Abstract
Background
Bariatric surgery seems to decrease bone mineral density and increase the risk of fatigue fractures. P1NP (bone formation) and βCTX (bone resorption) were recently validated as reference bone turnover markers (BTM).
Objective
To assess changes in bone remodeling in severely obese patients undergoing Roux-en-Y gastric bypass (RYGB) by using a new composite biomarker, the P1NP/βCTX ratio.
Methods
We prospectively collected blood samples preoperatively, at 1 month and at 1 year from 114 consecutive RYGB patients from 12/2012 to 04/2014. Repeated measures ANOVA and multiple regression were used for data analysis. Cumulative incidence of fractures was assessed in 06/2018.
Results
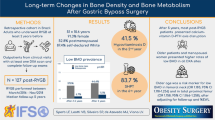
The P1NP/βCTX ratio decreased significantly (P < 0.001) from baseline to 1 month and 1 year (180 ± 6.6, 110 ± 4.1, and 132 ± 5.4). The 1-year P1NP/βCTX ratio did not correlate with BMI or ΔBMI, but inversely correlated with age (r = − 0.23, P = 0.014) and with hsCRP (r = − 0.26, P = 0.009), even after adjustment for age, sex, BMI, and lifestyle, and linearly correlated with albumin (r = 0.2, P = 0.037). At baseline, none of these correlations were detectable. Serum for all time-points was available from > 94% of the patients. At a median follow-up of 4.7 years, 8 patients (7.3%) had a bone fracture, all of them traumatic.
Conclusion
Following RYGB, bone remodeling increases, with a shift toward degradation. This effect seems to be weight-loss independent and shows a correlation with age, with the level of systemic inflammation, and with nutritional state. The risk of fractures should be assessed systematically in bariatric patients and measures of prevention should be improved accordingly.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The global obesity epidemic is expanding, with currently an estimated 2 billion people being overweight, a third of whom are obese [1]. Although the relationship of high fat/high sugar diets to bone health is unclear [2], obesity seems to shift bone homeostasis toward resorption and thus decreasing bone quality [3]. This might be related to deficiencies in nutrients important to bone health, especially calcium and vitamin D, and to parallel pathways that interact with bone remodeling and osteoclast differentiation, including bone-regulating hormones, systemic inflammation, oxidative stress, and the endocannabinoid system [4]. Furthermore, cardiovascular risk factors that are frequently associated to obesity such as impaired glucose tolerance and hypertriglyceridemia seem to have independently a detrimental effect on bone turnover [5].
While all three mainstay treatment avenues of obesity (weight loss pharmaceuticals, dietary restriction, and bariatric surgery [BS]) result in statistically significant reductions in hip bone mineral density (BMD) and in increased bone turnover, the strongest BMD reduction has been reported after BS [6]. Furthermore, recent studies showed that BS patients were more likely to suffer fractures than obese or non-obese controls [7, 8]. In a Canadian study, the median time to first fracture after BS was 3.9 years and occurred more frequently after biliopancreatic diversion than after Roux-en-Y gastric bypass (RYGB) or sleeve gastrectomy [7].
A number of factors may be involved in the reduction of BMD after BS. These include (1) “mechanical unloading” due to reduced biomechanical stress along the postoperative weight loss [9]; (2) elevated levels of serum parathyroid hormone secondary to inadequate calcium intake, intestinal calcium, and vitamin D malabsorption [10]; or 3) altered gastrointestinal and fat-derived hormone levels (PYY, GLP-1, leptin, adiponectin) [11, 12]. Via the amelioration of cardiovascular risk factors (especially fasting glycemia and triglyceridemia), BS also induces changes in favor of bone health [5, 13]. Smoking status, alcohol consumption, serum albumin levels, and physical activity further influence BMD, but the impact of BS on these parameters is not uniform [5, 14,15,16,17].
There is a need to stratify the fragility fracture risk in the obese population, both before and after BS, with fracture prevention strategies to be developed accordingly [18]. The standard reference method of BMD assessment is dual-energy X-ray absorptiometry [5]. However, the International Federation of Clinical Chemistry recently validated new biomarkers for bone turnover measurement to offset the need of ionizing radiation and expensive infrastructure [5, 14, 15, 19]. The two current reference bone turnover markers (BTM) are the serum procollagen type 1 N-terminal propeptide (P1NP), which correlates with bone formation, and the beta-C-terminal cross-linking telopeptide of type 1 collagen (βCTX), which increases in bone resorption. The P1NP/βCTX ratio has been shown in elderly patients to be a useful, simple, and inexpensive new biomarker for predicting osteoporotic fractures, with a threshold for increased risk < 100 [14]. The P1NP/βCTX ratio has not been previously studied in the bariatric population.
The aim of this study was to measure the P1NP/βCTX ratio in severely obese patients preoperatively and up to 1 year after RYGB, and to assess potential confounders that might influence it.
Patients and Methods
The present study followed the STrengthening the Reporting of OBservational studies in Epidemiology (STROBE) guidelines on the conduct and dissemination of observational studies [20].
Patients and Samples
We performed a single-center prospective observational study with 1-year follow-up for BTM. Blood samples from all consecutive morbidly obese patients undergoing laparoscopic proximal RYGB operation at the reference center for Bariatric Surgery of the Cantonal Hospital in Frauenfeld, Switzerland, between 12/2012 and 04/2014 were collected preoperatively as well as at 1 and 12 months postoperatively. Clinical data, body measurements, and information on co-morbidities were collected at similar time points. Missing data were obtained retrospectively from the electronic clinical information system. Due to lack of information on the effect of RYGB on the P1NP/βCTX ratio, a state-of-the-art sample size calculation could not be done a priori. Nevertheless, a sample size of n = 98 was aimed to detect a 10 ± 35 change in the P1NP/βCTX ratio with an α = 5% and a power of 0.8. One hundred fourteen consecutive patients were included in the study to ensure sufficient sample size even in case of incomplete follow-up. Information on bone fractures and compliance with vitamin D and Ca2+ supplementation were assessed systematically during regular follow-up and entered prospectively into the institutional bariatric database. Given the fact that the median time from BS to a fracture was reported to be around 4 years [7], we assessed fracture prevalence at a median follow-up of ~ 5 years by analyzing the database in 06/2018.
Surgical Technique
RYGB was performed as previously described [21]. Briefly, the stomach was transected creating a pouch of approximately 25-mL size. The jejunum was transected 50 cm distally to the doudenojejunal flexure. A stapled side-to-side jejunojejunostomy was created with a Roux limb length of 150 cm. The Roux limb was positioned antecolic and the gastrojejunostomy was performed using a stapler.
Calcium and Vitamin D Supplementation Protocol
Preoperatively, Ca2+ and vitamin D supplementation was not standardized. Postoperatively, standard supplementation consisted of daily Calcimagon D3 Forte© (1 g calcium +800 U Vitamin D) + 1500 U vitamin D in form of drops + 1 Supradyne energy© or Centrum A-Z© multivitamin pill (200 U vitamin D3 + 120 mg Ca2+). Patients who had a vitamin D level below 30 ng/l at any follow-up measurement received intramuscular vitamin D supplementation (300,000 U).
Laboratory Analysis of BTM
Venous blood was drawn after an overnight fast. Measurement of P1NP and βCTX was performed using electrochemiluminescence immunoassays applied on Cobas e-602 immunoassay autoanalyzer (Roche Diagnostics GmbH, Penzberg, Germany). Total imprecision (intra- and inter-assay) of each assay was assessed by measuring 20 replicates of quality control samples over 20 days. Total imprecision expressed as % coefficient of variation for P1NP and βCTX was less than 3.3 and 1.7, respectively.
Statistical Analysis
Baseline parameters were described as mean ± SD for continuous variables and as n for categorical variables. The evolution of continuous variables over time was assessed by repeated measures ANOVA with Bonferroni correction. Student’s t test was used to compare variables that were measured only at baseline and at 1-year (Hb1Ac and glycemia) and to compare subgroups. Not normally distributed variables were log-transformed for the parametric tests. The chi-square test was used to compare categorical variables. Associations between BTM at 1 year postoperatively and metabolic parameters were investigated using multiple regression analysis: partial correlations for continuous variables and univariate general linear model for categorical variables [5]. Four additive models were constructed to explore possible confounders: gender (model 1), + age (model 2), + body mass index (BMI) (model 3), + smoking and alcohol consumption (model 4). Linear and logarithmic correlations were performed to investigate clinically relevant associations. Cumulated hazard ratio of fracture incidence over time was calculated by the Kaplan-Meier survival function. The predictive value of BTM for fracture occurrence was assessed by receiver operating characteristic (ROC) curves. We hypothesized that for P1NP and P1NP/βCTX, lower values, whereas for βCTX, higher values would indicate a postoperative fracture. P values were two-tailed and < 0.05 was considered significant. Analyses were performed using SPSS Statistics, Version 25.0 (IBM, Armonk, New York, USA) and R software version 3.4.3 (The R foundation for Statistical Computing, Vienna, Austria).
Results
Missing data was minimal: the P1NP/βCTX ratio was obtained for all 3 time-points in > 94% of patients, 1-year BMI data were 100% complete, and data on bone-fractures were available for 96.5% of the patients.
Baseline Characteristics
Tables 1 and 2 show the baseline characteristics of all included patients (n = 114). Male patients were significantly older than females, had more often arthrosis, and presented lower alkaline phosphatase (AP) and high-density lipoprotein (HDL) values. Females had significantly higher low-grade inflammation based on high-sensitivity C-reactive protein (hsCRP) levels. Apart from the gonadic hormonal profiles, there were no other measured differences between male and female subgroups.
Postoperative Changes in Metabolic Markers and Sex-Hormones
Figure 1 shows the BTM and relevant parameters (BMI, vitamin D, hsCRP) that significantly changed postoperatively from baseline. Two hsCRP values over 100 mg/l at 1 month were not taken into consideration due to infectious postoperative complications. The mean pre-operative P1NP/βCTX ratio decreased from 180.8 ± 71.04 to 131.8 ± 57.8 by 1 year (P < 0.001). The lipid and the glycemic profiles also changed over 1 year: HDL increased to 1.5 ± 0.5 mmol/l (P < 0.001 between all time points), LDL decreased to 2.47 ± 0.8 mmol/l (P < 0.001 from baseline to 1 month, and p = 0.1 from 1 month to 1 year), triglycerides decreased to 1.19 ± 0.4 mmol/l (P < 0.001), total cholesterol decreased to 4.52 ± 0.99 mmol/l (P < 0.001 from baseline to 1 month, and P = 0.08 from 1 month to 1 year); fasted glycemia decreased to 5 ± 0.9 mmol/l (P < 0.001), Hb1Ac decreased to 5.2 ± 0.56% (P = 0.005). Testosterone levels changed in opposite directions according to gender subgroups: in females they decreased from 0.82 ± 0.4 to 0.61 ± 0.4 nmol/l (P < 0.001) and increased in males from 10.3 ± 4.2 to 17.86 ± 6 nmol/l (P < 0.001). At baseline, 67.9% of patients were vitamin D deficient (< 20 μg/l), compared to 42.6% at 1 month and 15.2% at 1 year (p < 0.001). The following serum parameters were not significantly different across time-points: albumin, AP, Ca2+ (total and albumin-corrected), parathyroid hormone (PTH), estradiol (neither in gender subgroups, nor overall).
Evolution of a BMI, b hsCRP, c Vitamin D, d P1NP E. ßCTX, and f P1NP/ßCTX ratio from baseline to 1 month and 1 year postoperatively (P = 0.006 for P1NP/ßCTX from 1 month to 1 year and P < 0.001 for all other comparisons). Boxes represent the interquartilar range and the median, whiskers show 1.5*inter-quartile range, and blue dots show individual values
Correlations of Bone Turnover Markers with Age and BMI
The P1NP/βCTX ratio at 1 year correlated negatively with age (r = − 0.23, P = 0.014), but was independent from 1-year BMI (P = 0.99) or from 1-year ΔBMI (P = 0.66) (Fig. 2). The baseline P1NP/βCTX ratio did not correlate with baseline BMI (P = 0.19) nor with age (P = 0.28). Both P1NP and βCTX correlated with BMI at baseline (r = 0.23, P = 0.014 and r = 0.25, P = 0.007, respectively), but there was no significant correlation between the 1-year value of these BTM and 1-year BMI (P > 0.3) or ΔBMI (P > 0.23).
Correlations of Bone Turnover Markers with Serum Markers of Metabolic Syndrome, Gonadic Hormones, and Bone Metabolism at 1 Year Postoperatively
Statistically significant correlations identified by crude and adjusted multiple regression between BTMs and serum values of metabolic syndrome and bone health are presented in Table 3. No significant correlation was found for vitamin D, alkaline phosphatase, Hb1Ac, glycemia, high-density lipoprotein, testosterone, and estrogen. The P1NP/βCTX ratio correlated inversely with hsCRP (r = − 0.26, P = 0.009) and significance remained after (a) adjusting for sex, age, BMI, and lifestyle factors, and also (b) when cases with hsCRP values > 5 g/l were excluded (potential occult infection on the day of sampling) (P = 0.043). The P1NP/βCTX ratio also correlated with serum albumin levels (r = 0.2, P = 0.037), although the correlation did not reach significance in the models adjusted for gender (P = 0.11) and age (P = 0.052). At 1 year, both P1NP and βCTX individually correlated with PTH levels (r = 0.22, P = 0.02, r = 0.23, P = 0.015, respectively). Further, P1NP also correlated with Ca2+ levels (r = 0.16, P = 0.009), and inversely correlated with hsCRP (r = − 0.2, P = 0.045) and trigliceridemia (r = − 0.29, P = 0.002). Most of the significant postoperative correlations were not present pre-operatively (Fig. 3), apart from baseline correlations between P1NP and PTH (r = 0.2, P = 0.037) and Ca2+ (r = 0.27, P = 0.003); as well as βCTX with PTH (r = 0.28, P = 0.003).
Postoperatively significant correlations of albumin, hsCRP, and triglycerides with bone turn over markers were non-significant at baseline: a correlation of the P1NP/βCTX ratio and albumin preoperatively (P = 0.33) and b at 1-year postoperatively (r = 0.2, P = 0.037); c correlation of the P1NP/βCTX ratio and hsCRP preoperatively (P = 0.67) and d at 1-year postoperatively (r = − 0.26, P = 0.009); e correlation of P1NP and hsCRP preoperatively (P = 0.12) and f at 1-year postoperatively (r = − 0.2, P = 0.045); g correlation of P1NP and triglycerides preoperatively (P = 0.38) and h at 1 year postoperatively (r = − 0.29, P = 0.002)
Bone Fractures
At a median follow-up of 4.7 years (range: from 3.2 to 5.9 years), eight patients (7.3% of those with available information on bone fractures) reported to have had a bone fracture since the operation. The cohort’s BMI at last follow-up was 29.7 ± 6.2 kg/m2, 81.6% of patients reported to take both Ca2+ and vitamin D supplementation, 9.7% were taking only Ca2+, 2.9% only vitamin D, and 5.8% did not comply with recommendations on supplementation. All fractures resulted unambiguously from an identifiable energy impact, such as a fall. Thus, none of the fractures could be classified as pathological or “fatigue.” Fractures occurred at the distal phalanx, distal forearm (n = 2), proximal humerus, nose, lumbar spine, tibial plateau, and at the 5th metatarsal. At last follow-up, the cumulative hazard ratio to develop a fracture after RYGB is shown on Fig. 4. Based on the ROC curves performed on BTM at 1 year postoperatively to assess their predictive value for bone fractures after RYGB, only the P1NP had a significant area under the curve (AUC = 0.75, P = 0.02, threshold = 80.4 ng/ml, specificity = 0.63, sensitivity = 0.88) (Electronic Supplementary Material 1).
Discussion
The main goal of this study was to investigate the short-term influence of RYGB on bone remodeling, represented by the P1NP/βCTX ratio. This is the first study investigating this new, composite biomarker in the context of BS. At 1-year postoperatively, the P1NP/βCTX ratio decreased significantly from baseline in a weight-loss independent fashion, on average by 27%. These observational findings further support previous evidence on the role of additional pathways beyond mechanical unloading that contribute to bone loss after BS [9, 22, 23]. Our findings are also in line with previous reports that independently analyzed P1NP and βCTX following BS and showed a postoperative increase in bone turnover [23,24,25,26,27,28,29,30].
The secondary aim of the study was to quantify the incidence of bone fractures ~ 5 years post-RYGB and to assess whether the P1NP/βCTX ratio could be used as a prediction marker for fractures. In this cohort, RYGB did not seem to increase the cumulative incidence of fractures compared to the findings of epidemiologic studies on the general population [31, 32]. The 5-year cumulative fracture incidence of 7.3% is comparable to the ~ 7% 5-year incidence observed in Scotland [31] and to the ~ 14% 10-year incidence reported in Germany [32]. In a large American cohort study, postbariatric fractures occurred at a similar incidence (6.4% of patients developed a fracture at 7.6 ± 5.6 postoperative years) as in our cohort; however, based on propensity score match with non-operated obese individuals, the authors concluded that BS doubled the fracture incidence [8]. In the present study, all reported fractures were trauma related and thus might be considered as randomly occurring unpredictable events. Surprisingly, among the BTMs at 1 year after RYGB, P1NP values alone had better sensitivity and specificity to predict a fracture than the P1NP/βCTX ratio. We assume that the absence of pathologic fractures in the cohort compromised the identification of an optimal P1NP/βCTX cut-off to predict bone fractures. Nevertheless, BTM might become a valuable marker of pathologic fracture risk if coupled with a reasonably long follow-up and adequate sample size.
Despite the fact that P1NP and βCTX measurements are readily available in inpatient and outpatient settings at an affordable price (102 CHF in Switzerland, in 2018 [33]), the reference values for the P1NP/βCTX ratio in healthy adults are currently unknown. In a geriatric population, the P1NP/βCTX ratio has been recently shown to have a major importance in assessing bone status compared to each of the markers taken alone, since well-balanced formation/resorption processes are protective against fractures [34]. Current clinical practice guidelines on vitamin and mineral supplementation regimens after BS differ among societies, and recommendations are mostly based on expert opinion [35]. Future high-quality randomized trials are needed to improve postbariatric pharmacotherapy of supplementation. These trials, along with observational cohort studies may be helpful in clarifying the role of BTM in assisting clinical decision-making, and thus, improving more-individualized prognosis and treatments.
The current study identified significant inverse correlations between the P1NP/βCTX ratio and age and postoperative hsCRP, and a positive correlation with postoperative albumin levels. Aging influences bone metabolism at a cellular level, the number and activity of osteoblasts decrease, while osteoclasts increase [36]. The effect of RYGB on BTM seems to affect all age groups. Based on the individual P1NP and βCTX data published by Beamish et al. in adolescents 2 years after RYGB [27], the P1NP/βCTX ratio is calculable: adolescents presented a similar trend in BTM as adults, with an observed decrease from 183 to 117 in males and from 124 to 98 in females.
Although the obesity-associated low-grade chronic inflammation exerts adverse effects on the skeleton [37], reports on associations of hsCRP with osteoporosis and/or osteopenia are inconsistent [38]. This study showed a high correlation between the P1NP/βCTX ratio and hsCRP only at 1 year, but not at baseline, suggesting that the influence of inflammatory state on bone health gains more importance postoperatively, and even infra-clinically elevated hsCRP levels might hallmark decreased BMD.
The correlation of albumin with the P1NP/βCTX ratio at 1-year emphasizes the importance of protein supplementation during the weight loss phase [36]. Indeed, some studies indicated that protein intake in the first year after surgery may be much lower than recommended, often closer to 0.5 instead of 1 to 1.5 g/body kg/day [39]. Contrarily to what was observed in healthy young patients, this study did not find any correlation between BTMs and glycemia or Hb1Ac [5].
RYGB may cause vitamin D and Ca2+ malabsorption, as the bypassed duodenum and proximal jejunum are the predominant sites of active transcellular 1,25(OH)2vitD-mediated Ca2+ uptake [39]. As recommended [7], the cohort in the present study received vitamin D supplementation. Accordingly, plasma vitamin D levels increased significantly postoperatively and the proportion of vitamin D-deficient patients decreased, while the Ca2+ and PTH levels remained unchanged. Interestingly, there was no correlation between vitamin D levels and BTM; however, PTH positively correlated with P1NP and βCTX both at baseline and at 1 year. Yet, our results emphasize the need of adherence for both patients and bariatric centers to existing clinical guidelines on postbariatric supplementation [40].
Alcohol consumption and smoking increase the risk of osteoporosis, whereas healthy diet and regular sport are beneficial for optimizing BMD [41]. In a large American bariatric cohort, tobacco use was the most important risk factor for postoperative fracture (OR = 3.1) [8]. Our database contained information on the patient’s harmful habits; however, it was incomplete for the protective lifestyle factors; therefore, we could adjust the analyses only for the noxious lifestyle parameters. Smoking cessation not only decreases perioperative morbidity, but also the risk of osteoporosis; therefore, it should be a part of the pre-operative bariatric checklist [42].
The main limitations of the study include the lack of comparison of serum BTM findings with the current diagnostic standard reference (bone absorptiometry) or with gut-hormones, ghrelin, or leptin. The lack of long-term follow-up and the lack of non-operated obese control group undergoing similar vitamin D and Ca2+ supplementation represent additional limitations. Further, our database did not contain information on postmenopausal status, which might have biased BTM values in the affected patient subgroup both pre- and postoperatively. This study included RYGB patients only; however, SG has recently become the most frequently performed BS procedure worldwide; therefore, future studies are needed to investigate its impact on bone turnover [43, 44].
Despite these limitations, this study is of clinical relevance, by introducing a new composite biomarker to assess bone remodeling in a bariatric cohort. The almost complete 1-year follow-up for BTM and 5-year follow-up for fracture incidence, the standardized surgical technique and standardized vitamin D supplementation are factors that increase the internal validity of the study. Another strength of the study is the multiple regression method with adjustments for potential confounders in the assessment of interactions of serum markers with the P1NP/βCTX ratio.
Conclusions
In conclusion, this study showed that bone remodeling is increased during the first year after RYGB. The postoperative P1NP/βCTX ratio decreased significantly, suggesting that bone quality becomes weaker after BS. The changes in BTM were already present at 1 month, and independent from the postoperative BMI loss. There was a high inverse correlation between the 1-year P1NP/βCTX ratio and hsCRP, and to a lower, but still significant extent a positive correlation with albumin levels. Further studies are needed to better understand the pathophysiological interplay between low-grade inflammatory state and bone remodeling and to assess the impact of decreased postoperative P1NP/βCTX ratio on long-term morbi-mortality. Bone health should receive a privileged attention in the obese population and adequate measures to prevent fatigue fractures would be necessary to support the optimal quality of life of bariatric patients.
References
Cuschieri S, Vassallo J, Calleja N, et al. The diabesity health economic crisis-the size of the crisis in a European island state following a cross-sectional study. Arch Public Health. 2016;74:52.
Tian L, Yu X. Fat, sugar, and bone health: a complex relationship. Nutrients. 2017;9(5). https://doi.org/10.3390/nu9050506.
Shapses SA, Pop LC, Wang Y. Obesity is a concern for bone health with aging. Nutr Res. 2017;39:1–13.
Soleymani T, Tejavanija S, Morgan S. Obesity, bariatric surgery, and bone. Curr Opin Rheumatol. 2011;23:396–405.
Pirila S, Taskinen M, Turanlahti M, et al. Bone health and risk factors of cardiovascular disease--a cross-sectional study in healthy young adults. PLoS One. 2014;9:e108040.
Harper C, Pattinson AL, Fernando HA, et al. Effects of obesity treatments on bone mineral density, bone turnover and fracture risk in adults with overweight or obesity. Horm Mol Biol Clin Investig. 2016;28:133–49.
Rousseau C, Jean S, Gamache P, et al. Change in fracture risk and fracture pattern after bariatric surgery: nested case-control study. BMJ. 2016;354:i3794.
Fashandi AZ, Mehaffey JH, Hawkins RB, et al. Bariatric surgery increases risk of bone fracture. Surg Endosc. 2018;32:2650–5.
Folli F, Sabowitz BN, Schwesinger W, et al. Bariatric surgery and bone disease: from clinical perspective to molecular insights. Int J Obes. 2012;36:1373–9.
Gregory NS. The effects of bariatric surgery on bone metabolism. Endocrinol Metab Clin N Am. 2017;46:105–16.
Yu EW, Wewalka M, Ding SA, et al. Effects of gastric bypass and gastric banding on bone remodeling in obese patients with type 2 diabetes. J Clin Endocrinol Metab. 2016;101:714–22.
Lenchik L, Register TC, Hsu FC, et al. Adiponectin as a novel determinant of bone mineral density and visceral fat. Bone. 2003;33:646–51.
Muller-Stich BP, Senft JD, Warschkow R, et al. Surgical versus medical treatment of type 2 diabetes mellitus in nonseverely obese patients: a systematic review and meta-analysis. Ann Surg. 2015;261:421–9.
Fisher A, Srikusalanukul W, Fisher L, et al. Lower serum P1NP/betaCTX ratio and hypoalbuminemia are independently associated with osteoporotic nonvertebral fractures in older adults. Clin Interv Aging. 2017;12:1131–40.
Spadola CE, Wagner EF, Dillon FR, et al. Alcohol and drug use among postoperative bariatric patients: a systematic review of the emerging research and its implications. Alcohol Clin Exp Res. 2015;39:1582–601.
Moser F, Signorini FJ, Maldonado PS, et al. Relationship between tobacco use and weight loss after bariatric surgery. Obes Surg. 2016;26:1777–81.
Zabatiero J, Smith A, Hill K, et al. Do factors related to participation in physical activity change following restrictive bariatric surgery? Obes Res Clin Pract: A qualitative study; 2017.
Savvidis C, Tournis S. Dede AD. Hormones (Athens): Obesity and bone metabolism; 2018.
Vasikaran S, Eastell R, Bruyere O, et al. Markers of bone turnover for the prediction of fracture risk and monitoring of osteoporosis treatment: a need for international reference standards. Osteoporos Int. 2011;22:391–420.
von Elm E, Altman DG, Egger M, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet. 2007;370:1453–7.
Weber M, Muller MK, Bucher T, et al. Laparoscopic gastric bypass is superior to laparoscopic gastric banding for treatment of morbid obesity. Ann Surg. 2004;240:975–82. discussion 82-3
Yu EW, Carmody JS, Brooks DJ, et al. Cortical and trabecular deterioration in mouse models of roux-en-Y gastric bypass. Bone. 2016;85:23–8.
Biagioni MFG, Mendes AL, Nogueira CR, et al. Bariatric Roux-En-Y gastric bypass surgery: adipocyte proteins involved in increased bone remodeling in humans. Obes Surg. 2017;27:1789–96.
Ivaska KK, Huovinen V, Soinio M, et al. Changes in bone metabolism after bariatric surgery by gastric bypass or sleeve gastrectomy. Bone. 2017;95:47–54.
Crawford MR, Pham N, Khan L, et al. Increased bone turnover in type 2 diabetes patients randomized to bariatric surgery versus medical therapy at 5 years. Endocr Pract. 2018;24:256–64.
Bredella MA, Greenblatt LB, Eajazi A, et al. Effects of Roux-en-Y gastric bypass and sleeve gastrectomy on bone mineral density and marrow adipose tissue. Bone. 2017;95:85–90.
Beamish AJ, Gronowitz E, Olbers T, et al. Body composition and bone health in adolescents after Roux-en-Y gastric bypass for severe obesity. Pediatr Obes. 2017;12:239–46.
Muschitz C, Kocijan R, Marterer C, et al. Sclerostin levels and changes in bone metabolism after bariatric surgery. J Clin Endocrinol Metab. 2015;100:891–901.
Costa TL, Paganotto M, Radominski RB, et al. Calcium metabolism, vitamin D and bone mineral density after bariatric surgery. Osteoporos Int. 2015;26:757–64.
Biagioni MF, Mendes AL, Nogueira CR, et al. Weight-reducing gastroplasty with Roux-en-Y gastric bypass: impact on vitamin D status and bone remodeling markers. Metab Syndr Relat Disord. 2014;12:11–5.
Court-Brown CM, Biant L, Bugler KE, et al. Changing epidemiology of adult fractures in Scotland. Scott Med J. 2014;59:30–4.
Rathmann W, Kostev K. Fracture risk in patients with newly diagnosed type 2 diabetes: a retrospective database analysis in primary care. J Diabetes Complicat. 2015;29:766–70.
Département fédéral de l‘intérieur Confédération suisse. Listes des analyses. Published by the Office fédéral de la santé publique, Bern; 1.1.2018. https://www.bag.admin.ch/bag/fr/home/versicherungen/krankenversicherung/krankenversicherung-leistungen-tarife/Analysenliste.html.
Fisher A, Fisher L, Srikusalanukul W, et al. Bone turnover status: classification model and clinical implications. Int J Med Sci. 2018;15:323–38.
Chakhtoura MT, Nakhoul N, Akl EA, et al. Guidelines on vitamin D replacement in bariatric surgery: identification and systematic appraisal. Metabolism. 2016;65:586–97.
Muschitz C, Kocijan R, Haschka J, et al. The impact of vitamin D, calcium, protein supplementation, and physical exercise on bone metabolism after bariatric surgery: the BABS study. J Bone Miner Res. 2016;31:672–82.
Cao JJ. Effects of obesity on bone metabolism. J Orthop Surg Res. 2011;6:30.
Huang JV, Schooling CM. Inflammation and bone mineral density: a Mendelian randomization study. Sci Rep. 2017;7:8666.
Xanthakos SA. Nutritional deficiencies in obesity and after bariatric surgery. Pediatr Clin N Am. 2009;56:1105–21.
Schafer AL, Weaver CM, Black DM, et al. Intestinal calcium absorption decreases dramatically after gastric bypass surgery despite optimization of vitamin D status. J Bone Miner Res. 2015;30:1377–85.
Sampson HW. Alcohol and other factors affecting osteoporosis risk in women. Alcohol Res Health. 2002;26:292–8.
Haskins IN, Amdur R, Vaziri K. The effect of smoking on bariatric surgical outcomes. Surg Endosc. 2014;28:3074–80.
Aminian A. Sleeve gastrectomy: metabolic surgical procedure of choice? Trends Endocrinol Metab. 2018;29:531–4.
Angrisani L, Santonicola A, Iovino P, et al. IFSO Worldwide Survey 2016 : Primary, Endoluminal, and Revisional procedures. Obes Surg. 2018;28:3783–94.
Acknowledgements
We are grateful to Lilian Roth, MD, for her help in the data collection process.
Communication
The Best Oral Presentation Prize was awarded to Daniel Gero for presenting this study at the 8th International Federation of Surgery of Obesity – European Chapter meeting in Athens, Greece on 19th May 2018.
Funding
The study was entirely funded by the assistant-professorship research grant awarded by the University of Zurich to Prof. Dr. med. Marco Bueter, PhD.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical Approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and cantonal research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed Consent
All enrolled patients provided written informed consent for voluntary participation in the study and to de-identified use of their health-related data for scientific purposes.
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The Best Oral Presentation Prize was awarded to Daniel Gero for presenting this study at the 8th International Federation of Surgery of Obesity – European Chapter meeting in Athens, Greece on 19th May 2018.
Electronic supplementary material
ESM 1.
Receiver Operating Characteristic curves to assess positive and negative predictive values of the burn turnover markers to predict a fracture after RYGB. A. P1NP at baseline (P = 0.379) and at 1-year (P = 0.02, AUC = 0.75, threshold = 80.4 ng/ml, specificity = 0.63, sensitivity = 0.88), B. ßCTX at baseline (P = 0.519) and at 1-year (P = 0.255), C. P1NP/ ßCTX at baseline (P = 0.041) and at 1-year (P = 0.159). (PDF 280 kb)
Rights and permissions
About this article
Cite this article
Muller, M.K., Gero, D., Reitnauer, D. et al. The Impact of Roux-en-Y Gastric Bypass on Bone Remodeling Expressed by the P1NP/βCTX Ratio: a Single-Center Prospective Cohort Study. OBES SURG 29, 1185–1194 (2019). https://doi.org/10.1007/s11695-018-03640-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11695-018-03640-3