Abstract
Background
Hypoglycemia is an increasingly recognized complication of bariatric surgery. Mechanisms contributing to glucose lowering remain incompletely understood. We aimed to identify differentially abundant plasma proteins in patients with post-bariatric hypoglycemia (PBH) after Roux-en-Y gastric bypass (RYGB), compared to asymptomatic post-RYGB.
Methods
Proteomic analysis of blood samples collected after overnight fast and mixed meal challenge in individuals with PBH, asymptomatic RYGB, severe obesity, or overweight recruited from outpatient hypoglycemia or bariatric clinics.
Results
The top-ranking differentially abundant protein at 120 min after mixed meal was fibroblast growth factor 19 (FGF-19), an intestinally derived hormone regulated by bile acid-FXR signaling; levels were 2.4-fold higher in PBH vs. asymptomatic post-RYGB (mean + SEM, 1094 ± 141 vs. 428 ± 45, P < 0.001, FDR < 0.01). FGF-19 ELISA confirmed 3.5-fold higher concentrations in PBH versus asymptomatic (360 ± 70 vs. 103 ± 18, P = 0.025). To explore potential links between increased FGF-19 and GLP-1, residual samples from other human studies in which GLP-1 was modulated were assayed. FGF-19 levels did not change in response to infusion of GLP-1 and PYY in overweight/obese individuals. Infusion of the GLP-1 receptor antagonist exendin 9–39 in recently operated asymptomatic post-RYGB did not alter FGF-19 levels after mixed meal. By contrast, GLP-1 receptor antagonist infusion yielded a significant increase in FGF-19 levels after oral glucose in individuals with PBH. While plasma bile acids did not differ between PBH and asymptomatic post-RYGB, these data suggest unique interrelationships between GLP-1 and FGF-19 in PBH.
Conclusions
Taken together, these data support FGF-19 as a potential contributor to insulin-independent pathways driving postprandial hypoglycemia in PBH.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Bariatric surgery is a powerful therapeutic approach for obesity and type 2 diabetes, yielding rapid postoperative improvements in glucose levels and allowing reduction in medications needed for diabetes, even before significant weight loss occurs [1,2,3,4]. One increasingly recognized complication of bariatric surgery is hypoglycemia, occurring after both Roux-en-Y gastric bypass (RYGB) and vertical sleeve gastrectomy [5, 6]. Post-bariatric hypoglycemia (PBH) can be strictly defined by hypoglycemia with neuroglycopenia, typically occurring 1 to 3 h after meals [7]. The etiology of PBH has not been fully elucidated, but both insulin-dependent and insulin-independent mechanisms may contribute. Insulin levels are inappropriately high at the time of hypoglycemia, potentially related to higher incretin hormones (including GLP-1) [7, 8], altered β cell glucose sensitivity [9], and reduced insulin clearance [7, 10]. Moreover, insulin-independent glucose uptake is increased in patients with PBH [11].
The goal of this study was to identify novel proteins which could contribute to hypoglycemia in patients with PBH. We analyzed the plasma proteome in samples collected in the fasting state and following mixed meal in individuals with PBH compared to asymptomatic post-RYGB and non-surgical individuals. We identified FGF-19 (Fgf-15 in rodents) as the most differentially regulated plasma protein in PBH.
Materials and Methods
Participants
The Joslin Diabetes Center Committee on Human Studies, the regional ethical committee of the capital region in Denmark, and Stanford University Institutional Review Board approved the study. Written informed consent was obtained from all participants.
Primary Analysis Cohort
For the index population, participants were recruited in four groups, and those with residual specimens available were included in this analysis: (A) 11 individuals who had undergone RYGB and presented with hypoglycemia (PBH), defined as documented severe hypoglycemia with neuroglycopenia (altered mental status requiring assistance, with or without seizure); (B) seven subjects who had undergone uncomplicated RYGB and denied hypoglycemic symptoms (“asymptomatic”); (C) three overweight subjects recruited from newspaper advertisement; and (D) four severely obese individuals being evaluated for bariatric surgery, recruited from local clinics, as previously described [11]. All subjects were weight stable for 6 months. Exclusion criteria included history of diabetes or other systemic illness, pregnancy, and use of medications affecting insulin sensitivity; overweight and severely obese subjects underwent a 2-h 75-g oral glucose tolerance test to confirm absence of diabetes [12].
After an overnight (> 8 h) fast, a liquid mixed meal (Ensure, 40 g carbohydrate, 9 g protein, 6 g fat, 240 mL; Abbott Laboratories, Abbott Park, IL) was consumed by participants in all four groups over 5 min. Dumping score was calculated using pre/post-meal pulse and hematocrit [12]. Blood samples collected before and at 30 and 120 min were used for proteomics analysis, the primary aim of this study.
Cohorts for Secondary Analyses
Detailed description of the prior clinical studies [8, 13, 14] which generated residual samples for secondary analysis in the present study are provided in Supplemental Methods.
Proteomics Analysis
Serum collected at baseline (fasting) and at 30 and 120 min following the mixed meal in the index population [7, 11] was utilized for proteomics analysis using SOMAscan, an aptamer-based approach to quantify 1129 proteins (SomaLogic, Inc., Boulder, CO) [15]. Each protein analyte is represented by a unique affinity-binding aptamer reagent. Serum samples are bound, washed, and hybridized to complementary sequences on a custom Agilent array; fluorescence intensity is related to protein quantity in the original sample. One of the 11 individuals with PBH and one of four severely obese individuals had analysis performed only at baseline and 30 min due to limited sample volume.
Additional Assays
Fasting glucose, cholesterol, HDL, triglycerides, and free fatty acids were measured by the clinical laboratory (Synchron CX3 and CX9; Beckman Coulter, Brea, CA), with hemoglobin A1c determined by HPLC (Tosoh 2.2; Tosoh Bioscience, San Francisco, CA) [7]. Immunoassays were performed in duplicate by commercial assay, including RIA for insulin (Diagnostic Systems Laboratories, Webster, TX). Serum FGF-19 was assayed by ELISA (FGF-19 Quantikine® DF1900; R&D Systems, Minneapolis, MN, USA, standard curve range 1.6–997 pg/mL). Fasting serum bile acids and their taurine (T) and glycine (G) conjugates were analyzed using HPLC tandem mass spectrometry and quantified using deuterium-labeled internal standards [16] in nine asymptomatic post-RYGB [17] and 10 PBH participants (data not previously reported).
Statistical Analysis
Data are presented as mean ± standard error of the mean. Comparisons among groups and linear associations between variables were analyzed using ANOVA/general linear model for continuous variables, with post hoc t tests using Tukey’s adjustment for multiple comparisons, or chi-square tests or Fisher’s exact test for comparison of proportions (GraphPad Prism, version 7.00 for Windows). Two-tailed P < 0.05 was considered significant. For SomaLogic data, differential expression analysis used the Bayesian linear modeling package limma [18], with correction for multiple testing using the false discovery rate (FDR); volcano plots were generated using ggplot2, all in R [19].
For paired samples, significance was assessed using repeated measures, two-way ANOVA, and Sidak’s correction for multiple comparisons (GraphPad). Linear mixed effects modeling of the influences on FGF-19 over time was performed on log-transformed FGF-19 data (PROC MIXED) in SAS v9.4 (2012 SAS Institute Inc., Cary, NC, USA).
Results
Clinical characteristics of the 25 individuals analyzed are provided in Table 1. Participants in all groups were of similar age. Sex distribution differed, with more women in the PBH group (P < 0.001). Current BMI and waist circumference were lower in post-RYGB vs. non-surgical groups (ANOVA P < 0.0001). Preoperative BMI and change in BMI (current minus preoperative) did not differ between PBH and asymptomatic. Hemoglobin A1c did not differ in any groups; insulin resistance (HOMA-IR), was significantly lower in both post-RYGB groups (ANOVA P < 0.0001).
Fasting plasma glucose was significantly lower in both post-RYGB groups (ANOVA P = 0.01, Table 1). Individuals with PBH had higher peak glucose (9.21 ± 0.94 mmol/L) compared to asymptomatic (7.66 ± 0.39 mmol/L), severely obese (6.83 ± 0.44 mmol/L), and overweight (7.71 ± 0.78 mmol/L) groups (ANOVA P = 0.02, Supplemental Fig. 1). Nadir glucose was lower in post-RYGB (PBH 4.27 ± 0.22; asymptomatic 3.94 ± 0.11 mmol/L) versus non-surgical groups (overweight 5.49 ± 0.22; severely obese 5.27 ± 0.33 mmol/L, ANOVA P < 0.001).
SOMAscan analysis of 1129 proteins revealed 17 differentially abundant proteins at all three time points in PBH as compared with asymptomatic individuals, including three with higher levels in PBH and 14 with lower (nominal P < 0.05, Supplemental Table 1).
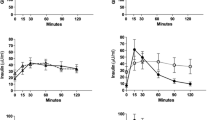
Since hypoglycemia in patients with PBH typically occurs 2–3 h after meals, we focused on those proteins altered at 120 min after mixed meal (Fig. 1, Supplemental Table 1). The protein with the greatest differential abundance between PBH and asymptomatic groups at 120 min was FGF-19, which was 2.4-fold higher in those with PBH (1094 ± 141 versus 428 ± 45 SOMAmer units; P < 1 × 10−5, FDR 0.01). FGF-19 was also significantly higher in PBH in the fasting state (487 ± 31 vs. 347 ± 25 SOMAmer units, P = 0.02) and at 30 min (540 ± 48 vs. 364 ± 25 SOMAmer units, P = 0.01) (Fig. 2a). Differences in FGF-19 were validated by ELISA, with 3.5-fold higher FGF-19 in PBH compared to asymptomatic post-RYGB at 120 min (360 ± 70 versus 103 ± 18 pg/mL, P < 0.05) (Fig. 2b). Differences remained after adjustment for sex.
Volcano plot of plasma proteomic data derived from blood samples collected at 120 min following mixed meal tolerance test. X-axis present log2 of ratio of protein content in PBH vs. asymptomatic post-RYGB, with proteins upregulated in PBH plotted to the right and proteins downregulated plotted to the left; y-axis indicates —log10P value
a Plasma FGF-19 levels in overweight (clear triangle), severely obese (black triangle), asymptomatic post-RYGB (clear square) and PBH (black circle), as measured by SomaLogic platform. Note that SomaLogic analysis was not performed at 120 min in overweight or severely obese groups as sample volume was not sufficient for analysis. At time 0, 30, and 120 min, P values for comparison between asymptomatic post-RYGB and PBH were 0.02, 0.01, and 6.88E-08, respectively. b ELISA analysis was performed in samples collected at 120 min after mixed meal. *P < 0.05, **P ≤ 0.01
Plasma FGF-19 levels did not correlate with age, BMI, surgical weight loss, postoperative duration, or dumping score (Supplemental Table 2). Interestingly, peak insulin levels at 30 min correlated positively with FGF-19 levels at 120 min in the combined post-RYGB group (r = 0.59, P = 0.01); this relationship was driven by participants with PBH (r = 0.71, P = 0.02) (Fig. 3), and was not significant in asymptomatic group (r = 0.35, P = 0.44).
FGF-19 correlates positively with insulin in asymptomatic post-RYGB (clear squares) and PBH (black circle). Insulin levels at 30 min (x-axis) and FGF-19 levels (y-axis) are strongly correlated in both bypass groups (r = 0.59, P = 0.01) (black solid line). The correlation coefficient is higher in participants with neuroglycopenia (r = 0.71, P = 0.02) (black dotted line)
Multivariable modeling was performed in the combined post-RYGB group to assess the relationship between candidate predictors and FGF-19. Predictors were systematically added and were retained if significant (P < 0.05) or inclusion caused > 15% change in estimated effect of a predictor already in the model. Modeling revealed significant differences in FGF-19 between PBH and asymptomatic groups (P < 0.01) and within both groups over time (P < 0.01), with a different relationship of FGF-19 to time (group by time interaction, P = 0.01). HOMA-IR, glucose and insulin, BMI, post-surgery change in BMI, lipids, dumping score, and pulse had no significant relationship with FGF-19 in this model.
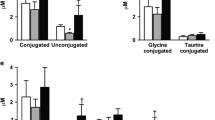
Since bile acids can activate FXR transcriptional activity and promote enterocyte FGF-19 secretion [20,21,22], we assessed fasting plasma bile acids in PBH (n = 14) and asymptomatic post-RYGB (n = 9). Total bile acids did not differ between groups (PBH, 9.6 ± 1.1 versus asymptomatic, 8.5 ± 1.5 μmol/L, P = 0.55) (Supplemental Fig. 2A). Moreover, the proportion of hydrophobic bile acids, previously linked to FXR agonist activity [20,21,22], did not differ between PBH and asymptomatic (82 ± 3 versus 79 ± 3%, P = 0.49) (Supplemental Fig. 2B/C).
Since increased GLP-1 contributes to PBH pathophysiology [8, 23, 24], we hypothesized that differences in FGF-19 might be mediated by increased GLP-1 or other incretins. We tested this by measuring FGF-19 levels in residual specimens from three additional studies in distinct populations in which GLP-1 was directly modulated (Supplemental Methods). First, we observed no impact of infusion of a mixture of GLP-1 and PYY3–36, as compared with placebo, on FGF-19 levels in overweight or obese men (mean BMI 29 + 3) (P = 0.300; ANOVA for time and treatment P = 0.002 and P = 0.11, respectively) [13] (Supplemental Fig. 3A). Secondly, to determine whether unique relationships between GLP-1 and FGF-19 might be present post-RYGB, we assessed the impact of the GLP-1 receptor antagonist Ex-9 on FGF-19 levels in 10 participants with recent (< 1 year) RYGB, without hypoglycemia [14]. There was no significant difference in FGF-19 levels at baseline or after mixed meal between Ex-9- and placebo-treated participants (ANOVA for time and treatment P = 0.053 and P = 0.60, respectively) (Supplemental Fig. 3B). Thirdly, we assessed the impact of Ex-9 on FGF-19 in PBH, given the possibility of interactions unique to this syndrome [8] (Fig. 4). Consistent with Ex-9 efficacy, peak post-meal insulin was lower with Ex-9 vs placebo (500 ± 139 vs 1493 ± 222 pmol/L, P < 0.001), and nadir glucose was higher (5.72 ± 0.33 vs 3.22 ± 0.33 mmol/L, P = 0.003). On both placebo and Ex-9 infusion days, plasma FGF-19 levels increased after oral glucose (placebo 51 ± 10 to 251 ± 45 ng/mL; Ex-9 151 ± 38 to 530 ± 54 ng/mL) (ANOVA for time, treatment, and their interaction: P < 0.001, P = 0.02, and P = 0.051, respectively) (Fig. 4), a pattern similar to the response after mixed meal in the primary cohort. However, in contrast to the lack of effect of Ex-9 in the asymptomatic early surgical cohort, infusion of Ex-9 in this PBH population resulted in higher mean FGF-19 levels at 90 and 120 min versus placebo (P = 0.001 and P = 0.018, respectively).
Plasma FGF-19 levels prior to and following a 75 g oral glucose load (ingested in portions over 20 min, marked by shaded area) in participants with PBH and neuroglycopenia. Participants received blinded infusion of either exendin-9-39 (black circles) or placebo (saline) (clear squares) in a crossover fashion. *p < 0.05, ***p < 0.0001 for comparison of placebo vs. exendin 9–39. ANOVA for time, treatment and the interaction of time and treatment p < 0.001, p = 0.02, and p = 0.0507, respectively
Discussion
We report the first proteomic analysis of plasma from patients with post-bariatric hypoglycemia, in comparison to asymptomatic post-RYGB individuals, and non-bariatric controls. The protein with the highest magnitude difference was the intestinally derived hormone FGF-19; FGF-19 was increased at all time points and 2.4-fold higher in PBH compared to asymptomatic at 120 min after mixed meal.
Increased FGF-19 in PBH is of particular interest as a candidate mediator of this syndrome, as FGF-19 (Fgf 15 in rodents) is secreted, largely by ileal enterocytes, in response to bile acid activation of the nuclear receptor FXR [25]. FGF-19 acts via hepatic FGFR4 receptors to inhibit Cyp7A1, the rate-limiting step of bile acid synthesis. Thus, this FXR-FGF-19 feedback loop can effectively sense and regulate bile acid homeostasis.
More recently, FGF-19 has been identified as a potent regulator of glucose and energy metabolism. Experimental increases in FGF-19 in rodents, either via injection or transgenic expression, results in lower glucose and improved glucose tolerance in both lean and obese mice, despite lower insulin levels [26,27,28,29]. Improved glucose metabolism may result in part from FGF-19 effects to reduce adiposity, increase brown adipose tissue, and increase energy expenditure [27]. FGF-19 also increases glucose effectiveness, defined as the ability of an increased concentration of glucose to promote its own disposal at basal insulin [28]. In humans, FGF-19 levels are inversely correlated with visceral fat accumulation [30]. Collectively, these data indicate that FGF-19 reduces glucose and improves metabolic homeostasis via multiple mechanisms.
FGF-19 is increased as early as 4 days after RYGB, with sustained increase at 6 weeks [31], 3 months [32], and further increases of > 50% at 1 year [33]. Such progressive increases in postprandial FGF-19 over time suggest that FGF-19 cannot explain early postoperative changes in glucose metabolism [33] but are consistent with strong links between diabetes improvement at 1 year postoperatively and both fasting and postprandial FGF-19 and bile acids [34]. Moreover, the time course of induction of FGF-19 fits well with the time course of PBH, which typically emerges clinically at 1–3 years postoperatively. Whether FGF-19 also contributes to the increased glucose effectiveness in PBH [11] and/or other mechanisms responsible for glucose lowering in PBH will be an important question for future studies.
The mechanisms responsible for increased FGF-19 in PBH remain unclear. Although FGF-19 is inversely associated with body weight in some studies [35], BMI in PBH participants did not differ from the asymptomatic post-RYGB control individuals in the primary study. Moreover, our multivariable analysis did not identify significant associations between demographic or available metabolic data and FGF-19 levels. We did observe a modest association between peak postprandial insulin (30 min) and later FGF-19 levels (120 min), raising the possibility that hyperinsulinemia might contribute to increased FGF-19. However, a prior study demonstrated that hyperinsulinemia, achieved by intravenous infusion during a euglycemic clamp, did not alter FGF-19 levels [36]; this suggests instead that other factors related to exaggerated prandial response in PBH could impact both insulin and FGF-19. Given the strong evidence implicating GLP-1 in excessive insulin secretion in PBH [7, 8], we analyzed FGF-19 levels in response to GLP-1 infusion or inhibition of its action in independent cohorts of overweight men, and in post-RYGB patients without and with PBH. Interestingly, our data indicate that GLP-1 signaling does not influence basal or meal-stimulated FGF-19 levels in either healthy individuals or at an early time point after RYGB. By contrast, blocking GLP-1 receptor signaling in individuals with longer postoperative duration and PBH yielded even higher FGF-19 levels [8, 24, 37]. These data suggest that GLP-1-dependent signals may limit FGF-19 secretion via mechanisms unique to PBH; such inhibitory interactions have not been previously reported.
Bile acids are recognized as potent regulators of the FXR-FGF-19 axis; increases in bile acids and altered composition may also contribute to efficacy of bariatric surgery [17, 38, 39]. Moreover, postoperative increases in serum total bile acids are paralleled by and correlated with increases in FGF-19 in multiple studies [33, 34, 40, 41]. Thus, unique and progressive perturbations in bile acid enterohepatic recirculation and metabolism over time could contribute to differential activation of FXR-FGF-19 signaling, particularly in the postprandial state, and contribute to observed increases in plasma FGF-19 levels in PBH.
While we do not find differences in fasting plasma bile acid composition between PBH and asymptomatic post-RYGB groups, it is possible that prandial plasma levels of bile acids or luminal bile acids with greater FXR agonism are increased in PBH. Bile acid composition and levels could also be modulated by RYGB-mediated microbiome changes [42]; microbial 7α-dehydroxylation could increase luminal conjugated bile acids, potentially yielding stronger agonists for FXR and promoting FGF-19 synthesis [38]. Comparing the gut microbiome, luminal bile acids, and neuroendocrine cell populations in patients with and without PBH will be challenging but important in future studies.
We acknowledge several limitations. Since our study is observational, we cannot determine whether the observed increases in FGF-19 are simply associated with hypoglycemia or other components of an exaggerated postprandial response, or play a pathogenic role in driving lower glucose in PBH. Differences in sex distribution, bariatric procedures, and post-surgical anatomy, such as roux limb length, and postoperative duration and dietary factors could influence enteroendocrine physiology. Our secondary analyses of FGF-19 in distinct experimental populations and conditions prevented direct comparisons across studies. Although we found no effect of PYY and GLP-1 infusion on circulating FGF-19 levels in healthy men, we cannot rule out that each incretin may have opposing effects. Future investigations will need to test the impact of each incretin individually.
In summary, we report identification of FGF-19 as the most significantly upregulated protein in PBH. Given that FGF-19 can potently reduce glucose, these data support a novel hypothesis that FGF-19 may be a key contributor to PBH, potentially via modulation of the bile acid-FXR-FGF-19 axis. Increases in FGF-19 do not appear to be a direct consequence of increased plasma incretins, as infusion of GLP-1/PYY did not increase FGF-19 levels acutely, and Ex-9 did not reduce FGF-19 levels in healthy or asymptomatic post-RYGB individuals. By contrast, antagonism of GLP-1 receptor signaling uniquely increased FGF-19 levels in PBH, indicating complex interactions between GLP-1 and FXR-FGF-19 signaling in PBH. Future studies of upstream pathways regulating both incretin and BA-FXR-FGF-19 pathways will be required to further define these complex interactions and to determine if this pathway could be a therapeutic target for PBH.
References
Mingrone G, Panunzi S, De Gaetano A, et al. Bariatric-metabolic surgery versus conventional medical treatment in obese patients with type 2 diabetes: 5 year follow-up of an open-label, single-centre, randomised controlled trial. Lancet. 2015;386(9997):964–73.
Schauer PR, Bhatt DL, Kirwan JP, et al. Bariatric surgery versus intensive medical therapy for diabetes - 5-year outcomes. N Engl J Med. 2017;376(7):641–51.
Halperin F, Ding SA, Simonson DC, et al. Roux-en-Y gastric bypass surgery or lifestyle with intensive medical management in patients with type 2 diabetes: feasibility and 1-year results of a randomized clinical trial. JAMA Surg. 2014;149(7):716–26.
Adams TD, Davidson LE, Litwin SE, et al. Weight and metabolic outcomes 12 years after gastric bypass. N Engl J Med. 2017;377(12):1143–55.
Patti ME, Goldfine AB. The rollercoaster of post-bariatric hypoglycaemia. Lancet Diabetes Endocrinol. 2016;4(2):94–6.
Goldfine AB, Patti ME. How common is hypoglycemia after gastric bypass? Obesity (Silver Spring). 2016;24(6):1210–1.
Goldfine AB, Mun EC, Devine E, et al. Patients with neuroglycopenia after gastric bypass surgery have exaggerated incretin and insulin secretory responses to a mixed meal. J Clin Endocrinol Metab. 2007;92(12):4678–85.
Craig CM, Liu LF, Deacon CF, et al. Critical role for GLP-1 in symptomatic post-bariatric hypoglycaemia. Diabetologia. 2017;60(3):531–40.
Salehi M, Gastaldelli A, D'Alessio DA. Beta-cell sensitivity to glucose is impaired after gastric bypass surgery. Diabetes Obes Metab. 2018;20(4):872–8.
Salehi M, Gastaldelli A, D'Alessio DA. Altered islet function and insulin clearance cause hyperinsulinemia in gastric bypass patients with symptoms of postprandial hypoglycemia. J Clin Endocrinol Metab. 2014;99(6):2008–17.
Patti ME, Li P, Goldfine AB. Insulin response to oral stimuli and glucose effectiveness increased in neuroglycopenia following gastric bypass. Obesity (Silver Spring). 2015;23(4):798–807.
Gavin III JR, Alberti KG, Davidson MB, et al. Report of the expert committee on the diagnosis and classification of diabetes mellitus. Diabetes Care. 1997;20(7):1183–97.
Schmidt JB, Gregersen NT, Pedersen SD, et al. Effects of PYY3-36 and GLP-1 on energy intake, energy expenditure, and appetite in overweight men. Am J Physiol Endocrinol Metab. 2014;306(11):E1248–56.
Svane MS, Jorgensen NB, Bojsen-Moller KN, et al. Peptide YY and glucagon-like peptide-1 contribute to decreased food intake after Roux-en-Y gastric bypass surgery. Int J Obes. 2016;40(11):1699–706.
Gold L, Ayers D, Bertino J, et al. Aptamer-based multiplexed proteomic technology for biomarker discovery. PLoS One. 2010;5(12):e15004.
Argmann CA, Houten SM, Champy MF, Auwerx J. Lipid and bile acid analysis. Curr Protoc Mol Biol. 2006; Chapter 29:Unit 29B.2. https://doi.org/10.1002/0471142727.mb29b02s75.
Patti ME, Houten SM, Bianco AC, et al. Serum bile acids are higher in humans with prior gastric bypass: potential contribution to improved glucose and lipid metabolism. Obesity (Silver Spring). 2009;17(9):1671–7.
Ritchie ME, Phipson B, Wu D, et al. Limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res. 2015;43(7):e47.
Team RDC. R: a language and environment for statistical computing: R Foundation for Statistical Computing; 2007. https://www.r-project.org/about.html.
Makishima M, Okamoto AY, Repa JJ, et al. Identification of a nuclear receptor for bile acids. Science. 1999;284(5418):1362–5.
Parks DJ, Blanchard SG, Bledsoe RK, et al. Bile acids: natural ligands for an orphan nuclear receptor. Science. 1999;284(5418):1365–8.
Wang H, Chen J, Hollister K, et al. Endogenous bile acids are ligands for the nuclear receptor FXR/BAR. Mol Cell. 1999;3(5):543–53.
Schirra J, Sturm K, Leicht P, et al. Exendin(9-39)amide is an antagonist of glucagon-like peptide-1(7-36)amide in humans. J Clin Invest. 1998;101(7):1421–30.
Salehi M, Gastaldelli A, D'Alessio DA. Blockade of glucagon-like peptide 1 receptor corrects postprandial hypoglycemia after gastric bypass. Gastroenterology. 2014;146(3):669–80 e2.
Xie MH, Holcomb I, Deuel B, et al. FGF-19, a novel fibroblast growth factor with unique specificity for FGFR4. Cytokine. 1999;11(10):729–35.
Fu L, John LM, Adams SH, et al. Fibroblast growth factor 19 increases metabolic rate and reverses dietary and leptin-deficient diabetes. Endocrinology. 2004;145(6):2594–603.
Tomlinson E, Fu L, John L, et al. Transgenic mice expressing human fibroblast growth factor-19 display increased metabolic rate and decreased adiposity. Endocrinology. 2002;143(5):1741–7.
Morton GJ, Matsen ME, Bracy DP, et al. FGF19 action in the brain induces insulin-independent glucose lowering. J Clin Invest. 2013;123(11):4799–808.
Ryan KK, Tremaroli V, Clemmensen C, et al. FXR is a molecular target for the effects of vertical sleeve gastrectomy. Nature. 2014;509(7499):183–8.
Hu X, Xiong Q, Xu Y, et al. Association of serum fibroblast growth factor 19 levels with visceral fat accumulation is independent of glucose tolerance status. Nutr Metab Cardiovasc Dis. 2018;28(2):119–25.
Pournaras DJ, Glicksman C, Vincent RP, et al. The role of bile after Roux-en-Y gastric bypass in promoting weight loss and improving glycaemic control. Endocrinology. 2012;153(8):3613–9.
Jansen PL, van Werven J, Aarts E, et al. Alterations of hormonally active fibroblast growth factors after Roux-en-Y gastric bypass surgery. Dig Dis. 2011;29(1):48–51.
Jorgensen NB, Dirksen C, Bojsen-Moller KN, et al. Improvements in glucose metabolism early after gastric bypass surgery are not explained by increases in total bile acids and fibroblast growth factor 19 concentrations. J Clin Endocrinol Metab. 2015;100(3):E396–406.
Nemati R, Lu J, Dokpuang D, et al. Increased bile acids and FGF19 after sleeve gastrectomy and Roux-en-Y gastric bypass correlate with improvement in type 2 diabetes in a randomized trial. Obes Surg. 2018;28:2672–86.
Gomez-Ambrosi J, Gallego-Escuredo JM, Catalan V, et al. FGF19 and FGF21 serum concentrations in human obesity and type 2 diabetes behave differently after diet- or surgically-induced weight loss. Clin Nutr. 2017;36(3):861–8.
Harris LLS, Smith GI, Mittendorfer B, et al. Roux-en-Y gastric bypass surgery has unique effects on postprandial FGF21 but not FGF19 secretion. J Clin Endocrinol Metab. 2017;102(10):3858–64.
Craig CM, Liu LF, Nguyen T, et al. Efficacy and pharmacokinetics of subcutaneous exendin (9-39) in patients with post-bariatric hypoglycaemia. Diabetes Obes Metab. 2018;20(2):352–61.
Tremaroli V, Karlsson F, Werling M, et al. Roux-en-Y gastric bypass and vertical banded gastroplasty induce long-term changes on the human gut microbiome contributing to fat mass regulation. Cell Metab. 2015;22(2):228–38.
Flynn CR, Albaugh VL, Cai S, et al. Bile diversion to the distal small intestine has comparable metabolic benefits to bariatric surgery. Nat Commun. 2015;6:7715.
Sachdev S, Wang Q, Billington C, et al. FGF 19 and bile acids increase following Roux-en-Y gastric bypass but not after medical management in patients with type 2 diabetes. Obes Surg. 2016;26(5):957–65.
Thoni V, Pfister A, Melmer A, et al. Dynamics of bile acid profiles, GLP-1, and FGF19 after laparoscopic gastric banding. J Clin Endocrinol Metab. 2017;102(8):2974–84.
Zhang H, DiBaise JK, Zuccolo A, et al. Human gut microbiota in obesity and after gastric bypass. Proc Natl Acad Sci U S A. 2009;106(7):2365–70.
Funding
This study received research grant funding from the American Society of Metabolic and Bariatric Surgery and Medimmune (both to MEP), a pilot award for plasma proteomic assay from SomaLogic (to MEP), T32 DK007260 and Hearst Fellowship (to CMM), American Diabetes Association 7–13-CE-17 (to ABG), RC1 DK086918 (to ABG), R56 DK095451 (to ABG), and P30 DK036836 (Joslin DRC). This work was conducted with support from Harvard Catalyst|The Harvard Clinical and Translational Science Center (National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health Award UL1 TR001102). NNF Center for Basic Metabolic Research, University of Copenhagen, NNF application number: 13563 (Novo Nordisk Foundation, Denmark), EliteForsk Rejsestipendiat (2016), The Danish Council for Independent Research (DFF–1333-00206A), European Research Council (Grant no.695069), Augustinus Foundation, and Aase og Ejnar Danielsens Fond. The following are the Stanford study funding sources: KL2 TR 001083, UL1 TR001085, and L30 TR001569-01 (to CMC), and a Fellow Pilot Award from the Stanford Translational Research and Applied Medicine (TRAM) Program of Stanford University School of Medicine (to CMC).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest Statement
Dr. Patti has consulted for Eiger Pharmaceuticals; has received investigator-initiated grant support from Janssen Pharmaceuticals, Medimmune, Sanofi, Astra-Zeneca, Jenesis, and Nuclea; has been a site investigator for XOMA; and acknowledges clinical trial research trial product support from Ethicon, Covidien, NovoNordisk, Nestle, and Dexcom within the past 5 years. Dr. Patti and Dr. Goldfine disclose a patent application for plasma proteins contributing to hypoglycemia. Dr. Mulla, Dr. Dreyfuss, Dr. Houten, Dr. Pan, Dr. Pober, Dr. Wewer Albrechtsen, Dr. Svane, Dr. Schmidt, Dr. Holst, Dr. Craig, and Dr. McLaughlin declare no potential competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(PPTX 3010 kb)
Rights and permissions
About this article
Cite this article
Mulla, C.M., Goldfine, A.B., Dreyfuss, J.M. et al. Plasma FGF-19 Levels are Increased in Patients with Post-Bariatric Hypoglycemia. OBES SURG 29, 2092–2099 (2019). https://doi.org/10.1007/s11695-019-03845-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11695-019-03845-0