Abstract
Background
This study aims to quantify changes in fibroblast growth factor 19 (FGF19) and bile acids (BAs) in patients with uncontrolled type 2 diabetes randomized to Roux-en-Y gastric bypass (RYGB) vs intensive medical management (IMM) and matched for similar reduction in HbA1c after 1 year of treatment.
Methods
Blood samples were drawn from patients who underwent a test meal challenge before and 1 year after IMM (n = 15) or RYGB (n = 15).
Results
Mean HbA1c decreased from 9.7 to 6.4 % after RYGB and from 9.1 to 6.1 % in the IMM group. At 12 months, the number of diabetes medications used per subject in the RYGB group (2.5 ± 0.5) was less than in the IMM group (4.6 ± 0.3). After RYGB, FGF19 increased in the fasted (93 ± 15 to 152 ± 19 pg/ml; P = 0.008) and postprandial states (area under the curve (AUC), 10.8 ± 1.9 to 23.4 ± 4.1 pg × h/ml × 103; P = 0.006) but remained unchanged following IMM. BAs increased after RYGB (AUC ×103, 6.63 ± 1.3 to 15.16 ± 2.56 μM × h; P = 0.003) and decreased after IMM (AUC ×103, 8.22 ± 1.24 to 5.70 ± 0.70; P = 0.01). No changes were observed in the ratio of 12α-hydroxylated/non-12α-hyroxylated BAs. Following RYGB, FGF19 AUC correlated with BAs (r = 0.54, P = 0.04) and trended negatively with HbA1c (r = −0.44; P = 0.09); these associations were not observed after IMM.
Conclusions
BA and FGF19 levels increased after RYGB but not after IMM in subjects who achieved similar improvement in glycemic control. Further studies are necessary to determine whether these hormonal changes facilitate improved glucose homeostasis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Bariatric surgery is currently the most effective method for attaining long-term weight reduction and has become a therapeutic option for the treatment of type 2 diabetes mellitus (T2DM) in obese patients [1–3]. Growing evidence indicates that several changes in neurohormonal regulators of energy balance and glucose homeostasis following Roux-en-Y gastric bypass surgery (RYGB) contribute to long-term weight loss and improvement in glucose control in individuals with T2DM [4–7]. The alterations in the direction and transit speed of nutrient flow most likely change the secretory pattern of gastrointestinal hormones involved in metabolic regulation [8].
Recently, the many positive metabolic effects of fibroblast growth factor 19 (FGF19), a protein produced by the mucosal cells of the terminal ileum, have been recognized. These include the following: (1) decreased gluconeogenesis, (2) increased glycogen and protein synthesis, (3) increased metabolic rate, (4) decreased adiposity, (5) regulation of gallbladder filling, and (6) regulation of bile acid (BA) homeostasis via feedback inhibition of the hepatic bile acid synthesis rate-governing enzyme CYP7A1 [9–13]. Indeed, FGF19 transgenic mice have decreased adiposity and are resistant to high-fat diet-induced weight gain. Infusion of recombinant FGF19 protein or transgenic FGF19 expression reduces hepatic lipid accumulation and improves insulin sensitivity in leptin-deficient and diet-induced obese mice [14, 15]. Moreover, targeted genetic disruption of Farnesoid X receptor (FXR) in mice substantially reduces the ability of vertical sleeve gastrectomy to reduce body weight and improve glucose tolerance [16].
FGF19 secretion is stimulated by BAs binding to the FXR on mucosal cells of the terminal ileum. Through FXR stimulation, BAs also decrease de novo lipogenesis and VLDL-TG export [17]. Other actions of BAs are mediated via the membrane G protein-coupled receptor TGR5. Through TGR5, BAs increase skeletal muscle and brown adipose tissue energy expenditure and stimulate secretion of the incretin, glucagon-like peptide-1 (GLP-1), which promotes satiety and stimulates β-cell insulin release [18–20]. The different chemical properties of BAs alter their biological functions. FXR is predominantly stimulated by CDCA and its conjugated derivatives [21], and DCA is a partial antagonist interfering with the ability of CDCA to activate FXR [22]. Haeusler et al. found that in healthy subjects, insulin resistance correlates with a higher ratio of fasting plasma 12a-hydroxylated/non-12a-hyroxylated; however, this increase was not found in T2DM subjects [23].
The many positive metabolic effects of FGF19 and BAs raise the question of whether they play a role in weight loss and improved glucose homeostasis following RYGB. Others have reported increased fasting FGF19 and BA levels in patients after RYGB [24–28]. Increased postprandial levels of BAs have also been reported [29] and appear to be specific to RYGB, and not weight loss per se, since they are not observed after laparoscopic adjustable gastric banding [30]. In T2DM patients, increases in fasting levels of FGF19 and BAs following RYGB are greater in those who experience remission of DM compared with diabetics who do not experience remission [31]. To date, studies comparing changes in both fasting and postprandial levels of FGF19 and BAs following RYGB vs changes brought about through intensive medical management (IMM) alone are lacking in patients with uncontrolled T2DM.
The primary aim of this study was to quantify changes in circulating levels of FGF19 and BAs in patients with T2DM following RYGB vs IMM treatment who have been matched for glycemic control. We hypothesized that levels of FGF19 and BAs would be significantly increased following RYGB, but not IMM despite similar improvement in glycemia.
Materials and Methods
Study Protocol
This investigation was an ancillary study to the Diabetes Surgery Study (DSS), a multicenter trial that randomized 120 patients with T2DM to intensive lifestyle and medical management with or without RYGB. The first 15 subjects (10 from Columbia University Medical Center and 5 from the University of Minnesota) who underwent RYGB and from whom plasma was available were studied. The first 15 IMM subjects (7 from Columbia University Medical Center and 8 from the University of Minnesota) who matched for mean 12-month HbA1C and change in HbA1C with the RYGB group were included as the control group. Detailed enrollment criteria, randomization methods, study protocol, and 12-month clinical outcomes have been reported [32, 33]. Key inclusion criteria were as follows: BMI 30−39.9 kg/m2; HbA1c ≥8 %; serum C-peptide level >1.0 ng/ml 90 min after a liquid meal of Ensure (237 ml, 250 kcal, 6 g fat, 40 g carbohydrate, 9 g protein); and willingness to accept randomization. The mixed meal test was repeated 1 year after interventions. IRB approval was obtained at each study site and all subjects signed written informed consent. The study was registered in Clinical Trials.gov: NCT00641251.
Assays
Serum insulin was measured with the Immulite Analyzer (Siemens, Los Angeles, CA). Total GLP-1 was measured by enzyme-linked immunosorbent assay (ELISA) (Millipore, St. Charles, MO). The sensitivity, intra-assay coefficient of variation, and inter-assay coefficient of variation were 1.5 pM, 2 %, and <12 %, respectively. FGF19 levels were determined by ELISA (R&D Systems, Minneapolis, MN) with a minimum detection limit of 1.2 pg/ml, and intra-assay and inter-assay coefficients of 6.5 and 5.5 %, respectively. BAs were measured by LC-MS with an assay sensitivity of 0.01 μM.
Calculations and Statistical Analysis
Data is presented as mean values ± SEM. Homeostasis model assessment of insulin resistance (HOMA-IR) was calculated as reported by Matthews et al. [34], and Matsuda Index was calculated as a measure of insulin sensitivity determined from the mixed meal challenge [35]. Differences at baseline and at 12 months between groups were assessed with t tests. Change from baseline within group was assessed using paired t tests. Between-group differences in change between baseline and 12 months were assessed using two-sample t tests. Statistical analysis was performed with Statistical Analysis Software (version 9.3, SAS Institute Inc., Cary, NC). A two-sided P value <0.05 was considered statistically significant.
Results
Subject Characteristics and Glucostatic Parameters
Characteristics of the 30 subjects before and 1 year after randomization to IMM or RYGB are shown in Table 1. At baseline, there were no differences in clinical characteristics such as age, weight, BMI, duration of DM, or the use of diabetes medications including insulin. Mean total body weight decreased by 11.4 ± 2.3 % and 28.9 ± 2.2 % for IMM and RYGB subjects, respectively. Both mean 12-month HbA1C and change in HbA1C were comparable between groups; however, the number of DM medications used per subject was significantly greater in the IMM group (Table 1). Fasting glucose and insulin decreased significantly in RYGB subjects, with only fasting glucose decreasing significantly in IMM subjects (Table 2). Insulin resistance as determined by HOMA-IR decreased from 14.1 ± 3.6 to 2.1 ± 0.9 in RYGB patients vs a nonsignificant decrease from 13.0 ± 2.5 to 7.0 ± 1.7 in IMM patients (P = 0.18 for between-group change). Similarly, Matsuda Index increased from 3.6 ± 1.0 to 11.1 ± 1.6 in RYGB patients vs an increase from 1.9 ± 0.3 to 5.7 ± 1.7 in IMM patients (P = 0.10 for between-group change). While area under the curve (AUC) for insulin did not change significantly, the pattern of secretion was altered after RYGB with an accelerated time to reach peak levels (Table 2, Fig. 1).
FGF19
After RYGB, fasting FGF19 levels increased by 62 %; there was an even more pronounced 96 % increase in the postprandial state as measured by AUC (Table 2, Fig. 2). There was no change in fasting or postprandial FGF19 levels in IMM subjects. Neither fasting nor AUC FGF levels at 12 months correlated with weight loss in IMM patients (r = 0.43 and 0.42 for weight loss vs fasting FGF and AUC FGF, respectively; P = 0.13 for both). Post-RYGB FGF19 levels also did not correlate with weight loss in the fasted (r = 0.08; P = 0.78) or postprandial state (r = 0.16; P = 0.56). At 12 months, there was a trend towards a negative association between FGF AUC levels and HbA1C (r = −0.44; P = 0.09) in RYGB subjects, which was not apparent in IMM subjects (r = 0.04; P = 0.88).
Bile Acids
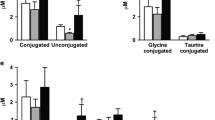
Following IMM, postprandial total BA levels decreased and fasting levels trended towards decreased levels as well (Table 2, Fig 3). Measurements of individual BAs are presented in Table 3. Following RYGB, total BAs increased most markedly in the postprandial state, with a 129 % increase in total BA AUC and a 64 % increase in total BA fasting levels (Table 2; Fig. 3). Changes were observed in the composition of BA species with a decrease after RYGB and an increase after IMM in secondary unconjugated BAs (Table 2). No changes were observed in the ratio of 12α-hydroxylated/non-12α-hyroxylated BAs. Prior to surgery, total BAs did not correlate with FGF19 levels. After surgery, there was a significant correlation of total BA and FGF19 levels in the fasted and postprandial states (r = 0.56, P = 0.03 and r = 0.54, P = 0.04, respectively) only in the RYGB group. There were no significant correlations between total BAs and HbA1c in either group.
Discussion
The objective of this study was to quantify glycemic control independent changes induced by RYGB compared to IMM in FGF19 levels, and from here to contextualize these changes with BA levels. We have demonstrated increases in fasting and postprandial levels of both FGF19 and total BAs that are specific to RYGB, and not simply improved glycemic control. Furthermore, there is an accelerated time to peak postprandial levels that is observed post-RYGB for both FGF19 and total BAs. These findings are consistent with findings of Wewalka et al., who also noted that improved glycemic control via IMM in T2DM patients did not increase fasting BA levels [36]. Though it is impossible to exclude the confounding effect of greater weight loss after RYGB, the study by Kohli et al., which controlled for weight loss and demonstrated significantly increased levels of BAs in non-diabetic patients undergoing RYGB but not LAGB, suggests that weight loss alone does not account for increased levels of BAs [30]. Furthermore, lack of correlation between FGF19 or BAs and weight change in RYGB patients suggests that metabolic effects mediated by FGF19 and BA are independent of total weight loss.
The hormone changes observed in this study may reflect increased efficiency of enterohepatic circulation following intestinal rerouting after RYGB. In the immediate postoperative period, a more efficient and undiluted flow of bile acids through the biliopancreatic loop isolated from food in the alimentary loop may result in a quicker and more robust uptake of bile acids into the terminal ileum, thereby leading to faster and increased postprandial FXR-mediated expression of FGF19. In fact, accelerated postprandial BA curves have been noted as early as 4 weeks post-RYGB [29]. Over time, BAs may have a trophic effect on ileal mucosa, as demonstrated by ileal mucosa hyperplasia following surgical translocation of the ileum to a location directly distal to the duodenum in rodent studies [37]. Mucosal hyperplasia, with presumably increased expression of BA transporters, may lead to increased BA reuptake and consequent FGF19 secretion, thereby sustaining increased levels of both hormones over time. Positive correlation between postoperative FGF19 and total BA levels highlights the potential post-RYGB interplay between these hormones. Given the negative feedback loop between FGF19 and bile acids, it is possible that the increased levels of FGF19 in turn suppress CYP7A1, thereby preventing sustained elevated levels of potentially toxic BAs.
Following RYGB, all BA species trended towards increased fasting levels and demonstrated statistically significant increases in AUC values (with the exception of the primary unconjugated subset, which only trended towards significance). These findings are consistent with findings reported by Kohli et al., who demonstrated a proportionate postprandial increase in all BA species, but differed from other studies that have reported decreases in the fasting taurine-conjugated fraction or no change in the AUC of the unconjugated fraction following RYGB [28–30]. Nevertheless, the proposed mechanism of enhanced enterohepatic efficiency is consistent with a more ubiquitous increase in postprandial levels of BA species. The percent composition of BA species did change in the fasted state, with an increase in primary unconjugated BAs and a decrease in secondary unconjugated BAs. Given that secondary BAs are produced in the distal small intestine or colon by bacterial enzymes, the intestinal rerouting and changes in intestinal microbiota known to accompany RYGB may certainly account for the decreased percent of secondary unconjugated BAs [38]. Lack of change in the ratio of 12a-hydroxylated/non-12a-hyroxylated bile acids despite improved insulin resistance was not unexpected given that the relative increase in this ratio has been associated with insulin resistance in healthy non-diabetic rather than diabetic subjects [23].
We did not detect a positive correlation between total BAs and GLP-1 that would be expected given the action of BAs on TGR5 (data not shown). Steinert et al. also did not find an association between postprandial plasma BAs and GLP-1 levels [39] although other groups have reported a positive association between peak GLP-1 levels and peak BA levels [24, 30]. These seemingly discrepant findings may be due to different timing of sample collections and/or composition of meal stimuli [40]. Nevertheless, our observation that BAs positively correlate with FGF19 and not GLP-1 raises the interesting possibility that the post-RYGB effects of BAs may occur predominantly through FXR rather than TGR5. Consistent with this hypothesis, Meyer-Gerspach et al. demonstrated that a physiological load of CDCA is a weak stimulus for GLP-1 with no insulinotropic GLP-1-dependent effects on glucose homeostasis or appetite perceptions [41]. Instead, intraduodenal infusion of CDCA resulted in an impressive dose-dependent increase in FGF19 and attenuated insulin release after an OGTT, leading them to similarly implicate the FXR pathway as the predominant means through which BAs exert glycemic control. Importance of FXR signaling has also been demonstrated in a rodent model of vertical sleeve gastrectomy, where despite increased circulating bile acids the ability of surgery to reduce body weight and improve glucose tolerance was significantly reduced in the absence of FXR [16].
A strength of this study is the similar improvement in HbA1c between groups which effectively rules out the possibility that improved glycemia is casual to the differences in BA and FGF19 levels. A limitation of the study is that greater weight loss is likely to have contributed to improvement in HbA1c after surgery. It is also difficult to compare groups given that fewer diabetes medications were required to achieve similar HbA1c reduction post-RYGB. Clearly, more studies are necessary to untangle the different mediators of glycemic control that occur with various methods of weight reduction.
References
Brolin RE. Bariatric surgery and long-term control of morbid obesity. JAMA. 2002;288(22):2793–6.
Dixon JB, Zimmet P, Alberti KG, et al. Bariatric surgery: an IDF statement for obese type 2 diabetes. Diabet Med. 2011;28(6):628–42.
American Diabetes Association. Approaches to glycemic treatment. Diabetes Care. 2015; 38 Suppl:S41-8.
Korner J, Bessler M, Cirilo LJ, et al. Effects of Roux-en-Y gastric bypass surgery on fasting and postprandial concentrations of plasma ghrelin, peptide YY, and insulin. J Clin Endocrinol Metab. 2005;90(1):359–65.
Korner J, Inabnet W, Conwell IM, et al. Differential effects of gastric bypass and banding on circulating gut hormone and leptin levels. Obesity. 2006;14(9):1553–61.
Korner J, Inabnet W, Febres G, et al. Prospective study of gut hormone and metabolic changes after adjustable gastric banding and Roux-en-Y gastric bypass. Int J Obes. 2009;33(7):786–95.
Nguyen KT, Korner J. The sum of many parts: potential mechanisms for improvement in glucose homeostasis after bariatric surgery. Curr Diab Rep. 2014;14(5):481.
Nguyen NQ, Debreceni TL, Bambrick JE, et al. Rapid gastric and intestinal transit is a major determinant of changes in blood glucose, intestinal hormones, glucose absorption and postprandial symptoms after gastric bypass. Obesity (Silver Spring). 2014;22(9):2003–9.
Inagaki T, Choi M, Moschetta A, et al. Fibroblast growth factor 15 functions as an enterohepatic signal to regulate bile acid homeostasis. Cell Metab. 2005;2(4):217–25.
Choi M, Moschetta A, Bookout AL, et al. Identification of a hormonal basis for gallbladder filling. Nat Med. 2006;12(11):1253–5.
Kir S, Beddow SA, Samuel VT, et al. FGF19 as a postprandial, insulin-independent activator of hepatic protein and glycogen synthesis. Science. 2011;331(6024):1621–4.
Kir S, Kliewer S, Mangelsdorf D. Roles of FGF19 in liver metabolism. Cold Spring Harbor Symp Quant Biol. 2011;LXXVI:1–6.
Wu AL, Coulter S, Liddle C, et al. FGF19 regulates cell proliferation, glucose and bile acid metabolism via FGFR4-dependent and independent pathways. PLoS One. 2011;6(3), e17868.
Fu L, John LM, Adams SH, et al. Fibroblast growth factor 19 increases metabolic rate and reverses dietary and leptin-deficient diabetes. Endocrinology. 2004;145(6):2594–603.
Tomlinson E, Fu L, John L, et al. Transgenic mice expressing human fibroblast growth factor-19 display increased metabolic rate and decreased adiposity. Endocrinology. 2002;143(5):1741–7.
Ryan KK, Tremaroli V, Clemmensen C, et al. FXR is a molecular target for the effects of vertical sleeve gastrectomy. Nature. 2014;509(7499):183–8.
Watanabe M, Houten SM, Wang L, et al. Bile acids lower triglyceride levels via a pathway involving FXR, SHP, and SREBP-1c. J Clin Invest. 2004;113(10):1408–18.
Watanabe M, Houten SM, Mataki C, et al. Bile acids induce energy expenditure by promoting intracellular thyroid hormone activation. Nature. 2006;439(7075):484–9.
Katsuma S, Hirasawa A, Tsujimoto G. Bile acids promote glucagon-like peptide-1 secretion through TGR5 in a murine enteroendocrine cell line STC-1. Biochem Biophys Res Commun. 2005;329(1):386–90.
Thomas C, Gioiello A, Noriega L, et al. TGR5-mediated bile acid sensing controls glucose homeostasis. Cell Metab. 2009;10(3):167–77.
Modica S, Gadaleta RM, Moschetta A. Deciphering the nuclear bile acid receptor FXR paradigm. Nuclear Recept Signal. 2010;8, e005.
Parks DJ, Blanchard SG, Bledsoe RK, et al. Bile acids: natural ligands for an orphan nuclear receptor. Science. 1999;284(5418):1365–8.
Haeusler RA, Astiarraga B, Camastra S, et al. Human insulin resistance is associated with increased plasma levels of 12alpha-hydroxylated bile acids. Diabetes. 2013;62(12):4184–91.
Patti ME, Houten SM, Bianco AC, et al. Serum bile acids are higher in humans with prior gastric bypass: potential contribution to improved glucose and lipid metabolism. Obesity (Silver Spring Md). 2009;17(9):1671–7.
Nakatani H, Kasama K, Oshiro T, et al. Serum bile acid along with plasma incretins and serum high-molecular weight adiponectin levels are increased after bariatric surgery. Metabolism. 2009;58(10):1400–7.
Jansen PL, van Werven J, Aarts E, et al. Alterations of hormonally active fibroblast growth factors after Roux-en-Y gastric bypass surgery. Dig Dis. 2011;29(1):48–51.
Pournaras DJ, Glicksman C, Vincent RP, et al. The role of bile after Roux-en-Y gastric bypass in promoting weight loss and improving glycaemic control. Endocrinology. 2012;153(8):3613–9.
Simonen M, Dali-Youcef N, Kaminska D, et al. Conjugated bile acids associate with altered rates of glucose and lipid oxidation after Roux-en-Y gastric bypass. Obes Surg. 2012;22(9):1473–80.
Ahmad NN, Pfalzer A, Kaplan LM. Roux-en-Y gastric bypass normalizes the blunted postprandial bile acid excursion associated with obesity. Int J Obes (2005). 2013;37(12):1553–9.
Kohli R, Bradley D, Setchell KD, et al. Weight loss induced by Roux-en-Y gastric bypass but not laparoscopic adjustable gastric banding increases circulating bile acids. J Clin Endocrinol Metab. 2013;98(4):E708–12.
Gerhard GS, Styer AM, Wood GC, et al. A role for fibroblast growth factor 19 and bile acids in diabetes remission after Roux-en-Y gastric bypass. Diabetes Care. 2013;36(7):1859–64.
Ikramuddin S, Korner J, Lee WJ, et al. Roux-en-Y gastric bypass vs intensive medical management for the control of type 2 diabetes, hypertension, and hyperlipidemia: the Diabetes Surgery Study randomized clinical trial. JAMA. 2013;309(21):2240–9.
Thomas AJ, Bainbridge HA, Schone JL, et al. Recruitment and screening for a randomized trial investigating Roux-en-Y gastric bypass versus intensive medical management for treatment of type 2 diabetes. Obes Surg. 2014;24(11):1875–80.
Matthews DR, Hosker JP, Rudenski AS, et al. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28(7):412–9.
Matsuda M, DeFronzo RA. Insulin sensitivity indices obtained from oral glucose tolerance testing: comparison with the euglycemic insulin clamp. Diabetes Care. 1999;22(9):1462–70.
Wewalka M, Patti ME, Barbato C, et al. Fasting serum taurine-conjugated bile acids are elevated in type 2 diabetes and do not change with intensification of insulin. J Clin Endocrinol Metab. 2014;99(4):1442–51.
Kohli R, Kirby M, Setchell KD, et al. Intestinal adaptation after ileal interposition surgery increases bile acid recycling and protects against obesity-related comorbidities. Am J Physiol Gastrointest Liver Physiol. 2010;299(3):G652–60.
Furet JP, Kong LC, Tap J, et al. Differential adaptation of human gut microbiota to bariatric surgery-induced weight loss: links with metabolic and low-grade inflammation markers. Diabetes. 2010;59(12):3049–57.
Steinert RE, Peterli R, Keller S, et al. Bile acids and gut peptide secretion after bariatric surgery: a 1-year prospective randomized pilot trial. Obesity (Silver Spring). 2013;21(12):E660–8.
Morton GJ, Kaiyala KJ, Foster-Schubert KE, et al. Carbohydrate feeding dissociates the postprandial FGF19 response from circulating bile acid levels in humans. J Clin Endocrinol Metab. 2014;99(2):E241–5.
Meyer-Gerspach AC, Steinert RE, Keller S, et al. Effects of chenodeoxycholic acid on the secretion of gut peptides and fibroblast growth factors in healthy humans. J Clin Endocrinol Metab. 2013;98(8):3351–8.
Acknowledgments
The investigators express gratitude to all study participants and recognize the contributions of the study coordinators, Joyce Schone, RD and Nyra Wimmergren, RN (University of Minnesota), Heather Bainbridge RD, CDN (Columbia University Medical Center), Avis Thomas, MS (statistician, University of Minnesota Data Coordinating Center), and Irene M. Conwell and Tiffany Thomas, Ph.D. (Columbia University Medical Center) for expert technical assistance.
Conflict of Interest
All authors have completed and submitted Disclosure of Potential Conflicts of Interest. Dr Billington reports receiving grant support from Covidien and personal support for consultancy from EnteroMedics Inc. Dr Connett reports receiving grant support from Covidien. Dr Ikramuddin serves as an advisory board member for Novo Nordisk, USGI, and Medica; consults for Metamodix Inc; and receives grant support from Covidien, EnteroMedics, and ReShape Medical. Dr Korner reports receiving institutional grant support from Covidien, travel expenses from American Board of Obesity Medicine, and personal support for serving on the Takeda Speaker Bureau and on the Scientific Advisory Board for Nutrisystem and consulting for the Office of Professional Misconduct and L.E.K Consulting.
Ethical Approval
All procedures involving human participants were in accordance with the ethical standards of the institutional research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Funding
The study was supported in part by Covidien, Mansfield, MA. The sponsoring agency had no role in the collection, management, analysis, and interpretation of the study data and had no part in the preparation of the manuscript. The sponsor was allowed to review the manuscript prior to submission but had no role in the decision to submit the manuscript for publication. Additional support was received from National Institutes of Health grant DK072011 (J.K.), the National Center for Advancing Translations Sciences Grant UL1 TR000040, and discretionary funds from Columbia University Medical Center (to J.K.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sachdev, S., Wang, Q., Billington, C. et al. FGF 19 and Bile Acids Increase Following Roux-en-Y Gastric Bypass but Not After Medical Management in Patients with Type 2 Diabetes. OBES SURG 26, 957–965 (2016). https://doi.org/10.1007/s11695-015-1834-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11695-015-1834-0