Abstract
Introduction
The goals of this experiment were to study therapeutic potential of intestinal electrical stimulation (IES) for obesity, its mechanisms involving gastrointestinal motility and hormones, and role of pulse width in diet-induced obese rats.
Methods
In a 4-week study, rats equipped with one pair of electrodes at the duodenum were assigned to receive either a sham or IES of varied pulse widths in a sequential way. Food intake was measured daily and body weight measured weekly. Blood samples were collected for the measurement of glucagon-like peptide-1 (GLP-1). Solid gastric emptying (GE) and small bowel transit (SIT) tests were performed at the end of the experiment.
Results
The results of the study were as follows: (1) Daily food intake, not affected by IES of 0.3 ms, was pulse width-dependently reduced by 1.9 g with 1 ms and by 5.7 g with 3 ms. Accordingly, body weight was pulse width-dependently reduced by 2.4 g with 1 ms and by 12.8 g with 3 ms compared to a gain of 5.6 g in sham. (2) GLP-1 level was elevated by both 0.3 and 3 ms at 15 min, but was elevated only with 3 ms at 60 min. (3) GE was delayed to 52.3 % by IES of 3 ms but not 0.3 ms, compared to that at 64.4 % with sham IES. (4) Compared to the geometric center of 7.0 with sham IES, SIT was accelerated by 3 ms to 7.8 but not by 0.3 ms.
Conclusion
IES pulse width-dependently reduces food intake and body weight, attributed to the delay of gastric emptying and the acceleration of small bowel transit, as well as the enhancement of GLP-1 secretion.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Obesity, ultimately resulting from an imbalance between energy expenditure and caloric intake, is one of the most prevalent public health problems worldwide. The annual allocation of healthcare resources for obesity and related comorbidities projected to exceed $150 billion in the USA [1]. Behavioral modification, pharmacotherapy, and surgical treatment are current therapeutic strategies for the treatment of obesity. However, surgical treatment is the only long-term effective therapy with satisfactory sustained weight loss. Due to invasiveness and procedure-induced complications, the bariatric surgery is typically limited to a small fraction of patients with morbid obesity [2, 3].
Gastric electrical stimulation (GES) using implantable devices has been investigated and proposed as a safer, less invasive, and more tolerable alternative to existing bariatric surgeries. Several single-arm clinical trials have shown promising results in food intake and weight loss in GES-treated patients [4–7]. However, randomized placebo-controlled clinical studies did not yield significant weight loss effects on obese patients [8]. Due to the limitations of the available implantable stimulators, GES for treating obesity patients was confined to a narrow range of stimulation parameters with pulse width below 1 ms, despite several animal studies indicating that wider pulse widths are more effective in modulating gastric motility, reduce food intake, and body weight [9, 10].
Several recent studies have suggested that intestinal electrical stimulation (IES) may also be a potential method to treat obesity or type 2 diabetes [11–14]. In normal rats, IES was reported to accelerate intestinal transit and reduce nutrient absorption [11]. IES with long pulses (≥100 ms) was found to delay gastric emptying and reduce food intake in normal dogs [12] and normal and obese rats [13]. Clinically, however, it is impractical to develop an implantable pulse generator that is capable of delivering pulses with a width of 100 ms or wider. It is therefore important to study whether IES can be delivered using pulse trains with much narrower pulse width and whether IES with pulse trains of reasonable pulse width has a therapeutic potential for treating obesity. Furthermore, it is of equally great importance to investigate whether IES of pulse trains is able to alter gastrointestinal motility and hormones.
The aims of this study were therefore (1) to explore the therapeutic potential of IES for obesity, (2) to investigate the dependency of its anti-obesity effects on pulse width as observed in GES for obesity and 3) mechanisms involving gastrointestinal motility (gastric emptying and intestinal transit) and gut hormone in diet-induced obese (DIO) rats.
Materials and Methods
Animals
Thirty 9-week-old male Sprague-Dawley rats were purchased from Charles River Laboratories (Kingston, NY). They were given 1 week for the acclimation upon arrival at the facility while being fed with a lab chow diet (Lab Diet 5002; PMI Nutrition International, Brentwood, MO). Then the rats were fed ad libitum on a high-fat diet (Research Diets D12451, 4.73 kcal/g, 45 % of kcal from fat) that is widely used to induce obesity [15]. After 8 weeks, 16 rats with the heaviest body weight were selected as DIO rats and used in experiment 1 for the optimization of stimulation parameters and experiment 3 for gastric emptying and small bowel transit. The other 14 rats were used for experiments 2 and 3. The housing room was humidity- and temperature-controlled (20–22 °C) and maintained on a fixed 12-h/12-h light-dark cycle. The animals had free access to water in their home cages at all times.
Surgery Procedure
A midline laparotomy was performed under 2–3 % isoflurane to implant one pair of stainless steel cardiac pacing wires (Medtronic, Minneapolis, MN). The tip of the wire was exposed and shaped in a small circle and purse-sutured on the serosal surface of the duodenum (3 cm from the pylorus). The distance between two electrodes in a pair was around 1.0 cm. The electrode wires were then externalized percutaneously on the back of the neck through a silicone and Dacron button (Instech laboratories, Plymouth Meeting, PA) that was sutured under the skin. The electrode wires passed through a tether spring attached to the silicone and Dacron to a tether arm attached to the lid of the rat home cage. This tether system allows continuous electrical stimulation to be delivered to the rat via an external stimulator while the rat could move freely in the home cage. Cefazolin (30 mg/kg) and buprenorphine (0.05 mg/kg) were given for 2 days after surgery for the control of postoperative pain and infection. The rats were allowed to completely recover in their home cages for 7–10 days before the experiment.
Experiment 1: Pulse Width Optimization
The first experiment was to compare the effects of IES with varied pulse widths on food intake and body weight. After the recovery period, the rats were housed in cages equipped with a BioDAQ continuous food intake monitoring system (Research Diets Inc., New Brunswick, NJ) and provided access to the high-fat diet and water ad libitum. After a 2-week acclimation period, food intake was continuously and automatically monitored by the system for the next 4 weeks. In the 4-week study, 16 DIO rats were randomized into a 4 × 4 Latin square crossover design in which each rat was assigned to receive a randomized sequence of four treatments: sham IES, IES with pulse width of 0.3 ms, 1 ms, and 3 ms. Each lasted five consecutive days in 1 week and the other 2 days in the week were allowed for the washout of the possible residual effect of the previous treatment. IES was applied during the 12 h in dark (6:00 pm–6:00 am) using a digital pulse generator (World Precision Instrument, Sarasota, FL). Rats in the sham IES group had electrode wires connected but received no stimulation. IES parameters other than the pulse width were fixed at 40 Hz, 0.6 s-on, 0.9 s-off, 3 mA, biphasic. The pulse width was set at 0.3 ms, 1 ms, or 3 ms. A food hopper with food pellet was weighted automatically by a computer via an electronic strain gage transducer. The difference in the food hopper weight between 6:00 pm (initial) and 6:00 am (final) was calculated as the nocturnal food intake while the difference between 6:00 am (initial) and 6:00 pm (final) was calculated as the diurnal food intake. Little to no spillage was recorded during the experiments. The rats were weighed once a week at 2–4 pm on the first day of each treatment. The ending weight was taken at 2–4 pm on the day after the final treatment session and was used to compute weight change over the 28-day treatment period.
Experiment 2: Acute Effects of IES on Postprandial Plasma GLP-1 Concentration
This experiment was to investigate the acute effect of IES on postprandial plasma glucagon-like peptide-1 (GLP-1) concentration. Nine rats were chronically implanted with intestinal electrodes for IES and randomized into 3 × 3 Latin square crossover design in which each rat was assigned to receive a randomized sequence of three treatments: sham IES, IES with pulse width of 0.3 ms, or 3 ms. After being fasted for 14 h, rats were given glucose (1 g/kg, 20 % in water) by gavage and then received one of the three treatments for 1 h. Experimental sessions were performed at an interval of 4 days. Blood samples were collected at rat tail vein under light anesthesia of isoflurane at baseline (15 min before oral gavage), 15 min, and 60 min after gavage of glucose using chilled EDTA tubes containing aprotinin and diprotin A (Sigma Aldrich, St. Louis, MO). Plasma was stored at −80 °C until further analysis. Plasma GLP-1 was quantified using a commercial ELISA kit (Sigma-Aldrich, St. Louis, MO). The assay was carried out according to the instructions provided by the manufacturer.
Experiment 3: Solid Gastric Emptying and Small Bowel Transit
The method for assessing solid gastric emptying was based on a method previously described [16]. Thirty rats implanted with chronic implanted electrodes for IES were randomized into three groups: sham IES, IES with pulse width of 0.3 ms, or 3 ms. After being fasted in cages with metal-wired mesh for 18 h with free access to water, rats were given access to 2 g of high-fat diet pellets and all rats finished the food within less than 5 min. Right after the feeding, rats were given 1.5 ml phenol red (0.5 mg/ml in saline) by gavage and received one of the three treatments for 90 min. Rats were then euthanized under anesthesia and the abdomen was opened and the stomach and the whole intestine were surgically isolated and removed. The gastric content was recovered from the stomach which was air dried for 48 h and then weighed. Solid gastric emptying was calculated according to the following formula: Gastric emptying (%) = [1 − (dried gastric content in g / 2 g)] × 100.
The method for phenol red analysis was based on a previous method [17]. The small intestine was divided into 10 equal segments and placed in one cup with 50 ml of 0.1 N NaOH and homogenized. After 24 h, 2.5 ml of supernatant was added to in a tube with 0.25 ml of trichloroacetic acid (20 % wt/vol). After centrifugation (1000g for 15 min), 1.5 ml of the supernatant was added to 2 ml of NaOH (0.5 N) to develop the maximum intensity of color. The amount of phenol red in each of the intestinal segment was measured at a wavelength of 560 nm by a spectrophotometer. The intestinal transit was determined by the geometric center (GC) defined as follows: GC = ∑ (Pn n) for n = 1, 2, 3,…10, where Pn is the percentage of phenol red in segment n and n is the number of the segment. Any phenol red found in the colon was included in segment 10.
Statistics and Analysis
The average daily, nocturnal, and diurnal food intake for each animal was calculated for each test day. A mean of the 5 days under each treatment was calculated for each animal and used in statistical analyses. Data are expressed as mean ± SE. One-way repeated measurement ANOVA was used to compare the difference of food intake among groups followed by post hoc Fisher’s test. One-way ANOVA was used to compare the difference of gastric emptying (GE), small bowel transit (SIT), and GLP-1 among groups followed by post hoc Fisher’s test. p value less than 0.05 was considered as significant.
Results
Pulse Width-Dependent Inhibitory Effect of IES on Food Intake and Body Weight
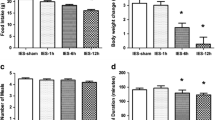
Mean daily food intake was significantly lower under the active IES treatments, compared to that under sham stimulation. Mean daily food intake over the 28-day experiment was 18.4 ± 0.7 g in the sham IES group rats, not affected by IES of 0.3 ms (18.2 ± 0.5 g, p = 0.816). However, in rats treated with wider pulse width, daily food intake was reduced to 16.5 ± 0.7 g in IES of 1 ms group (p = 0.019, vs. sham) and further to 12.8 ± 0.7 g (p < 0.001, vs. sham) under IES of 3 ms. As shown in Fig. 1a, an apparent linear trend in food intake reduction with increasing pulse widths was observed and the reduction was significant for the tested three pulse widths (1 ms vs. 0.3 ms, p = 0.033, 3 ms vs. 0.3 ms and 1 ms: p < 0.001).
Food intake and body weight changes by intestinal electrical stimulation (IES) with varied pulse widths. Sixteen male Sprague-Dawley rats fed with high-fat diet were chronically implanted with one pair of electrodes at the duodenum. IES parameters other than the pulse width were fixed at 40 Hz, 0.6 s on/0.9 s off, 3 mA, biphasic. Rats were assigned to receive either a sham or IES for 1 week in a sequential way. a IES pulse width-dependently reduced daily food intake. b IES pulse width-dependently reduced nocturnal food intake. c IES had no effect on diurnal food intake. d IES pulse width-dependently reduced body weight
We further analyzed the nocturnal and diurnal food intake in all groups of rats. As shown in Fig. 1b, a significant difference was observed among the nocturnal food intake among the treatments. IES of 3 ms significantly decreased the nocturnal food intake by 4.3 g in comparison to that in sham IES group (p < 0.001), and IES of 1 ms marginally decreased the nocturnal food intake. However, in Fig. 1c, no difference was observed in diurnal food intake among the treatments (p = 0.160).
Changes in body weight under different treatments were consistent with the food intake results. In Fig. 1d, while body weight increased slightly over 5 days of treatment with sham IES or IES of 0.3 ms, it declined by 2.4 g under IES of 1 ms (p = 0.019 vs. sham, p = 0.033 vs. 0.3 ms) and 12.8 g under IES of 3 ms (p < 0.001 vs. sham or 0.3 ms). The difference in mean weight change between IES of 1 and 3 ms was also significant (p < 0.001).
IES Increased Postprandial GLP-1 Pulse Width-Dependently
IES increased GLP-1 at 15 min after oral glucose with both 3 and 0.3 ms, and the effect was sustained only with IES of 3 ms. The plasma concentration of GLP-1 at baseline did not differ among three groups, ranging from 322 to 335 pg/ml. It was increased at 15 min after glucose gavage in every group (Fig. 2). IES significantly elevated plasma GLP-1 concentration to 539 pg/ml with 0.3 ms (p = 0.005 vs. sham) and to 565 pg/ml with 3 ms (p = 0.001 vs. sham), compared to the slight increase with sham IES at 15 min after gavage. No difference was found between IES of 0.3 ms and IES of 3 ms (p = 0.645, n = 9). The GLP-1 level remained elevated at 443 pg/ml with IES of 3 ms (p < 0.001 vs. sham, p = 0.012 vs. 0.3 ms) while that in sham IES and 0.3 ms decreased and returned to the basal level at 60 min.
Change of postprandial plasma GLP-1 under sham or intestinal electrical stimulation (IES) of varied pulse widths. After being fasted for 12 h, rats were given glucose (1 g/kg) by oral gavage and immediately after received one of the three treatments. Blood samples were collected at rat tail vein at baseline (15 min before oral gavage), 15 min, and 60 min. Postprandial plasma GLP-1 concentrations were elevated by both 0.3 and 3 ms 15 min after gavage compared that in sham. However, the elevation was only observed in 3-ms group 60 min after gavage
Pulse Width-Dependent Effects of IES on Gastrointestinal Transit
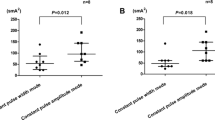
IES of 3 ms but not 0.3 ms delayed gastric emptying and accelerated small intestinal transit. As shown in Fig. 3a, compared to the percentage of gastric emptying with sham IES (64.4 ± 2.4 %, n = 10), IES of 0.3 ms had no significant effect (61.5 ± 1.8 %, n = 10, p = 0.673) while IES of 3 ms significantly delayed gastric emptying to 52.3 ± 2.8 % (p = 0.003, n = 10). The effect of IES of 3 ms on reducing the gastric emptying was also statistically significant in comparison to that with short-pulse treatments (p = 0.026 vs. 0.3 ms).
Effect of intestinal electrical stimulation (IES) with varied pulse widths on gastric emptying and small bowel transit. a Solid gastric emptying was delayed with increased pulse width as 3 ms. b Small bowel transit, defined as geometric center, was accelerated with increased pulse width as 3 ms. c The recovery of phenol red at segments 9 and 10 is significantly increased by IES of 3 ms, compared to that in sham
Small bowel transit was accelerated by IES with increased pulse width. As shown in Fig. 3b, compared to the GC in the sham IES group (7.0 ± 0.2, n = 10), IES of 0.3 ms had no significant effect (7.2 ± 0.2, n = 10, p = 0.764) while IES of 3 ms significantly accelerated the small bowel transit to 7.8 ± 0.2 (p = 0.004, n = 10). The effect of IES of 3 ms on accelerating the small bowel transit was also statistically significant in comparison to that with IES of 0.3 ms (p = 0.022 vs. 0.3 ms). Since the GLP-1-secreting L cell is thought to mainly locate at distal ileum and colon, the percentage of phenol red recovery at segments 9 and 10 was also calculated: 22.3 ± 1.9 % in the sham group and 43.7 ± 6.5 % in the 3 ms group (n = 10, p = 0.003), Fig. 3c.
Discussion
In the present study, we have found that IES with pulse width of 3 ms but not 0.3 ms significantly reduced food intake and body weight in rats fed with high-fat diet. We further investigated the effect of IES with varied pulse widths on gastrointestinal motility and the involvement of gut hormone GLP-1. The results showed that the delayed gastric emptying, accelerated small bowel transit, and increased GLP-1 secretion were associated with IES with wider pulse width of 3 ms but not 0.3 ms. These findings suggest a therapeutic potential of IES and mechanisms involving gastrointestinal motility and hormones, and that wider pulse of 3 ms is needed for the IES to be effective in treating obesity.
Different methodologies including patterns of stimuli, placement of electrodes, and delivery time of stimuli have been used for IES. Based on pulse width, IES can be mainly classified as long-pulse stimulation, short-pulse stimulation, and pulse-train stimulation [18]. Existing implantable devices which typically deliver stimuli of less than 1-ms pulse width have been used for cardiac pacing or neural stimulation. Short-pulse implantable device such as the Enterra® therapy (Medtronic, Minneapolis, MN, USA), employing a short-pulse protocol (14 Hz, 0.1 s on/5.0 s off, 5 mA, pulse width 0.33 ms), has no acute effects on gastric slow waves or gastric emptying, but is capable of improving symptoms of nausea and vomiting via the modulation of vagal afferent pathway [19, 20]. However, considering differences in cell capacitance and components of ion channels between neurons and smooth muscle cells, long pulses or trains of long pulses are required to effectively modulate gastrointestinal smooth muscle cell functions [21]. Long-pulse stimulation is composed of repetitive single pulses with a pulse width of 10–600 ms and a stimulation frequency in the vicinity of the physiological frequency of the intestinal slow wave. IES with long pulse of 50 ms was reported to entrain or pace the intestinal slow waves and accelerate intestinal transit in canine [22], and IES with long pulse of 100 ms has an inhibitory effect on gastric emptying and food intake in rats [13]. However, the stimulations used in these studies were of long pulses which need much greater energy, and no commercial implantable stimulator is available to deliver electrical stimulation with such long pulses [23]. In the present study, IES with short pulses (0.3–3 ms) was applied at a frequency of 40 cycles per minute, which is a frequency in the vicinity of the intrinsic slow wave activity of duodenum in rat [24]. These parameters were chosen based on a previous GES study in which GES with a pulse width wider than 2 ms and a sufficiently high pulse frequency than 40 Hz was reported to be able to evoke action potentials in smooth muscle cells [25].
Although there is a lack of clinical studies on the therapeutic potential of IES for obesity, some promising results from canine and swine studies suggested that IES with wider pulses might be a viable therapy for obesity. In rats, IES was reported to reduce food intake and body weight in both lean- and diet-induced obese rats although a higher stimulation of energy was required in diet-induced obese rats to achieve the similar body weight loss as that in the control rats [13]. However, long pulses (100 to 300 ms) were used in these studies and therefore it is practical for clinic applications due to difficulties in developing such kind of implantable device. In the present study, all rats were fed with high-fat diet and these DIO-prone rats were used for the 4 week’s food intake and body weight loss experiment. In addition, in previous weight loss studies, rats were trained or acclimated to time-restricted feeding in custom-built restrainers with access to a surplus of food pellets for 2 h each day. Though these experiments were well designed and the rats were allowed enough time for the acclimation, it is still unclear whether the restricted feeding would have some effects on food intake when stimulation is off, and there is no study showing any residential effect of IES on food intake or weight control. In the present study, rats were allowed to move freely in their home cages during stimulation and had free access to food and water all day even when stimulation was off. The experimental results in this study showed that the food intake when the stimulation was off at daytime was not different between the sham and IES groups, demonstrating that the reduction of food intake and body weight was not attributed to adverse effects caused by electrical stimulation. From the relationship between food intake/body weight and pulse width shown in Fig. 1a–d, pulse width above 1 ms is recommended for treating obesity while wider pulse like 3 ms is preferred.
In a recent rodent study, IES was found to decrease the expression of ghrelin, a prominent hunger hormone in gastric tissue, and increase the expression of cholecystokinin, one of the major satiety hormones, in duodenal tissue [26]. GLP-1 is an insulinotropic hormone which has been shown to inhibit food intake and induce weight loss in humans [27, 28]. Both the peripheral effect on gastric emptying [27] and the central effect on appetite regulation [10, 11] contribute to the anorectic effect of GLP-1. In the present study, we investigated the effect of IES with varied pulse width on the secretion of GLP-1 stimulated by oral glucose. Compared to sham IES which only slightly increased GLP-1 secretion at 15 min after oral glucose, IES of both 0.3 and 3 ms significantly enhanced the secretion of GLP-1. IES was reported to modulate neuronal activities in the nucleus of the solitary tract and hypothalamus in rats via the activation of vagal afferent pathway [29, 30]. This rapid rise in GLP-1 secretion which was augmented by IES may be mediated indirectly through a neuro/endocrine pathway, rather than through forwarding the nutrient for the direct interactions of the luminal contents with L cells [31]. L cell in the distal intestine was reported to be activated by the sense of nutrients in the upper gut intestine via neural activity [31]. A recent study in isolated rodent small intestine showed that GLP-1 release was enhanced by local IES response to luminal nutrients in the intestines [32]. In anesthetized rats, nutrient infusion into the duodenum increased plasma GLP-1 levels which was further augmented by electrical stimulation at the duodenum [33]. At 60 min, the plasma GLP-1 remained high in the rats treated with IES of 3 ms but returned to baseline in the rats with sham IES or IES of 0.3 ms. This elevated plasma GLP-1 level in the IES of 3 m group was speculated to be attributed to the accelerative effect of IES of 3 m on intestinal transit, which brought more nutrients to the distal ileum and resulted in the release of GLP-1 from L cells in the distal ileum.
Mechanically, IES with appropriate settings is able to modulate gastrointestinal motility. According to the stimulation location in the small intestine, IES can be classified as antegrade or forward (IES via proximal intestine) stimulation and retrograde or backward stimulation (IES via distal intestine) [18]. Retrograde IES was designed to delay intestinal transit of the proximal small intestine or to increase absorption in various canine models of intestinal dysmotility such as short bowel syndrome, Roux stasis syndrome, and dumping syndrome [34–37]. Antegrade IES was reported to accelerate intestinal transit slowed by fat-induced ileal brake [22] or pretreatment of Nω-nitro-l-arginine in canine [38] or in obese-prone pigs [39]. Although antegrade IES with long pulses of 50–150 ms was reported to entrain or pace intrinsic intestinal slow waves when IES is delivered at a frequency in the vicinity of the intrinsic slow waves [40–42], IES with trains of short pulses (2–5 ms) was equally [39] or more effective [11] than IES with long pulses (200 ms) in accelerating jejunal transit. The present study was different from previous studies in a number of factors. First, IES was applied at a train frequency of 40 cycles per minute, which is the frequency of the intrinsic intestinal slow wave in rats. Second, the present study was performed in DIO rats instead of regular rats. Third, the present study was performed in freely moving animals instead of anesthetized or restrained rats. Last, the stimuli were composed of trains of short pulses instead of repetitive single pulses. All these indicate that the accelerating effect on small bowel transit by the optimized IES is more clinically relevant as accelerated transit may reduce absorption [43] and also increase the flow of nutrient to the distal ileum to stimulate the release of GLP-1 from L cells. We found that wide pulse IES, not short pulse as 0.3 ms, increased the small bowel transit. This result was consistent with the finding from gastric electrical stimulation, in which wider pulse was reported to be more effective in depolarizing smooth muscle and modulating gastric motility [9, 25].
Gastric motility also participates in the control of appetite and satiety, and the modulation in gastric motor functions in obesity could be a potential factor in the maintenance of weight or the development of obesity. Acceleration in gastric emptying of solid food, which precipitates hunger and frequent eating, was reported in obese subjects [44–46] and DIO rats [16, 47]. IES with long pulses of 100 to 300 ms was noted to reduce gastric tone and delay gastric emptying in canine [48, 49] and rats [13]. In the present study, IES of 3 ms was able to delay solid gastric emptying in addition to its effect on intestinal transit. IES-induced gastric distention and delayed gastric emptying were reported to correlate with reduced food intake [13] and thus the delayed gastric emptying observed in this study is also believed to play a role in reduced food intake and body weight.
Taking together, IES pulse width-dependently reduces food intake and body weight, and the mechanisms underlying the inhibitory effect may include a number of factors such as delayed gastric emptying and accelerated small bowel transit, as well as increased secretion of GLP-1.
References
Hurt RT, Kulisek C, Buchanan LA, et al. The obesity epidemic: challenges, health initiatives, and implications for gastroenterologists. Gastroenterol Hepatol. 2010;6:780–92.
Crookes PF. Surgical treatment of morbid obesity. Annu Rev Med. 2006;57:243–64.
Sagar PM. Surgical treatment of morbid obesity. Br J Surg. 1995;82:732–9.
Favretti F, De Luca M, Segato G, et al. Treatment of morbid obesity with the transcend implantable gastric stimulator (IGS): a prospective survey. Obes Surg. 2004;14:666–70.
Greenstein RJ, Belachew M. Implantable gastric stimulation (IGS) as therapy for human morbid obesity: report from the 2001 IFSO symposium in Crete. Obes Surg. 2002;12 Suppl 1:3S–5.
Cigaina V. Gastric pacing as therapy for morbid obesity: preliminary results. Obes Surg. 2002;12 Suppl 1:12S–6.
D’Argent J. Gastric electrical stimulation as therapy of morbid obesity: preliminary results from the French study. Obes Surg. 2002;12 Suppl 1:21S–5.
Shikora SA, Bergenstal R, Bessler M, et al. Implantable gastric stimulation for the treatment of clinically severe obesity: results of the SHAPE trial. Surg Obes Relat Dis. 2009;5:31–7.
Zhang J, Tang M, Chen JD. Gastric electrical stimulation for obesity: the need for a new device using wider pulses. Obesity. 2009;17:474–80.
Zhang J, Maude-Griffin R, Zhu H, et al. Gastric electrical stimulation parameter dependently alters ventral medial hypothalamic activity and feeding in obese rats. Am J Physiol Gastrointest Liver Physiol. 2011;301:G912–8.
Sun Y, Chen J. Intestinal electric stimulation decreases fat absorption in rats: therapeutic potential for obesity. Obes Res. 2004;12:1235–42.
Yin J, Ouyang H, Chen JD. Potential of intestinal electrical stimulation for obesity: a preliminary canine study. Obesity. 2007;15:1133–8.
Yin J, Zhang J, Chen JD. Inhibitory effects of intestinal electrical stimulation on food intake, weight loss and gastric emptying in rats. Am J Physiol Regul Integr Comp Physiol. 2007;293:R78–82.
Aberle J, Busch P, Veigel J, et al. Duodenal electric stimulation : results of a first-in-man study. Obes Surg. 2016;26:369–75.
Farley C, Cook JA, Spar BD, et al. Meal pattern analysis of diet-induced obesity in susceptible and resistant rats. Obes Res. 2003;11:845–51.
Li S, Maude-Griffin R, Pullan AJ, et al. Gastric emptying and Ca(2+) and K(+) channels of circular smooth muscle cells in diet-induced obese prone and resistant rats. Obesity. 2013;21:326–35.
Sallam HS, Oliveira HM, Gan HT, et al. Ghrelin improves burn-induced delayed gastrointestinal transit in rats. Am J Physiol Regul Integr Comp Physiol. 2007;292:R253–7.
Yin J, Chen JD. Mechanisms and potential applications of intestinal electrical stimulation. Dig Dis Sci. 2010;55:1208–20.
Abell T, McCallum R, Hocking M, et al. Gastric electrical stimulation for medically refractory gastroparesis. Gastroenterology. 2003;125:421–8.
Chen JD, Qian L, Ouyang H, et al. Gastric electrical stimulation with short pulses reduces vomiting but not dysrhythmias in dogs. Gastroenterology. 2003;124:401–9.
Du P, Li S, O’Grady G, et al. Effects of electrical stimulation on isolated rodent gastric smooth muscle cells evaluated via a joint computational simulation and experimental approach. Am J Physiol Gastrointest Liver Physiol. 2009;297:G672–80.
Chen JD, Lin HC. Electrical pacing accelerates intestinal transit slowed by fat-induced ileal brake. Dig Dis Sci. 2003;48:251–6.
Zhang J, Chen JD. Pacing the gut in motility disorders. Curr Treat Options Gastroenterol. 2006;9:351–60.
Ouyang X, Li S, Foreman R, et al. Hyperglycemia-induced small intestinal dysrhythmias attributed to sympathovagal imbalance in normal and diabetic rats. Neurogastroenterol Motil. 2015;27:406–15.
Li S, Chen JD. Cellular effects of gastric electrical stimulation on antral smooth muscle cells in rats. Am J Physiol Regul Integr Comp Physiol. 2010;298:R1580–7.
Xu J, McNearney TA, Chen JD. Gastric/intestinal electrical stimulation modulates appetite regulatory peptide hormones in the stomach and duodenum in rats. Obes Surg. 2007;17:406–13.
Gutzwiller JP, Goke B, Drewe J, et al. Glucagon-like peptide-1: a potent regulator of food intake in humans. Gut. 1999;44:81–6.
Naslund E, Gutniak M, Skogar S, et al. Glucagon-like peptide 1 increases the period of postprandial satiety and slows gastric emptying in obese men. Am J Clin Nutr. 1998;68:525–30.
Sun Y, Qin C, Foreman RD, et al. Intestinal electric stimulation modulates neuronal activity in the nucleus of the solitary tract in rats. Neurosci Lett. 2005;385:64–9.
Zhang J, Zhu H, Chen JD. Central neuronal mechanisms of intestinal electrical stimulation: effects on duodenum distention-responsive (DD-R) neurons in the VMH of rats. Neurosci Lett. 2009;457:27–31.
Rocca AS, Brubaker PL. Role of the vagus nerve in mediating proximal nutrient-induced glucagon-like peptide-1 secretion. Endocrinology. 1999;140:1687–94.
Schwartz A, Ort T, Kajekar R, et al. Electrical stimulation of the isolated rat intestine in the presence of nutrient stimulus enhances glucagon-like peptide-1 release. Physiol Meas. 2010;31:1147–59.
Sandoval D, Dunki-Jacobs A, Sorrell J, et al. Impact of intestinal electrical stimulation on nutrient-induced GLP-1 secretion in vivo. Neurogastroenterol Motil. 2013;25:700–5.
Cranley B, Kelly KA, Go VL, et al. Enhancing the anti-dumping effect of Roux gastrojejunostomy with intestinal pacing. Ann Surg. 1983;198:516–24.
Layzell T, Collin J. Retrograde electrical pacing of the small intestine—a new treatment for the short bowel syndrome? Br J Surg. 1981;68:711–3.
Miedema BW, Kelly KA. The Roux stasis syndrome. Treatment by pacing and prevention by use of an ‘uncut’ Roux limb. Arch Surg. 1992;127:295–300.
Reiser SB, Schusdziarra V, Bollschweiler E, et al. Effect of enteric pacing on intestinal motility and hormone secretion in dogs with short bowel. Gastroenterology. 1991;101:100–6.
Wang WF, Yin JY, De Dz Chen J. Acceleration of small bowel transit in a canine hypermotility model with intestinal electrical stimulation. J Dig Dis. 2015;16:135–42.
Xu X, Lei Y, Chen JD. Duodenum electrical stimulation delays gastric emptying, reduces food intake and accelerates small bowel transit in pigs. Obesity. 2011;19:442–8.
Lin X, Peters LJ, Hayes J, et al. Entrainment of segmental small intestinal slow waves with electrical stimulation in dogs. Dig Dis Sci. 2000;45:652–6.
Bjorck S, Kelly KA, Phillips SF. Mechanisms of enhanced canine enteric absorption with intestinal pacing. Am J Physiol. 1987;252:G548–53.
Collin J, Kelly KA, Phillips SF. Absorption from the jejunum is increased by forward and backward pacing. Br J Surg. 1979;66:489–92.
Sun Y, Chen JD. Intestinal electric stimulation accelerates whole gut transit and promotes fat excrement in conscious rats. Int J Obes. 2009;33:817–23.
Tosetti C, Corinaldesi R, Stanghellini V, et al. Gastric emptying of solids in morbid obesity. Int J Obes Relat Metab Disord. 1996;20:200–5.
Wright RA, Krinsky S, Fleeman C, et al. Gastric emptying and obesity. Gastroenterology. 1983;84:747–51.
Zahorska-Markiewicz B, Jonderko K, Lelek A, et al. Gastric emptying in obesity. Hum Nutr Clin Nutr. 1986;40:309–13.
Zhang J, Sha W, Zhu H, et al. Blunted peripheral and central responses to gastric mechanical and electrical stimulations in diet-induced obese rats. J Neurogastroenterol Motil. 2013;19:454–66.
Ouyang H, Yin J, Chen JD. Gastric or intestinal electrical stimulation-induced increase in gastric volume is correlated with reduced food intake. Scand J Gastroenterol. 2006;41:1261–6.
Zhao X, Yin J, Chen J, et al. Inhibitory effects and mechanisms of intestinal electrical stimulation on gastric tone, antral contractions, pyloric tone, and gastric emptying in dogs. Am J Physiol Regul Integr Comp Physiol. 2009;296:R36–42.
Acknowledgments
This work was supported by a VA merit grant (I01BX002010) to JC.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interests.
Ethical Approval
The study was performed according to the National Institutes of Health Guidelines on the use of laboratory animals and approved by the Animal Care and Use Committee of the Veterans Affairs Medical Center (Oklahoma City, OK). All applicable institutional and/or national guidelines for the care and use of animals were followed.
Informed Consent
Does not apply.
Rights and permissions
About this article
Cite this article
Li, S., Chen, J.D.Z. Pulse Width-Dependent Effects of Intestinal Electrical Stimulation for Obesity: Role of Gastrointestinal Motility and Hormones. OBES SURG 27, 70–77 (2017). https://doi.org/10.1007/s11695-016-2238-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11695-016-2238-5