Abstract
Background
Sleeve gastrectomy (SG) and Roux-en-Y gastric bypass (RYGB) are both effective bariatric procedures to treat type 2 diabetes (T2DM) and obesity. The contribution of changes in bile acids (BAs) and fibroblast growth factor19 (FGF19) to such metabolic improvements is unclear.
Methods
We examined associations between changes in BAs, FGF19 (fasting and prandial), with changes in body weight, glycemia, and other metabolic variables in 61 obese patients with T2DM before and 1 year after randomization to SG or RYGB.
Results
Weight loss and diabetes remission (defined by HbA1c < 39 mmol/mol [< 5.7%] in the absence of glucose-lowering therapy) after RYGB and SG was similar (mean weight loss − 29 vs − 31 kg, p = 0.50; diabetes remission proportion 37.5 vs 34%, p = 1.0). Greater increments in fasting and prandial levels of total, secondary, and unconjugated BAs were seen after RYGB than SG. Fasting and prandial increases in total (r = − 0.3, p = 0.01; r = − 0.2, p = 0.04), secondary (r = − 0.3, p = 0.01; r = − 0.4, p = 0.01) and unconjugated BA (r = − 0.3, p = 0.01; r = 0.4, p < 0.01) correlated with decreases in HbA1c, but not weight. Changes in 12α-OH/non 12α-OH were positively associated with prandial glucose increments (r = 0.2, p = 0.03), HbA1c (r = 0.3, p = 0.01), and negatively associated with changes in insulinogenc index (r = − 0.3, p = 0.01). Only changes in prandial FGF19 were negatively associated with HbA1c (r = − 0.4, p < 0.01) and visceral fat (r = − 0.3, p = 0.04).
Conclusions/interpretation
The association between increases in secondary, unconjugated BAs and improvements in HBA1c (but not weight) achieved after both RYGB and SG suggest manipulation of BA as a potential strategy for controlling T2DM through weight-independent means.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Bariatric surgery is considered to be the most effective treatment for obesity-related type 2 diabetes (T2DM) [1]. The two most common types of bariatric surgery are sleeve gastrectomy (SG) and Roux-en-Y gastric bypass (RYGB) [2]. Despite different anatomical alterations to the gut (resection of the stomach in SG vs disconnection of most of the stomach and duodenum from the passage of food in RYGB), both types of bariatric surgery achieve similar diabetes remission [3,4,5], although we have recently reported superior 1-year weight loss after banded RYGB [6]. The physiological mechanisms underlying these beneficial effects from bariatric surgery-related reduced gastric size and altered nutrient flow are thought to include changes to bile acid metabolism, gut hormones, gut microbiota, and vagal nerve signaling [7,8,9].
Of these mechanisms, changes to bile acid (BA) metabolism have been demonstrated to be of critical importance in animal studies. A rat study showed that bile diversion via inserting a catheter between the bile duct and the mid-jejunum for 4–5 weeks reduced weight and improved insulin resistance [10]. The main pathway for BA signaling is through binding to the farnesoid X receptor (FXR), without which, the weight loss and diabetes remission effects of SG were no longer sustained in FXR gene knock-out mice with diet-induced obesity [11].
BAs are thought to contribute to improvement of T2DM through a number of molecular pathways that impact on glucose metabolism: increased hepatic glycogenesis, energy expenditure via stimulatory effect on adaptive thermogenesis, gut hormones, and lipids due to the mutual relationship between BA metabolism and intestinal microbiome [8, 12]. BA signaling after binding to FXR induces secretion of fibroblast growth factor19 (FGF19) from enterocytes [13]. Transgenic FGF19 mice have lower fat content but higher brown fat-related energy expenditure and did not become obese or develop diabetes on a high-fat diet [14]. There is some evidence that FGF19 levels increase following RYGB [15, 16] and after SG [17] although it is not clear whether this correlates with any metabolic improvements seen.
Human studies showed that BAs are increased after RYGB caused by increased delivery of BAs to the distal gut [9], particularly in the postprandial state. However, it is not clear whether this increase in fasting or postprandial BAs occurs to a similar extent after SG [17,18,19,20], and to what extent such increases correspond with metabolic improvements seen. Since both types of surgery have distinct effects on gastric size and nutrient flow, yet produce similar T2DM remission, we aimed to investigate the changes in fasting and postprandial BAs and FGF19 1 year after either SG or RYGB. We reasoned that if BAs and FGF19 were important in achieving metabolic improvements seen after bariatric surgery, then changes in BAs and FGF19 after both types of surgery should correlate with changes in weight, glycemia/diabetes remission, lipid levels, appetite, and resting energy expenditure (REE) among obese people with T2DM pre-operatively.
Materials and Methods
Study Design and Subjects
The current study included data from 61 of 114 participants who took part in a single-center, prospective, randomized, double-blind (subjects and assessor) parallel design study comparing the effectiveness of laparoscopic SG and banded RYGB for the treatment of morbid obesity and T2DM at North Shore Hospital, Auckland, New Zealand. These 61 participants provided additional oral glucose tolerance test blood samples and appetite assessments at baseline and 1 year as described previously [6] and shown in supplementary Figure 1. In brief, the full randomized clinical trial consisted of 114 subjects who were randomized 1:1 (using computer generated random number codes) to either SG or RYGB, if they met the following eligibility criteria: aged between 20 and 55 years with T2DM for at least 6-month duration, 35 < BMI < 65 kg/m2 for at least 5 years and were suitable surgical candidates for either SG or RYGB. Exclusion criteria were current smokers, C-peptide more than 350 pmol/l, gestational diabetes, pregnancy, type 1 diabetes, or other type of diabetes related to chronic pancreatitis, oral steroid therapy. Primary outcome was T2D remission (defined by HbA1c < 42 mmol/mol [< 6%] in the absence of glucose-lowering therapy) at 5 years. Secondary outcomes included changes in body composition, food intake, appetite scores, and various mechanistic investigation using stored blood samples at baseline and 1 and 5 years [6]. All procedures performed were in accordance with the ethical standards of the New Zealand Health and Disability Ethics committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all individual participants included in the study. This study was prospectively registered at Australian New Zealand Clinical Trial Registry ANZCTR (ACTRN12611000751976) and retrospectively registered at https://clinicaltrials.gov (NCT01486680).
Surgical Protocol
All subjects scheduled for either SG or RYGB were prescribed three servings daily of very low-calorie diet (Optifast Nestle, Vevey, Switzerland) plus vegetables for a 2-week period prior to the surgery. All surgeries were performed at a single center in North Shore Hospital, Auckland, New Zealand. The surgical protocol has been described previously [6]. SG involved a longitudinal resection of the stomach against a 32-French bougie from just lateral to the angle of His to 2 cm proximal to the pylorus. RYGB was performed with a 100-cm antecolic Roux limb with hand-sewn pouch (gastro) jejunostomy, a 50-cm biliopancreatic limb, and a hand-sewn small bowel anastomosis (jejunojejunostomy).
Data Collection
All participants in this sub-study were assessed in the Body Composition Laboratory at the University of Auckland. Participants were assessed after an overnight fast at baseline (1–3 days pre-operatively), and at 1 year post-operatively. Any glucose-lowering treatments were stopped for 24 h before these assessments. Participants wore light clothing and were barefoot when height and weight were measured. Total body fat (TBF), fat free mass (FFM), bone mineral content (BMC), and abdominal and visceral fat were measured by dual-energy X-ray absorptiometry (DXA, model iDXA, software V.15, GE-Lunar, Madison, Wisconsin, USA). REE was measured by Deltatrac Metabolic Monitor (MBM-100, Datex Instruments, Helsinki, Finland). Just before OGTT (fasted state), participants were asked to self-rate and record how hungry they were, how full did they feel, how strong was their desire to eat, and how much food could they eat using a visual analogue scale as described previously [21].
All patients had a standard oral glucose tolerance test with 75-g glucose load. Blood samples were collected at fasting, then at 15, 30, 60, 90, and 120 min after taking a 75-g glucose drink.
Biochemical Assays
Samples from fasting and all 5 postprandial time points were used to measure glucose and insulin and AUC of BAs were measured up to 60 min. Plasma lipids were only measured in the fasting state. FGF19 was measured at fasting and 120-min time points.
All 13 individual BAs were measured by a liquid chromatography–tandem mass spectrometry (LC-MS/MS) method described previously [22] with slight modifications to achieve best detection and quantification using an Agilent 6420 triple quadrupole LC-MS system (Agilent Technologies, Santa Clara, CA, USA).
FGF19 levels were measured by a commercially available human FGF19 sandwich ELISA kit (RayBiotech, Inc. GA, USA) with intra-assay CV, 4.5% and inter-assay CV, 6.5%.
Lipid profile, plasma glucose, and insulin were determined by an autoanalyzer (Roche Diagnostics, Basel, Switzerland) based on Roche manufacturer’s protocols. HbA1C was measured by high-performance liquid chromatography (Bio-Rad Laboratories, Hercules, CA, USA).
Definition of Variables and Mathematical Modeling
BAs were categorized based on their mechanism of synthesis and potential metabolic signaling significance, as total BAs, primary and secondary BAs, conjugated and unconjugated BAs, 12α-hydroxylation (12 α-OH) and non 12α-hydroxylation (non 12 α-OH) BAs and their ratios, glycine and taurine BAs. The computation formulae are described in supplemental Table 1. Concentrations of BAs and their computed forms are presented in picogram per milliliter.
Diabetes indices included homeostatic model assessment of insulin resistance (HOMA-IR) and beta cell function (HOMA-B), Matsuda insulin sensitivity index (ISI), insulinogenic index (IGI), and quantitative insulin sensitivity check index (QUICKI) calculated as previously described [23].
Statistical Analysis
Statistical Package for the Social Sciences (SPSS) version 22 was used for statistical analysis. Sample size determination for the full clinical trial is described in the published protocol [6]. No sample size calculation was conducted for this mechanistic sub-study. For normally distributed data, the results are presented as mean ± standard deviation (SD) unless stated otherwise. Comparisons within and between surgical groups were examined using paired and unpaired Student’s t tests for normally distributed data and, for non-normally distributed data, Wilcoxon signed-ranks test and Mann–Whitney U test, respectively. Correlation analysis was performed using Pearson or Spearman rank tests as appropriate. Categorical data were compared using Fisher’s exact test. Areas under the curve were calculated with the trapezoidal rule. ANCOVA was performed to compare variables at 1 year between SG and RYGB, adjusting for baseline values and gender. Linear mixed model analysis was utilized to compare changes in REE between SG and RYGB, adjusting for covariates. OGTT data were compared at 1 year between SG and RYGB using mixed model analysis. Logarithmic transformation was used for non-normally distributed data. If two-tailed p value was less than 0.05, it was considered as significant.
Data Availability Statement
Datasets generated and analyzed during the current study are available from the corresponding authors upon reasonable request.
Results
Baseline Characteristics of Participants
Table 1 tabulates clinical characteristics of all participants pre-operatively. All had T2DM and 55 out of 61 patients (~ 90%) were treated by glucose-lowering treatments before surgery. There were no significant differences between fasting and prandial BA fractions and FGF19 between four participants who had previous cholecystectomy and the remaining participants at baseline (supplemental Table 2).
Changes in Diabetes Indices and Lipids 1 Year After SG and RYGB
The proportions achieving diabetes remission (defined by HbA1C < 39 mmol/mol (5.7%)) in the absence of glucose-lowering medications 1 year after SG and RYGB were 34.5% (10/29) and 37.5% (12/32), respectively, and not significantly different between types of surgery (p = 1.00). Table 2 shows that in the presence of reduced weight and reduced glucose-lowering treatments, fasting glucose did not change, but the glucose AUC and HbA1c improved after both SG and RYGB. All other diabetes indices showed significant improvements after both procedures but were not significantly different between SG and RYGB.
Fasting lipid profile improved after both SG and RYGB, except there were no changes in total cholesterol and LDL-C a year after RYGB. There were no significant differences between SG and RYGB.
Weight, Body Composition, Resting Energy Expenditure, and Appetite Changes
Table 2 shows that at 1 year after both types of bariatric surgery, weight and BMI reduced significantly from baseline. After adjusting for baseline values and gender weight and BMI (for both p = 0.03), TBF (p = 0.019), visceral (p = 0.026), and trunk fat (p = 0.009), but not abdominal fat (p = 0.14), were significantly lower after RYGB compared to SG. REE significantly reduced a year after SG and RYGB compared with baseline (− 318 ± 364 vs. − 276 ± 431 kcal/day, respectively) with no difference between the groups after adjusting for gender, age, and changes in FFM and TBF (p = 0.51). Of all four appetite rating scores, only the perception of the amount of food that could be consumed declined significantly: − 12 mm (− 22–8.0), p = 0.04, after SG and − 15 mm (− 50–7.0), p = 0.007, after RYGB, with no significant difference between SG and RYGB (p = 0.3).
Changes in Fasting and Post-OGTT Levels of BAs and FGF19 after SG and RYGB
The changes in fasting BAs 1 year after SG and RYGB (relative to baseline values) are shown in Table 3. Total bile acids (both primary and secondary) increased after both types of surgery, but to a greater extent after RYGB. Both secondary unconjugated BAs (DCA and LCA) increased after both types of surgery, but LCA increased significantly more after RYGB (p < 0.0001). Increases in secondary conjugated BAs (THDCA>GUDCA>TUDCA) occurred to a similar extent after both types of surgery. There was a greater increase in taurine BAs than glycine BAs after both types of surgery, but the increase in taurine BAs was greater after RYGB (p = 0.04). There was a greater increase in non 12 α-OH species than 12 α-OH species after both types of surgery, but the increase in none 12 α-hydroxylated species was greater after RYGB (p = 0.0002).
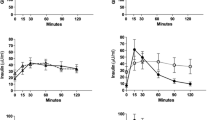
All AUCs0–60min of BA fractions significantly increased a year after both SG and RYGB (all p < 0.01) except AUC0–60min of glycine after RYGB (p = 0.1) (Fig. 1). The changes of post-glucose BA profiles over 60 min, relative to baseline BA profiles, with their corresponding changes in AUC over 1 year are shown in Fig. 2. The increases in post-glucose AUC0–60min for total BAs, secondary BAs, and unconjugated BAs at 1 year were greater after RYGB compared to SG (Fig. 2).
Changes in BAs fractions (log-transformed data) between baseline and 1 year post-operatively in patients who underwent sleeve gastrectomy (SG) or Roux-en-Y gastric bypass (RYGB) as time course during OGTT and as AUC0–60min. Data are mean ± SE. P values show the group × time interaction effect. *p < 0.05, **p < 0.01, ***p < 0.0001 for between-surgery comparisons at each time point and changes in AUC0–60min between SG and RYGB
Fasting and 2 h FGF19 significantly increased 1 year after both surgeries and were not significantly different between SG and RYGB at 1-year follow-up (Table 2). No significant associations were observed between 1-year changes of fasting FGF19 and BA fractions (data not shown).
Differences in BA and FGF19 by Diabetes Remission Status
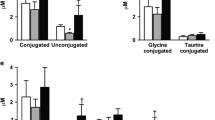
Fasting levels and AUC0–60min of all BA groups were similar at baseline among those who subsequently achieved diabetes remission and those who did not (Fig. 3a, b). At 1 year, fasting levels and prandial AUC0–60min of all BA groups were similar by diabetes remission status (Fig. 3c, d). Similarly, no significant differences observed in the changes in fasting levels and AUC0–60min of all BA groups who achieved diabetes and those who did not (Fig. 3e, f).
Concentrations of bile acid (BA) fractions at fasting (a) and AUC during OGTT (b) before surgery. Concentrations of bile acid (BA) fractions at fasting (c) and AUC during OGTT (d) at 1 year after surgery. Changes in concentrations of bile acid (BA) fractions between baseline and 1 year after surgery at fasting (e) and AUC during OGTT (f). Dotted bars represent those who achieved diabetes remission at 1 year after surgery, non-dotted bars represent those who did not achieve diabetes remission. All data are log-transformed and shown as mean ± SE
There were no significant differences in fasting or 2-h prandial levels of FGF19 by diabetes remission status (supplemental Figure 2).
Associations Between Changes in Metabolic Characteristics and Changes in Fasting and Prandial BA Groups
Changes in BMI or HOMA-IR were not associated with changes in any BA groups either in fasting or prandial states (Table 4). Only changes in HbA1c showed consistent correlations with changes in several BA groups in both fasting and prandial states. HbA1c was negatively associated with fasting and AUC0–60min of total BA, secondary BA, and unconjugated BAs. Changes in HbA1c were also negatively associated with changes in fasting primary BAs, and positively correlated with changes in fasting ratio of 12α-OH/non 12α-OH BAs.
Other metabolic characteristics showed several correlations only with fasting BA groups. Changes in visceral fat were negatively associated with changes in fasting total BAs and primary BAs. Changes in abdominal fat were positively associated with changes in fasting glycine BAs. Changes in 12α-OH/non 12α-OH were positively associated with changes in glucose AUC and negatively associated with changes in IGI.
Changes in glucose AUC0–120min were negatively associated with changes in AUC0–60min of total BAs, secondary BAs, and unconjugated BAs. Changes in triglycerides and HDL were negatively associated with changes in AUC0–60min non 12α-OH. Changes in REE were positively associated with changes in AUC0–60min taurine BAs. Changes in the appetite score of the perceived amount of food that could be eaten were negatively associated with AUC0–60min primary BAs.
There were no significant associations seen between changes in fasting FGF19 with changes in any clinical and metabolic characteristics (Table 4). However, changes in prandial AUC0–120min of FGF19 were negatively associated with HbA1c and visceral fat.
Discussion
The key findings from this study are (1) most fasting BA groups increased after both SG and RYGB, but to a greater extent after RYGB; (2) OGTT time profiles of many BAs differed between two surgeries with significantly greater prandial AUC increments in total BA, secondary BA, and unconjugated BA seen after RYGB than SG; (3) changes in HbA1c negatively correlated with both fasting and prandial changes in total BA, secondary and unconjugated BAs; (4) changes in several other metabolic outcomes, except for BMI and HOMA-IR, correlated with either fasting or prandial changes in BA groups; (5) fasting and 2 h FGF19 increased after both surgeries, but these changes were not associated with changes in total or postprandial BAs; (6) only changes in postprandial FGF19 were negatively correlated with changes in HbA1c and visceral fat.
Plasma BAs After Different Types of Bariatric Surgery
We found a greater increment in both fasting and prandial levels of total, secondary, and unconjugated BA measured 1 year after RYGB than after SG, while the composition of certain BA groups such as taurine BA and non 12 α-OH, which were higher after RYGB in the fasting state, was not higher after RYGB in the postprandial state. These results from a relatively large cohort of obese patients with T2DM pre-operatively make a unique contribution to the existing literature on the composition of plasma BA changes that occur with these two different types of bariatric surgery when measured in both the fasting and postprandial states. Most studies which have examined postprandial plasma BAs either after SG [19, 24] or after RYGB [19, 25,26,27,28,29,30,31] have reported similar increases in postprandial levels as in fasting levels among those without T2DM. Only one small study investigated the pattern of change in fasting and prandial BA composition directly between RYGB compared with SG, among 14 obese people without T2DM [19], and reported that both fasting and postprandial total BAs increased after RYGB but only fasting total BA increased after SG at 1 year post-operatively, with all individual BA following the same pattern [19]. Only one RYGB study investigated those with T2DM [31].
Given primary BA unconjugation and transformation to secondary BA occurs by gut microbiota, it is likely that these specific gut microbiota reactions are enhanced by the presence of the biliopancreatic limb that receives only bile but not food, and may result in discordant changes in BA metabolism during the fasting and postprandial state. Biliopancreatic diversion (BPD) has been reported to produce even greater increments in unconjugated BAs than RYGB [32, 33], suggesting that the longer biliopancreatic limb in BPD leads to greater increases in primary BA unconjugation through such gut microbial reactions. However, no [28], or only a single BA species (GLCA) [34], has been shown to be elevated after adjustable gastric banding, which produces the least impact on gut anatomy, and unlike SG does not permit more rapid delivery of bile to the distal small intestine.
Several early post-bariatric studies (mainly in patients without T2DM) report that total BAs do not rise as soon as 1 week after RYGB [19, 27, 35] and SG [19], while others have found no increase at 1 month post RYGB [31] or 1–6 months after SG [19, 36]. It is unlikely, therefore, that increases in BAs are essential for glycemic benefits which are already observed at very early time points after both RYGB and SG types of surgery [37]. However, BAs may contribute to sustaining these metabolic improvements which are superior after BPD, RYGB, and SG than after gastric banding [2].
BAs and Metabolic Changes After Bariatric Surgery
Besides the collective role of BAs in lipid absorption, the individual BAs are thought to respond to ingested nutrients and to influence glucose metabolism and body weight regulation. While we found greater increases in fasting and postprandial total, secondary, and unconjugated BA were associated with greater decreases in HbA1c, there were no BA groups associated with weight loss. BA levels at baseline did not differ between those who subsequently did or did not achieve diabetes remission. Greater increases in fasting and postprandial total, secondary, and unconjugated BA were associated with greater decreases in HbA1c, while greater increases in postprandial total, secondary, and unconjugated BAs were associated with lower glucose AUC. This is consistent with secondary BA being predominant activators of the TGR5 pathway which is thought to influence glucose metabolism [38, 39]. Other studies have shown that higher 12α-OH/non 12α-OH BAs are associated with insulin resistance as measured by euglycemic insulin clamp [40]. While we did not find this ratio was associated with HOMA-IR in our study, we found higher 12α-OH/non 12α-OH was associated with lower IGI and higher glucose AUC. The association of changes in postprandial taurine conjugated BA with changes in REE and the changes in fasting glycine conjugated BA with changes in abdominal fat are in line with previous studies [41].
Most previous reports have focused on correlations between the achieved fasting BA levels with the clinical state post-operatively among patients without T2DM [19, 24,25,26,27,28,29, 35, 36, 41, 42]. Fasting total BA levels have been reported to be negatively correlated with BMI [19, 31, 33], glycemia [19, 42], and lipids [33, 42], while many other studies reported no such associations with BMI [31, 41,42,43], glycemia [20, 44, 45], lipids [20, 44], or insulin resistance [36, 44, 45]. Only one study of RYGB and biliopancreatic diversion reported a positive correlation between the change in fasting total BA with weight loss [33]. Weight loss itself does not result in significant BA changes given that in the presence of similar acute 20% weight loss achieved after RYGB and gastric banding, no increase in BAs were found to occur after gastric banding in contrast to a marked increase in BA observed after RYGB [28].
Plasma FGF19 After Different Types of Bariatric Surgery
Glycine or taurine conjugated primary BAs are actively transported in the terminal ileum, while unconjugated BAs diffuse across in a gradient-dependent way [46] after which they bind to FXR and lead to hepatic FGF19 production that is secreted into the portal vein and negatively feeds back to inhibit hepatic BA synthesis. The reason why the elevation in FGF19 does not limit the increase in several BA species after most types of bariatric surgery, other than after gastric banding, is unclear. We found an increase in FGF19 after both RYGB and SG consistent with most other studies which have measured FGF19 after RYGB [15, 16, 31, 44] or after SG, although among patients without T2DM, either at 1 month [24], 6 months [36], or 1–2 years after [17]. However, one study did not find this increased after RYGB at 3 months in 13 participants with T2DM [44]. Interestingly, FGF19 was found to be continuously elevated between 3 and 12 months after gastric banding [34]. A positive correlation between fasting and/or prandial FGF19 and fasting and/or prandial BA has been reported in several studies [31, 34, 44], but not in others [15, 25], as we also found.
FGF19 and Metabolic Changes After Bariatric Surgery
We found that only changes in prandial FGF19 were negatively correlated with changes in HbA1c and visceral fat. Most other studies have found no correlations between the fasting FGF19 level and any achieved clinical variable after bariatric surgery such as BMI [15, 43], or insulin resistance [34, 36, 43, 44], or inflammatory markers [44]. The few studies examining prandial FGF19 after bariatric surgery have also reported no correlations with any achieved clinical variable such as glucose tolerance [44], fasting glucose, or C-peptide [25].
Strengths and Weaknesses
One of the limitations of the current and most previous studies is that serum BAs were measured; however, peripheral BA levels correlate with portal venous samples in fasting and postprandial states and reflect what is present in the enterohepatic circulation. We did not investigate links between serum BAs and serum gut peptides, which could mediate the changes we observed between BAs and glycemic responses. The large number of correlation analyses we performed were not corrected for multiple testing and so these individual results need to be verified in other studies. However, this is the largest study to evaluate changes in BAs and FGF19 with changes in clinical and metabolic outcomes after both RYGB and SG among obese people with T2DM pre-operatively.
Conclusion
The association of SG- and RYGB-induced increases in prandial total, secondary, and unconjugated BA and prandial FGF19 with improvements in glycemia, but not weight at 1 year among those with pre-surgery T2DM, suggests that boosting these factors could be a target for novel medical therapies in T2DM. Further such studies are required to prove a causal role of bile acids in glycemic improvement.
References
Association AD. Standards of medical care in diabetes—2017: summary of revisions. Diabetes Care. 2017;40:S4–5.
Buchwald H, Oien DM. Metabolic/bariatric surgery worldwide 2011. Obes Surg. 2013;23:427–36.
Schauer PR, Bhatt DL, Kirwan JP, et al. Bariatric surgery versus intensive medical therapy for diabetes—5-year outcomes. N Engl J Med. 2017;376:641–51.
Keidar A, Hershkop KJ, Marko L, et al. Roux-en-Y gastric bypass vs sleeve gastrectomy for obese patients with type 2 diabetes: a randomised trial. Diabetologia. 2013;56:1914–8.
Cho J-M, Kim HJ, Menzo EL, et al. Effect of sleeve gastrectomy on type 2 diabetes as an alternative treatment modality to Roux-en-Y gastric bypass: systemic review and meta-analysis. Surg Obes Relat Dis. 2015;11:1273–80.
Murphy R, Clarke MG, Evennett NJ, et al. Laparoscopic sleeve gastrectomy versus banded Roux-en-Y gastric bypass for diabetes and obesity: a prospective randomised double-blind trial. Obes Surg. 2017; 1–10
Kaska L, Sledzinski T, Chomiczewska A, et al. Improved glucose metabolism following bariatric surgery is associated with increased circulating bile acid concentrations and remodeling of the gut microbiome. World J Gastroenterol. 2016;22:8698–719.
Vítek L, Haluzík M. The role of bile acids in metabolic regulation. J Endocrinol. 2016;228:R85–96.
Penney N, Kinross J, Newton R, et al. The role of bile acids in reducing the metabolic complications of obesity after bariatric surgery: a systematic review. Int J Obes. 2015;39:1565–74.
Kohli R, Setchell KD, Kirby M, et al. A surgical model in male obese rats uncovers protective effects of bile acids post-bariatric surgery. Endocrinology. 2013;154:2341–51.
Ryan KK, Tremaroli V, Clemmensen C, et al. FXR is a molecular target for the effects of vertical sleeve gastrectomy. Nature. 2014;509:183–8.
Taoka H, Yokoyama Y, Morimoto K, et al. Role of bile acids in the regulation of the metabolic pathways. World J Diabetes. 2016;7:260–70.
Schaap FG. Role of fibroblast growth factor 19 in the control of glucose homeostasis. Curr Opin Clin Nutr Metab Care. 2012;15:386–91.
Fu L, John LM, Adams SH, et al. Fibroblast growth factor 19 increases metabolic rate and reverses dietary and leptin-deficient diabetes. Endocrinology. 2004;145:2594–603.
Gerhard GS, Styer AM, Wood GC, et al. A role for fibroblast growth factor 19 and bile acids in diabetes remission after Roux-en-Y gastric bypass. Diabetes Care. 2013;36:1859–64.
Sachdev S, Wang Q, Billington C, et al. FGF 19 and bile acids increase following Roux-en-Y gastric bypass but not after medical management in patients with type 2 diabetes. Obes Surg. 2016;26:957–65.
Haluzíková D, Lacinová Z, Kaválková P, et al. Laparoscopic sleeve gastrectomy differentially affects serum concentrations of FGF-19 and FGF-21 in morbidly obese subjects. Obesity. 2013;21:1335–42.
Escalona A, Muñoz R, Irribarra V, et al. Bile acids synthesis decreases after laparoscopic sleeve gastrectomy. Surg Obes Relat Dis. 2016;12:763–9.
Steinert RE, Peterli R, Keller S, et al. Bile acids and gut peptide secretion after bariatric surgery: a 1-year prospective randomized pilot trial. Obesity. 2013;21:E660–8.
Nakatani H, Kasama K, Oshiro T, et al. Serum bile acid along with plasma incretins and serum high–molecular weight adiponectin levels are increased after bariatric surgery. Metabolism. 2009;58:1400–7.
Stubbs RJ, Hughes DA, Johnstone AM, et al. The use of visual analogue scales to assess motivation to eat in human subjects: a review of their reliability and validity with an evaluation of new hand-held computerized systems for temporal tracking of appetite ratings. Br J Nutr. 2000;84:405–15.
Tagliacozzi D, Mozzi AF, Casetta B, et al. Quantitative analysis of bile acids in human plasma by liquid chromatography-electrospray tandem mass spectrometry: a simple and rapid one-step method. Clin Chem Lab Med. 2003;41:1633–41.
Muniyappa R, Lee S, Chen H, et al. Current approaches for assessing insulin sensitivity and resistance in vivo: advantages, limitations, and appropriate usage. Am J Physiol Endocrinol Metab. 2008;294:E15–26.
Khan FH, Shaw L, Zhang W, et al. Fibroblast growth factor 21 correlates with weight loss after vertical sleeve gastrectomy in adolescents. Obesity. 2016;24:2377–83.
Schmidt JB, Pedersen SD, Gregersen NT, et al. Effects of RYGB on energy expenditure, appetite and glycaemic control: a randomized controlled clinical trial. Int J Obes. 2013;40:281–90.
De Giorgi S, Campos V, Egli L, et al. Long-term effects of Roux-en-Y gastric bypass on postprandial plasma lipid and bile acids kinetics in female non diabetic subjects: a cross-sectional pilot study. Clin Nutr. 2014;34:911–7.
Ahmad N, Pfalzer A, Kaplan L. Roux-en-Y gastric bypass normalizes the blunted postprandial bile acid excursion associated with obesity. Int J Obes. 2013;37:1553–9.
Kohli R, Bradley D, Setchell KD, et al. Weight loss induced by Roux-en-Y gastric bypass but not laparoscopic adjustable gastric banding increases circulating bile acids. J Clin Endocrinol Metab. 2013;98:E708–12.
Werling M, Vincent RP, Cross GF, et al. Enhanced fasting and post-prandial plasma bile acid responses after Roux-en-Y gastric bypass surgery. Scand J Gastroenterol. 2013;48:1257–64.
Steinert R, Feinle-Bisset C, Geary N, et al. Digestive physiology of the pig symposium: secretion of gastrointestinal hormones and eating control. J Anim Sci. 2013;91:1963–73.
Dutia R, Embrey M, O’Brien S, et al. Temporal changes in bile acid levels and 12α-hydroxylation after Roux-en-Y gastric bypass surgery in type 2 diabetes. Int J Obes. 2015;39:806–13.
Ferrannini E, Camastra S, Astiarraga B, et al. Increased bile acid synthesis and deconjugation after biliopancreatic diversion. Diabetes. 2015;64:3377–85.
Risstad H, Kristinsson JA, Fagerland MW, et al. Bile acid profiles over 5 years following gastric bypass and duodenal switch—results from a randomized clinical trial. Surg Obes Relat Dis. 2017;13:1544–53.
Thöni V, Pfister A, Melmer A, et al. Dynamics of bile acid profiles, GLP-1 and FGF19 after laparoscopic gastric banding. J Clin Endocrinol Metab. 2017;102:2974–84.
Pournaras DJ, Glicksman C, Vincent RP, et al. The role of bile after Roux-en-Y gastric bypass in promoting weight loss and improving glycaemic control. Endocrinology. 2012;153:3613–9.
Belgaumkar AP, Vincent RP, Carswell KA, et al. Changes in bile acid profile after laparoscopic sleeve gastrectomy are associated with improvements in metabolic profile and fatty liver disease. Obes Surg. 2016;26:1195–202.
Yip S, Signal M, Smith G, et al. Lower glycemic fluctuations early after bariatric surgery partially explained by caloric restriction. Obes Surg. 2014;24:62–70.
Fiorucci S, Distrutti E. Bile acid-activated receptors, intestinal microbiota, and the treatment of metabolic disorders. Trends Mol Med. 2015;21:702–14.
Chiang JY. Bile acids: regulation of synthesis. J Lipid Res. 2009;50:1955–66.
Haeusler RA, Astiarraga B, Camastra S, et al. Human insulin resistance is associated with increased plasma levels of 12α-hydroxylated bile acids. Diabetes. 2013;62:4184–91.
Simonen M, Dali-Youcef N, Kaminska D, et al. Conjugated bile acids associate with altered rates of glucose and lipid oxidation after Roux-en-Y gastric bypass. Obes Surg. 2012;22:1473–80.
Patti ME, Houten SM, Bianco AC, et al. Serum bile acids are higher in humans with prior gastric bypass: potential contribution to improved glucose and lipid metabolism. Obesity. 2009;17:1671–7.
Albaugh VL, Flynn CR, Cai S, et al. Early increases in bile acids post Roux-en-Y gastric bypass are driven by insulin-sensitizing, secondary bile acids. J Clin Endocrinol Metab. 2015;100:E1225–33.
Jørgensen NB, Dirksen C, Bojsen-Møller KN, et al. Improvements in glucose metabolism early after gastric bypass surgery are not explained by increases in total bile acids and fibroblast growth factor 19 concentrations. J Clin Endocrinol Metab. 2014;100:E396–406.
Albaugh VL, Banan B, Ajouz H, et al. Bile acids and bariatric surgery. Mol Asp Med. 2017;56:75–89.
Dietschy JM. Mechanisms for the intestinal absorption of bile acids. J Lipid Res. 1968;9:297–309.
Ferrebee CB, Dawson PA. Metabolic effects of intestinal absorption and enterohepatic cycling of bile acids. Acta Pharm Sin B. 2015;5:129–34.
Vincent RP, Omar S, Ghozlan S, et al. Higher circulating bile acid concentrations in obese patients with type 2 diabetes. Ann Clin Biochem. 2013;50:360–4.
Wewalka M, Patti M-E, Barbato C, et al. Fasting serum taurine-conjugated bile acids are elevated in type 2 diabetes and do not change with intensification of insulin. J Clin Endocrinol Metab. 2014;99:1442–51.
Acknowledgments
We thank all the participants who took part in this study and the larger clinical research team who made this study possible. We would like to thank Dr. Ashveen Nand from Auckland University of Technology for the assistance in LC-MS/MS analysis and Dr. Ian Ong from North Shore Hospital, Waitemata District Health Board, for the assistance with ascertainment of cholecystectomy status from clinical records.
Funding
The main clinical trial was funded by Waitemata District Health Board. Additional grant for biochemical analyses was obtained from the Maurice Wilkins Centre for Biodiscovery. The funders had no role in the analyses, interpretation of findings, manuscript review or decision to submit the manuscript for publication.
Author information
Authors and Affiliations
Contributions
R.M., J.L., and L.P. conceived the project and designed study. R.N., M.B., D.D., J.L., and L.P. performed sample and/or data collection. R.N., J.L., L.P., and R.M. analyzed the data. R.N., R.M., L.P., and J.L. wrote the manuscript. All authors have read and agreed with the final version of this manuscript.
Corresponding authors
Ethics declarations
Conflict of Interest
The authors declare that they have no conflicts of interest.
Ethical Approval Statement
All procedures performed in this study involving human participants were in accordance with the ethical standards of the New Zealand national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed Consent Statement
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Nemati, R., Lu, J., Dokpuang, D. et al. Increased Bile Acids and FGF19 After Sleeve Gastrectomy and Roux-en-Y Gastric Bypass Correlate with Improvement in Type 2 Diabetes in a Randomized Trial. OBES SURG 28, 2672–2686 (2018). https://doi.org/10.1007/s11695-018-3216-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11695-018-3216-x