-surgical variations in metabolic hormones [12].
Anyhow, patients involved in severe obesity can be treated via the management of bariatric surgery as a renowned and established therapy. Laparoscopic sleeve gastrectomy and laparoscopic Roux-en-Y gastric bypass are the most commonly performed procedures [13]. Due to the conflicting results of the previous studies on the complications and consequences of all types of surgeries for obesity, the aim of this study was to sum up those results via a systematic review.
Materials and Methods
Search Strategy
A comprehensive search adhering to the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) guidelines [14] was performed by using the databases of PubMed, Web of Science, EMBASE, and Scopus to identify the English articles published until November 1, 2017. The following keywords were used in this search: [(“Bariatric surgery” OR “obesity surgery” OR “gastric bypass” OR “Roux-en-Y gastric bypass” OR “sleeve gastrectomy” OR “adjustable gastric band” OR “Duodenal switch” OR “Lap band” OR “gastric balloon”)] AND [(“vascular function” OR “endothelial function” OR endothelin-1 OR “nitric oxide” OR e-selectin OR p-selectin OR l-selectin OR sVCAM-1 OR sICAM-1 OR “plasminogen activator inhibitor-1” OR PAI-1 OR fibrinolysis OR “thrombomodulin”) OR (inflammation OR inflammat* OR CRP OR hs-CRP OR IL-6 OR fibrinogen OR TNF-alpha OR TNF OR TNF-α OR “soluble TNF-receptor” OR (sTNF-R)-1 OR (sTNF-R)-2 OR IFN-ɤ) OR (“Visceral fat” OR “visceral adiposity” OR “abdominal fat” OR “Intra-Abdominal Fat”) OR (“insulin sensitivity” OR chemerin OR Hb-A1C OR HOMA-IR OR “insulin resistance” OR hypergylsemia OR hyperinsulinemia) OR (adipokine* OR chemerin OR visfatin OR adiponectin OR leptin OR apelin OR omentin OR vaspin) OR (“metabolic hormone*” OR obestatin OR ghrelin OR GLP-1 OR PYY OR peptide-YY OR “pancreatic polypeptide” OR insulin) OR mortality]. Additionally, the references of the extracted articles were reviewed to obtain any other related articles.
Study Selection
After identifying a total of 4552 articles and removing the duplicates, 2786 records were reviewed by title and abstract to determine their eligibility for inclusion in the project. Then, the full texts of 950 articles were reviewed and the articles of interest were selected according to the inclusion criteria.
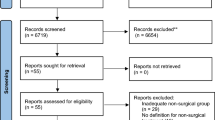
The inclusion criteria were as follows: [1] cohort studies comparing the outcomes before and after the surgery, [2] studies on adult patients (18–80 years old), and [3] the studies published in English. The exclusion criteria were as follows: [1] animal studies; [2] lack of access to full-text articles; [3] randomized clinical trials, case reports, and review articles; and [4] studies on adolescents. The selection process of the articles is presented in Fig. 1.
Data Extraction and Quality Assessment
Data extraction from each included paper was performed by three independent authors (M. Kh., A.M., and R.A.) based on author’s name, publication year, journal name, study population, country of the study population, sex, sample size, procedure type, follow-up period, and outcomes before and after bariatric surgery. The same eligibility criteria were applied to the full-text articles. Any disagreements between the authors for selecting the studies were resolved via a discussion with another reviewer (M. N.). Also, we would contact the authors if there were any areas in need of clarification. The inter-reviewer agreement was determined by calculating the kappa-statistic (κ). The agreement was very good for the abstracts and titles (κ = 0.82), as well as for full-text screening (κ = 0.75). Each included study was evaluated by the nine-star Newcastle–Ottawa Scale (NOS) in terms of quality. The mentioned scale consists of the three parts of participant selection, comparability of the study groups, and outcome assessment provided by follow-up adequacy [15].
Statistical Analysis
The data were analyzed using STATA, version 12.0 (STATA Corporation, College Station, TX, USA). Mean Difference (MD) and a Confidence Interval (CI) of 95% were utilized as measures for the estimated effects of the outcomes of interest. Calculations of the MDs and Standard Deviations (SDs) of the included studies were followed. The available data would be employed if the studies did not directly provide the required SDs.
The statistical heterogeneity between the studies was assessed using Cochran’s Q test (from chi-square) and the I2 statistic, which was able to measure the inconsistency across the results of the studies and describe the proportion of the total variations based on their estimates due to the presence of heterogeneity rather than sampling errors. In detail, the I2 values of 0, < 30, 30–60, and > 60% indicated no, low, moderate, and high heterogeneities, respectively [16].
Publication bias was assessed in more than three studies using the Egger’s test and graphically presented by the funnel plots of mean difference vs. standard error. The visual inspections of the asymmetries of the funnel plots were performed to address any possible small-study effects, while the Egger’s test was done to deal with the publication bias over and above any subjective evaluations. P < 0.10 was considered statistically significant [17].
All the outcomes were analyzed based on surgical procedure and follow-up time. The procedures were categorized according to the restrictive (gastric bypass, gastroplasty, and sleeve gastrectomy), malabsorptive (Bilio-Pancreatic Diversion (BPD) with/without Duodenal Switch (DS)), and mixed restrictive and malabsorptive operations (gastric bypass and few surgical treatment groups) [18]. The follow-up times were divided into short-term (< 12 months), medium-term (12–60 months), and long-term (> 60 months) follows-up [19].
For each outcome of interest, the related effect size (mean effect after the surgery minus mean effect before the surgery) was used in the analysis. Due to the different surgical procedures and follow-up durations, as well as large related results (14 variables and a total of 126 reports), only the results of highest efficacy were reported in the present study.
Results
Study Characteristics
Four thousand five hundred fifty-two papers were initially retrieved from the electronic databases, from among which 771 studies were included in the meta-analysis after excluding some of them due to their inconsistencies with the inclusion criteria (FBS: n = 154; HbA1c: n = 137; HOMA-IR: n = 114; insulin level: n = 91; leptin: n = 48; ghrelin: n = 47; CRP: n = 41; adiponectin: n = 39; GLP-1: n = 26; PYY: n = 25; IL-6: n = 22; TNF-α: n = 19; sICAM-1: n = 4; and PAI-1: n = 4) (Fig. 1). The main characteristics are summarized in Table 1. We only included the prospective human studies, which were written in English and their full texts were accessible. The minimum and maximum follow-up periods were 15 days and 84 months, respectively. The quality scores of the included studies based on NOS had a range of 5–7.
Meta-Analysis Results of the Outcomes of Interest
Fasting Blood Sugar
A total of 154 eligible studies were included in the analysis. The results related to the medium-term follow-up and mixed restrictive and malabsorptive methods were reported. A forest plot was used to illustrate the effect sizes before and after the surgery in each study and the pooled effect sizes (Fig. 2). A random effect model was utilized to combine the results of the studies because of their heterogeneity (Q = 1744.7, P < 0.0001, and I2 = 97.4%). The pooled analysis revealed that bariatric surgery had been associated with a significant decrease in the fasting blood sugar levels of obese adults (ES = − 48.4, 95% CI (− 54.4, − 42.3), and P value < 0.0001). The funnel plot showed evidence of publication bias (Fig. 3).
HbA1c
One of the most commonly reported biomarkers was HbA1c, which was pre- and post-operatively determined in 137 studies. The mean HbA1c levels were found to have been reduced in obese patients (ES = − 4.537, 95% CI (− 5.553, − 3.522), and P value < 0.0001) after more than 60 months of surgically induced weight losses by the mixed restrictive and malabsorptive methods (Fig. 4). We evaluated the funnel plot for any evidence of publication bias and unfortunately found it to be unsymmetrical, indicating a publication bias (Fig. 5).
HOMA-IR
There was a significant decrease in HOMA-IR level in patients undergoing a malabsorptive surgery within a medium-term follow-up (ES = − 7.285, 95% CI (− 7.975, − 6.595), and P value < 0.0001) (Fig. 6). No significant heterogeneity was found between the studies (Q = 0.22, P value = 0.642) by using the chi-square test prior to the analysis and pooling of the results. To this goal, the fixed effect model was employed to provide the pooled estimates of the studies with no adjustments for heterogeneity. Finally, no publication bias was found through the funnel plot (Fig. 7).
Insulin
Ninety-one studies had reported insulin levels in the obese patients undergoing bariatric surgeries. Among them, only 3 studies presented the results relevant to the malabsorptive surgery procedure after 12–60 months. The pooled results displayed significant decreases in the means of insulin levels after bariatric surgery (ES = − 110.332, 95% CI (− 156.652, − 64.011), and P value < 0.0001) (Fig. 8). The results indicated a heterogeneity between the studies (I2 = 92.2%, P value < 0.001). The funnel plot showed some evidence of publication bias (Fig. 9).
Leptin
We found a significant reduction in leptin level associated with malabsorptive bariatric surgery after less than 12 months (ES = − 46.265, 95% CI (− 64.627, − 27.903), and P value < 0.0001) (Fig. 10). The heterogeneity among these studies was statistically significant (I2 = 95.1%, P value < 0.0001) (Fig. 11).
Adiponectin
From among the included studies, 39 studies were found to report changes in adiponectin levels before and after bariatric surgeries. The pooled results of 8 sub-group studies demonstrated significant increases in adiponectin levels after a medium-term bariatric surgery based on the mixed method (ES = 11.965, 95% CI (4.403, 19.527) and P value = 0.002) (Fig. 12). Publication bias was evidenced through the funnel plot (Fig. 13). This could be due to the underestimated means and variances provided by the small sample sizes and probable oversight of some studies.
Ghrelin
A reduction in ghrelin level was mostly seen after 12–60 months based on the restrictive method of bariatric surgery, but it was not significant (ES = − 64.241, 95% CI (− 213.609, − 85.128), and P value = 0.399) (Fig. 14). Again, the funnel plot showed some evidence of publication bias (Fig. 15).
GLP-1
GLP-1 levels were obtained from 26 studies, from among which only 5 studies were included in the sub-group analysis based on the mixed surgery procedure and 12–60 months of follow-up. The pooled analysis revealed that bariatric surgery was associated with increased GLP-1 levels in obese participants (ES = 4.247, 95% CI (− 0.923, 9.417), and P value = 0.107) as represented in Fig. 16. A funnel plot was drawn to evaluate any publication bias (Fig. 17).
PYY
After including 25 studies in the assessment of PYY level, 2 studies fell in the sub-group of the restrictive procedure and 12–60 months of follow-up. Their pooled results depicted no significant enhancements in PYY levels (ES = 42.33, 95% CI (− 25.64, 110.30), and P value = 0.222) (Fig. 18). Publication bias was discovered in the funnel plot (Fig. 19).
CRP
Among the 41 studies reporting CRP data, 9 studies were classified into the sub-group of the mixed surgical procedure accompanied by 12–60 months of follow-up. Significant reductions were seen in CRP values after bariatric surgery (ES = − 6.629, 95% CI (− 8.582, − 4.677), and P value < 0.0001) (Fig. 20). Again, the random effect model was applied to combine the results of the studies due to their heterogeneities (Fig. 21).
IL-6
Figure 22 illustrates the pooled results of 8 studies in the sub-group of the mixed surgical procedure with 12–60 months of follow-up. Significant decreases can be observed in the IL-6 levels of obese patients after bariatric surgery (ES = − 5.433, 95% CI (− 7.157, − 3.709), and P value < 0.0001). The funnel plot exhibits a heterogeneity between the studies (Q = 430.52, I2 = 98.4%, and P value < 0.0001) (Fig. 23).
TNF-α
We found a significant reduction in TNF-α levels associated with the mixed procedure of bariatric surgery after 12–60 months of follow-up (ES = − 12.197, 95% CI (− 16.352, − 8.043), and P value < 0.0001) (Fig. 24). Unfortunately, the heterogeneity among these studies was statistically significant (I2 = 98.5%, P value < 0.0001) (Fig. 25).
PAI-1
Another significant decrease in the desired variables was the reduction of PAI-1 level (ES = − 15.103, 95% CI (− 18.916, − 11.290), and P value < 0.0001) within less than 12 months after the mixed surgical procedure (Fig. 26). A funnel plot was drawn for the evaluation of publication bias, but no bias was found (Fig. 27).
sICAM-1
Three out of 4 studies reporting the results of sICAM-1 levels after bariatric surgery demonstrated a reduction most frequently occurring to this variable within less than 12 months after the mixed surgical procedure (ES = − 39.037, 95% CI (− 68.662, − 9.411), and P value = 0.010) (Fig. 28). The funnel plot depicted some evidence of publication bias (Fig. 29).
Discussion
Bariatric surgery was found to have a positive effect on insulin resistance, adipokines, inflammation, and vascular function, but its effects on metabolic hormones were insignificant. Moreover, the surgical treatments of obesity had increased GLP-1 and PYY levels and decreased ghrelin level, thus reducing appetite and resulting in further weight loss. HbA1c, insulin level, FBS, and HOMA-IR were used as IR indicators. Our pooled analyses showed significant decreases in FBS, HbA1c, HOMA-IR, and insulin levels after bariatric surgery.
In a similar meta-analysis conducted on more than 22,000 bariatric surgeries by Buchwald et al., 989 diabetic patients were included. Diabetes was completely reversed in the majority of these patients (> 83%) [20]. Thus, bariatric surgery can be highly recommended for diabetes remission [21].
In a 52-week study, de Moura et al. [22] demonstrated substantial improvements in diabetes status besides showing that the subjects’ percentages of HbA1c with HbA1c < 7% at the baseline had improved from 4.5 to 73.0% in the final study assessment, independent of the baseline value. Although the underlying mechanism for the improvement of type 2 diabetes is not completely clear in bariatric surgery, the rapid decreases in the IR indicators suggests that weight loss is not solely responsible for the betterment [22]. Changes of GI anatomy [23,24,25]; changes in gastrointestinal hormones, such as incretins [26,27,28]; and bypassing dysregulated neuroendocrine signaling between the proximal intestine and pancreas may play some roles in the improvement of insulin sensitivity [22]. However, C-peptide level, insulin use, and duration of diabetes prior to bariatric surgery can be some indicators of diabetes severity and determinants of its remission after the surgery [29].
In this study, leptin and adiponectin were used to assess changes in the levels of adipokines after bariatric surgery. Our analysis indicated a significant decrease and increase in leptin and adiponectin levels associated with malabsorptive bariatric surgery, respectively.
Leptin as an adipocyte-derived hormone secreted in a diurnal and pulsatile pattern [30] plays an important role in appetite regulation [31]. Its secretion decreases after weight loss [32, 33]. Several studies have demonstrated reduced serum leptin levels after bariatric surgery [34,35,36]. Downregulation of leptin gene expression after bariatric surgery may play a role in normalizing its level and insulin sensitivity [34].
In a study conducted by Camastra et al., the 24-h leptin levels were normalized by a 26% weight reduction based on the BPD technique 6 months after the surgery [37]. In another study, the diurnal secretion of leptin was normalized 1 year after Roux-en-Y gastric bypass surgery [38].
Leptin may mediate both arterial and venous thromboses by changing PAI-1 and platelet aggregation levels in the overweight and obese patients [39, 40]. Several studies have indicated that leptin has a critical role in cardiovascular diseases by acting on different types of immune cells and enhancing the release of inflammatory cytokines [41, 42], while adiponectin as an adipocyte-secreted adipokine has a key role in vascular protection. Moreover, it has anti-atherosclerotic and antithrombotic effects by suppressing the pro-inflammatory events and stimulating the production of anti-inflammatory cytokines [43,44,45]. Also, it has a crucial impact on the modulation of insulin sensitivity. Its decreased secretion in some diseases, such as obesity and diabetes [46], precedes reduced insulin sensitivity [47].
Ghrelin, GLP-1, and PYY levels were assessed to show any changes in metabolic hormones. Although the results of our study revealed a decreased ghrelin level and increased GLP-1 and PYY levels in post-bariatric surgery, they were not statistically significant.
Ghrelin, which is mainly produced by the gastric fundus, has an important role in suppressing adiponectin, blocking hepatic insulin signaling, and lowering insulin secretion at pharmacological doses; hence, gastric fundus removal through bariatric surgery may result in reduced ghrelin production and improved insulin sensitivity [48, 49].
Ghrelin acts as a diabetogenic factor in several other ways like enhancing epinephrine, growth hormone, and cortisol [50, 51]. Thus, its suppression may lead to ameliorated glucose homeostasis [21].
GLP-1 as an incretin hormone secreted from the gut can promote the postprandial insulin responses [52]. Caloric restriction cannot be the cause of GLP-1 secretion due to the fact that nutrient intakes are needed to stimulate GLP-1 release. The reduced GLP-1 secretion before bariatric surgery can be explained by GLP-1 resistance, which shows a functional deficiency state leading to uncontrolled glycemia [53,54,55]. After bariatric surgery, different mechanisms contribute to the rapid improvement of glycemic control. Moreover, the augmented GLP-1 level induces satiety, which can result in further weight loss [21].
Peptide YY (PYY) is a hormone secreted by L-cells in the distal gastrointestinal tract after food intake. Similar to GLP-1, the reduced PYY secretion before bariatric surgery indicates PYY resistance leading to uncontrolled appetite and glucose homeostatsis [21].
Following bariatric surgery, an increase in PYY secretion could be due to accelerated gastric emptying and earlier contact of chyme with the L cells [56].
CRP, IL-6, and TNF-α were used to assess the levels of inflammation in the studies included in this meta-analysis. Our pooled analysis was indicative of the association of bariatric surgery with significantly improved inflammation. The greatest impact was observed in patients undergoing the mixed procedure of surgery after 12–60 months of follow-up. Adipose tissue is recognized as an endocrine organ and a rich source of the so-called “adipocytokines,” including TNF-α and Il-6. It has been also shown to produce CRP [57]. A significant weight loss and ameliorated inflammatory profile in severely obese patients have been recently reported by Illán Gómez et al. to be resulted from gastric bypass surgery [58]. Bariatric surgery has significantly improved the obese patients’ chronic low-grade inflammatory states and consequently led to their BMI and weight reductions as a result of continuously decreasing hs-CRP and IL-6 concentrations [59,60,61]. Some studies [62], but not others [63, 64], have reported the lowered serum levels of TNF-α and IL-6 after the weight loss occurrence in morbidly obese patients. The following mechanisms have been already suggested for low-grade inflammation relevant to obesity: fat tissue serving as a significant source of pro-inflammatory (TNF and IL-6) and anti-inflammatory (adiponectin) cytokines, insulin resistance syndrome interfering with the anti-inflammatory effect of insulin to induce inflammation [65], and oxidative stress occurring in obesity mainly due to excessive macronutrient intakes or enhanced metabolic rates leading to inflammatory responses [66]. Indeed, obese or normal people may undergo inflammation, augmented generation of reactive oxygen species (ROS), and reduced vascular reactivity when subjected to glucose or lipid loads [67]. Other previous studies have further shown that bariatric surgery can lower systemic inflammation in obese patients [68, 69]. Besides revealing a profound impact on the patients’ levels of systemic inflammation after bariatric surgery, the study conducted by Arismendi et al. [70] was indicative of the effectiveness and safety of this kind of treatment in highly obese individuals. These advantages may be attributed to the declined macrophage infiltration in adipose tissue and altered pro-inflammatory responses of macrophage phenotypes after a weight loss [71]. The involved mechanisms are further suggestive of the pathobiology of obesity based on the systemic inflammation of obese patients.
In a cardiovascular event, a tissue-type plasminogen activator (t-PA) is acutely secreted from endothelial cells causing the initiation of intravascular fibrinolysis and thrombus formation. Fibrin degradation is ensured via t-PA activation of plasminogen to plasmin. t-PA is mainly inhibited by PAI-1, the enhanced levels of which in the plasma would result in impaired thrombus removal triggered by the hypofibrinolysis state [72]. Furthermore, a robust correlation has been evidenced between BMI and the plasma levels of PAI-1, which is probably engaged in the development of adipose tissue. In several investigations, PAI-1 levels have been reported to be effectively declined by fat mass reduction via surgical resection or through a diet [73,74,75,76]. In this study, ICAM-1 was shown to serve as the major molecule contributing to leukocyte adhesion to endothelium at sites of inflammation. ICAM-1 increase described in obese patients can be partially due to its enhanced expression in adipocytes [77]. Reduced ICAM-1 concentrations were also verified by the study of Ziccardi et al. on obese women losing at least 10% of their original weights after a 1-year multidisciplinary program of weight reduction through exercising, behavioral counseling, and dietary guidelines [78]. These findings have not been consistently reported in the different studies [79].
This meta-analysis had the following limitations: first, achievement of completely different outcomes of the assessment methods employed for the included studies, probably leading to a bias and affecting the statistical power analysis; second, inability to explore the publication bias due to the relatively small number of articles (sample size) included in each sub-group; and third, presence of highly heterogeneous studies despite the use of a random effect model for their partial rectification.
Conclusion
In conclusion, the reviewed observational studies provided consistent evidence that bariatric surgery is beneficial in morbidly obese patients. Although treating obesity in a surgical way can lead to some complications in the involved patients, the resulted weight loss is generally safe and effective in this process. The prospective randomized studies should evaluate if these changes may occur to the biochemical markers as well.
References
Yusuf S, Hawken S, Ôunpuu S, et al. Obesity and the risk of myocardial infarction in 27 000 participants from 52 countries: a case-control study. Lancet. 2005;366(9497):1640–9.
Wilson PW, D'agostino RB, Sullivan L, et al. Overweight and obesity as determinants of cardiovascular risk: the Framingham experience. Arch Intern Med. 2002;162(16):1867–72.
Libby P, Ridker PM, Maseri A. Inflammation and atherosclerosis. Circulation. 2002;105(9):1135–43.
Ross R. Atherosclerosis-an inflammatory diseaseN Engl J Med 1999; 340: 2115-26. CrossRef Google Scholar 1999.
Blankenberg S, Barbaux S, Tiret L. Adhesion molecules and atherosclerosis. Atherosclerosis. 2003;170(2):191–203.
Lewczuk P, Dzienis-Str S, Kowalska I, et al. Elevated soluble intercellular adhesion molecule-1 levels in obesity: relationship to insulin resistance and tumor necrosis factor-[alpha] system activity. Metabolism. 2002;51(1):75–8.
Steffen B, Steffen L, Tracy R, et al. Obesity modifies the association between plasma phospholipid polyunsaturated fatty acids and markers of inflammation: the Multi-Ethnic Study of Atherosclerosis. Int J Obes. 2012;36(6):797–804.
Vaughan D. PAI-1 and atherothrombosis. J Thromb Haemost. 2005;3(8):1879–83.
Ito H, Ohshima A, Inoue M, et al. Weight reduction decreases soluble cellular adhesion molecules in obese women. Clin Exp Pharmacol Physiol. 2002;29(5–6):399–404.
Adami GF, Scopinaro N, Cordera R. Adipokine pattern after bariatric surgery: beyond the weight loss. Obes Surg. 2016;26(11):2793–801.
Finelli C, Padula MC, Martelli G, et al. Could the improvement of obesity-related co-morbidities depend on modified gut hormones secretion. World J Gastroenterol. 2014;20(44):16649–64.
Barja-Fernández S, Folgueira C, Castelao C, Leis R, Casanueva FF, Seoane LM. Peripheral signals mediate the beneficial effects of gastric surgery in obesity. Gastroenterol Res Pract 2015;2015.
Hydock CM. A brief overview of bariatric surgical procedures currently being used to treat the obese patient. Critical Care Nursing Quarterly. 2005;28(3):217–26.
Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol. 2009;62(10):e1–34.
Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25(9):603–5.
Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ: British Medical Journal. 2003;327(7414):557.
Sterne JA, Egger M, Smith GD. Systematic reviews in health care: investigating and dealing with publication and other biases in meta-analysis. BMJ (Clinical research ed). 2001;323(7304):101–5.
Vairavamurthy J, Cheskin LJ, Kraitchman DL, et al. Current and cutting-edge interventions for the treatment of obese patients. Eur J Radiol. 2017;93:134–42.
Chouillard E, Younan A, Alkandari M, et al. Roux-en-Y fistulo-jejunostomy as a salvage procedure in patients with post-sleeve gastrectomy fistula: mid-term results. Surg Endosc. 2016;30(10):4200–4.
Buchwald H, Avidor Y, Braunwald E, et al. Bariatric surgery: a systematic review and meta-analysis. JAMA. 2004;292(14):1724–37.
Peterli R, Wölnerhanssen B, Peters T, et al. Improvement in glucose metabolism after bariatric surgery: comparison of laparoscopic Roux-en-Y gastric bypass and laparoscopic sleeve gastrectomy: a prospective randomized trial. Ann Surg. 2009;250(2):234–41.
de Moura EG, Martins BC, Lopes GS, et al. Metabolic improvements in obese type 2 diabetes subjects implanted for 1 year with an endoscopically deployed duodenal-jejunal bypass liner. Diabetes Technol Ther. 2012;14(2):183–9.
Wickremesekera K, Miller G, Naotunne TD, et al. Loss of insulin resistance after Roux-en-Y gastric bypass surgery: a time course study. Obes Surg. 2005;15(4):474–81.
Ballantyne G, Farkas D, Laker S, et al. Short-term changes in insulin resistance following weight loss surgery for morbid obesity: laparoscopic adjustable gastric banding versus laparoscopic Roux-en-Y gastric bypass. Obes Surg. 2006;16(9):1189–97.
Deitel M, Crosby RD, Gagner M. The first international consensus summit for sleeve gastrectomy (SG), New York City, October 25–27, 2007. Obes Surg. 2008;18(5):487–96.
Cummings DE, Overduin J, Shannon MH, et al. Hormonal mechanisms of weight loss and diabetes resolution after bariatric surgery. Surg Obes Relat Dis. 2005;1(3):358–68.
Camastra S, Gastaldelli A, Mari A, et al. Early and longer term effects of gastric bypass surgery on tissue-specific insulin sensitivity and beta cell function in morbidly obese patients with and without type 2 diabetes. Diabetologia. 2011;54(8):2093–102.
Rubino F, Schauer PR, Kaplan LM, et al. Metabolic surgery to treat type 2 diabetes: clinical outcomes and mechanisms of action. Annu Rev Med. 2010;61:393–411.
Dogan K, Betzel B, Homan J, et al. Long-term effects of laparoscopic Roux-en-Y gastric bypass on diabetes mellitus, hypertension and dyslipidaemia in morbidly obese patients. Obes Surg. 2014;24(11):1835–42.
Heptulla R, Smitten A, Teague B, et al. Temporal patterns of circulating leptin levels in lean and obese adolescents: relationships to insulin, growth hormone, and free fatty acids rhythmicity. The Journal of Clinical Endocrinology & Metabolism. 2001;86(1):90–6.
Considine RV, Sinha MK, Heiman ML, et al. Serum immunoreactive-leptin concentrations in normal-weight and obese humans. N Engl J Med. 1996;334(5):292–5.
Gumbau V, Bruna M, Canelles E, et al. A prospective study on inflammatory parameters in obese patients after sleeve gastrectomy. Obes Surg. 2014;24(6):903–8.
Siejka A, Jankiewicz-Wika J, Kołomecki K, et al. Long-term impact of vertical banded gastroplasty (VBG) on plasma concentration of leptin, soluble leptin receptor, ghrelin, omentin-1, obestatin, and retinol binding protein 4 (RBP4) in patients with severe obesity. Cytokine. 2013;64(2):490–3.
Edwards C, Hindle AK, Fu S, et al. Downregulation of leptin and resistin expression in blood following bariatric surgery. Surg Endosc. 2011;25(6):1962–8.
van Dielen FM, van ‘tVeer C, Buurman WA, Greve JWM. Leptin and soluble leptin receptor levels in obese and weight-losing individuals. The Journal of Clinical Endocrinology & Metabolism. 2002;87(4):1708–16.
Faraj M, Havel PJ, Phélis S, et al. Plasma acylation-stimulating protein, adiponectin, leptin, and ghrelin before and after weight loss induced by gastric bypass surgery in morbidly obese subjects. The Journal of Clinical Endocrinology & Metabolism. 2003;88(4):1594–602.
Camastra S, Manco M, Frascerra S, et al. Daylong pituitary hormones in morbid obesity: effects of bariatric surgery. Int J Obes. 2009;33(1):166.
Cummings DE, Weigle DS, Frayo RS, et al. Plasma ghrelin levels after diet-induced weight loss or gastric bypass surgery. N Engl J Med. 2002;346(21):1623–30.
Cugno M, Castelli R, Mari D, et al. Inflammatory and prothrombotic parameters in normotensive non-diabetic obese women: effect of weight loss obtained by gastric banding. Intern Emerg Med. 2012;7(3):237–42.
Eržen B, Šabovič M. In young post-myocardial infarction male patients elevated plasminogen activator inhibitor-1 correlates with insulin resistance and endothelial dysfunction. Heart Vessel. 2013;28(5):570–7.
Piestrzeniewicz K, Łuczak K, Goch JH. Factors associated with C-reactive protein at the early stage of acute myocardial infarction in men. Cardiology journal. 2009;16(1):36–42.
Martin SS, Qasim A, Reilly MP. Leptin resistance: a possible interface of inflammation and metabolism in obesity-related cardiovascular disease. J Am Coll Cardiol. 2008;52(15):1201–10.
Messier V, Karelis AD, Prud'homme D, et al. Identifying metabolically healthy but obese individuals in sedentary postmenopausal women. Obesity. 2010;18(5):911–7.
Iyer A, Fairlie DP, Prins JB, et al. Inflammatory lipid mediators in adipocyte function and obesity. Nat Rev Endocrinol. 2010;6(2):71.
Netto BDM, Bettini SC, Clemente APG, et al. Roux-en-Y gastric bypass decreases pro-inflammatory and thrombotic biomarkers in individuals with extreme obesity. Obes Surg. 2015;25(6):1010–8.
Weyer C, Funahashi T, Tanaka S, et al. Hypoadiponectinemia in obesity and type 2 diabetes: close association with insulin resistance and hyperinsulinemia. The Journal of Clinical Endocrinology & Metabolism. 2001;86(5):1930–5.
Stefan N, Vozarova B, Funahashi T, et al. Plasma adiponectin concentration is associated with skeletal muscle insulin receptor tyrosine phosphorylation, and low plasma concentration precedes a decrease in whole-body insulin sensitivity in humans. Diabetes. 2002;51(6):1884–8.
Papailiou J, Albanopoulos K, Toutouzas KG, et al. Morbid obesity and sleeve gastrectomy: how does it work? Obes Surg. 2010;20(10):1448–55.
Zhou D, Jiang X, Ding W, et al. Impact of bariatric surgery on ghrelin and obestatin levels in obesity or type 2 diabetes mellitus rat model. Journal of diabetes research. 2014;2014
Davenport AP, Bonner TI, Foord SM, et al. International Union of Pharmacology. LVI. Ghrelin receptor nomenclature, distribution, and function. Pharmacol Rev. 2005;57(4):541–6.
Murphy KG, Dhillo WS, Bloom SR. Gut peptides in the regulation of food intake and energy homeostasis. Endocr Rev. 2006;27(7):719–27.
Chronaiou A, Tsoli M, Kehagias I, et al. Lower ghrelin levels and exaggerated postprandial peptide-YY, glucagon-like peptide-1, and insulin responses, after gastric fundus resection, in patients undergoing Roux-en-Y gastric bypass: a randomized clinical trial. Obes Surg. 2012;22(11):1761–70.
le Roux CW, Welbourn R, Werling M, et al. Gut hormones as mediators of appetite and weight loss after Roux-en-Y gastric bypass. Ann Surg. 2007;246(5):780–5.
Rodieux F, Giusti V, D'alessio DA, et al. Effects of gastric bypass and gastric banding on glucose kinetics and gut hormone release. Obesity. 2008;16(2):298–305.
Laferrère B, Teixeira J, McGinty J, et al. Effect of weight loss by gastric bypass surgery versus hypocaloric diet on glucose and incretin levels in patients with type 2 diabetes. The Journal of Clinical Endocrinology & Metabolism. 2008;93(7):2479–85.
Peterli R, Steinert RE, Woelnerhanssen B, et al. Metabolic and hormonal changes after laparoscopic Roux-en-Y gastric bypass and sleeve gastrectomy: a randomized, prospective trial. Obes Surg. 2012;22(5):740–8.
Saltiel AR, Olefsky JM. Inflammatory mechanisms linking obesity and metabolic disease. J Clin Invest. 2017;127(1):1–4.
Illán Gómez F, Gonzálvez Ortega M, Aragón Alonso A, et al. Obesidad, inflamación y función endotelial: efectos de la pérdida de peso tras cirugía bariatrica. Nutr Hosp. 2016;33(6):1340–6.
Forsythe LK, Wallace JM, Livingstone MBE. Obesity and inflammation: the effects of weight loss. Nutr Res Rev. 2008;21(2):117–33.
Hagman DK, Larson I, Kuzma JN, et al. The short-term and long-term effects of bariatric/metabolic surgery on subcutaneous adipose tissue inflammation in humans. Metabolism-Clinical and Experimental. 2017;70:12–22.
Mottaghi A, Mirmiran P, Delshad H, et al. Effect of different obesity phenotypes on incidence of chronic kidney disease in Tehranian adults. J Am Coll Nutr. 2016;35(7):587–96.
Dandona P, Weinstock R, Thusu K, et al. Tumor necrosis factor-α in sera of obese patients: fall with weight loss. The Journal of Clinical Endocrinology & Metabolism. 1998;83(8):2907–10.
Laimer M, Ebenbichler C, Kaser S, et al. Markers of chronic inflammation and obesity: a prospective study on the reversibility of this association in middle-aged women undergoing weight loss by surgical intervention. Int J Obes. 2002;26(5):659.
Kopp H-P, Kopp C, Festa A, et al. Impact of weight loss on inflammatory proteins and their association with the insulin resistance syndrome in morbidly obese patients. Arterioscler Thromb Vasc Biol. 2003;23(6):1042–7.
Dandona P, Aljada A, Mohanty P, et al. Insulin inhibits intranuclear nuclear factor κB and stimulates IκB in mononuclear cells in obese subjects: evidence for an anti-inflammatory effect? The Journal of Clinical Endocrinology & Metabolism. 2001;86(7):3257–65.
Davì G, Guagnano MT, Ciabattoni G, et al. Platelet activation in obese women: role of inflammation and oxidant stress. JAMA. 2002;288(16):2008–14.
Tripathy D, Mohanty P, Dhindsa S, et al. Elevation of free fatty acids induces inflammation and impairs vascular reactivity in healthy subjects. Diabetes. 2003;52(12):2882–7.
Jiménez A, Casamitjana R, Flores L, et al. Long-term effects of sleeve gastrectomy and Roux-en-Y gastric bypass surgery on type 2 diabetes mellitus in morbidly obese subjects. Ann Surg. 2012;256(6):1023–9.
Morínigo R, Casamitjana R, Delgado S, et al. Insulin resistance, inflammation, and the metabolic syndrome following Roux-en-Y gastric bypass surgery in severely obese subjects. Diabetes Care. 2007;30(7):1906–8.
Arismendi E, Rivas E, Agustí A, et al. The systemic inflammome of severe obesity before and after bariatric surgery. PLoS One. 2014;9(9):e107859.
Cancello R, Henegar C, Viguerie N, et al. Reduction of macrophage infiltration and chemoattractant gene expression changes in white adipose tissue of morbidly obese subjects after surgery-induced weight loss. Diabetes. 2005;54(8):2277–86.
Flevaris P, Vaughan D, editors. The role of plasminogen activator inhibitor type-1 in fibrosis. Seminars in thrombosis and hemostasis; 2017: Thieme Medical Publishers.
Khan SS, Lloyd-Jones DM, Chan C, Liu K, Cushman M, Kestenbaum B, et al. Association of plasminogen activator inhibitor-1 with prevalent and incident obesity is independent of inflammatory markers: the Multi-Ethnic Study of Atherosclerosis (MESA). Am Heart Assoc; 2015.
Alessi M, Peiretti F, Morange P, et al. Production of plasminogen activator inhibitor 1 by human adipose tissue: possible link between visceral fat accumulation and vascular disease. Diabetes. 1997;46(5):860–7.
Tschoner A, Sturm W, Engl J, et al. Plasminogen activator inhibitor 1 and visceral obesity during pronounced weight loss after bariatric surgery. Nutrition, metabolism, and cardiovascular diseases : NMCD. 2012;22(4):340–6.
Morel O, Luca F, Grunebaum L, Jesel L, Meyer N, Desprez D, et al. Short-term very low-calorie diet in obese females improves the haemostatic balance through the reduction of leptin levels, PAI-1 concentrations and a diminished release of platelet and leukocyte-derived microparticles. International journal of obesity (2005). 2011 Dec;35(12):1479–86.
Bosanská L, Michalský D, Lacinová Z, et al. The influence of obesity and different fat depots on adipose tissue gene expression and protein levels of cell adhesion molecules. Physiol Res. 2010;59(1):79.
Ziccardi P, Nappo F, Giugliano G, et al. Reduction of inflammatory cytokine concentrations and improvement of endothelial functions in obese women after weight loss over one year. Circulation. 2002;105(7):804–9.
Vázquez LA, Pazos F, Berrazueta JR, et al. Effects of changes in body weight and insulin resistance on inflammation and endothelial function in morbid obesity after bariatric surgery. The Journal of Clinical Endocrinology & Metabolism. 2005;90(1):316–22.
Acknowledgements
Research reported in this publication was supported by the Elite Researcher Grant Committee under award number 958714 from the National Institutes for Medical Research Development (NIMAD), Tehran, Iran.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Author 2 reports grants from National Institutes for Medical Research Development (NIMAD), during the conduct of the study. All of the other authors report no conflict of interest.
Informed Consent Statement
Informed consent does not apply.
Ethical Approval Statement
This article does not contain any studies with human participants or animals performed by any of the authors.
Rights and permissions
About this article
Cite this article
Khosravi-Largani, M., Nojomi, M., Aghili, R. et al. Evaluation of all Types of Metabolic Bariatric Surgery and its Consequences: a Systematic Review and Meta-Analysis. OBES SURG 29, 651–690 (2019). https://doi.org/10.1007/s11695-018-3550-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11695-018-3550-z