Abstract
Background
The aim of this study was to demonstrate feasibility and safety of a new electric duodenal stimulation system (EDS, BALANCE) in humans. Secondary objectives were to evaluate the effect on glycemic control and weight loss in patients with obesity and type 2 diabetes mellitus (T2DM).
Methods
In an open-labeled, prospective, single-arm, non-randomized multicenter study, 12 obese T2DM patients with a mean HbA1c of 8.0 % received laparoscopic implantation of the BALANCE duodenal stimulating device. Adverse events, changes in glycemic control, cardiovascular parameters, and weight were collected. The follow-up period after implantation was 12 months.
Results
Device related severe adverse events did not occur. Mean HbA1c decreased by 0.8 % (p = 0.02) and mean fasting blood glucose level (FBG) was reduced by 19 % (p = 0.038) after the 12 months. Mean HDL level increased from 44 to 48 mg/dl (p = 0.033).
Conclusions
EDS is a feasible and safe procedure. Positive effects on T2DM and some cardiovascular parameters (HDL, weight) were seen. However, further prospective randomized blinded studies are needed in order to evaluate the potential of this new minimally invasive method.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Type 2 diabetes mellitus (T2DM) is a chronic disease with many severe complications. More than a third of a billion people are affected worldwide. In the next two decades, the global prevalence is expected to rise by about 75 % especially in Asian countries [1].
As early as in 1987, Walter Pories et al. were able to show that Roux-en-Y gastric bypass (RYGB) has a beneficial effect on diabetes [2]. Newer data confirm an overall remission rate of as high as 78.1 % depending on the definition used for diabetes remission [3].
Patients usually become euglycemic 10 days or less after surgery. Therefore, weight loss itself seems to play only a minor role in diabetes improvement and additional weight-independent mechanisms are likely. Rubino and Gagner postulated that bypassing the duodenum and proximal jejunum avoids secretion of anti-incretin factors and an earlier arrival of food in the ileum induces higher incretin levels. Both lead to an improvement of blood glucose levels [4, 5].
Intestinal electrical stimulation (IES) can affect gastric passage [6]. Electric stimulation has also been shown to reduce food intake, decrease blood glucose levels, and induce weight loss in animals [7, 8]. Moreover, Khawaled et al. demonstrated a decrease of gastric emptying and an increase of intestinal flow in rats treated with duodenal electrical stimulation [9].
The hypothesis underlying our study was that an implanted electric duodenal stimulation system (EDS, BALANCE system) is able to mimic the effects of a gastric bypass through electrical stimulation of the duodenal wall and lead to similar effects in humans as seen in rats [9]. It was our aim to investigate the feasibility and safety of the EDS implanted in humans. Moreover, as a secondary objective, it was our intention to evaluate the BALANCE system’s effects on glycemic control and weight loss in patients with obesity and type 2 diabetes mellitus.
Methods
The study was approved by local ethics committee (PV 3033). All procedures performed were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all individual participants included in the study. Primary objectives were to evaluate feasibility, safety, and technical performance. Secondary objectives were to investigate a potential effect on glucose control, body weight, and cardiovascular risk parameters (LDL, HDL, triglycerides, and blood pressure) in obese patients with T2DM. Inclusion and exclusion criteria are listed in Table 1.
The study was designed in a multicenter, open-label, single arm, non-randomized fashion. Patients were recruited from three different centers. All centers used the same standardized study protocol. The number of intention to treat was 12. Visits included baseline screening, laparoscopic implantation of the device, nine follow-up appointments, and laparoscopic explantation (Table 2). Baseline screening and follow-up visits consisted of physical examination, collection of blood samples, and additional patient data concerning concomitant medications and adverse events. Pregnancy tests were performed at each visit for women of child-bearing potential.
Patients
Twelve patients were included in the study. Each patient had to be on a stable antidiabetic mediation for at least 3 months prior to study inclusion. Medication is shown in Table 3. According to the protocol, antidiabetic medication was not changed during the study. Patients were advised not to change eating habits or physical activity levels to rule out possible effects of lifestyle modification.
Baseline characteristics are presented in Table 4. Mean age was 46.6 years, 33 % were male. Mean body mass index (BMI) and weight at baseline were 43 kg/m2 and 131.8 kg, respectively. Mean HbA1c at baseline was 8.4 %.
BALANCE System
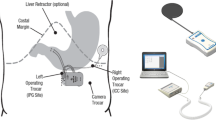
The duodenal pacer (BALANCE system) consists of the implantable device and external parts (Fig. 1). The implantable device contains one bipolar lead connected via two electrodes to an implantable pulse generator (IPG). The external parts of the system consist of a wireless wand that receives and transmits data from the IPG to a remote control and a computer system.
Implantation Procedure
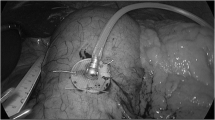
Both electrodes were implanted laparoscopically and under endoscopic control into the subserosal layer of the anterior duodenal wall 2 cm behind the pylorus for a length of approximately 1 cm. Electrodes were placed in parallel with a 1-cm gap in between and secured by hemoclips at the distal end. The lead was fixed by an additional suture as seen in Fig. 2.
Following lead implantation, the IPG was implanted into a subcutaneous pocket and electrodes were connected. After the implantation procedure, the system was tested by measuring the impedance of the electrodes. Electrical activity was recorded from the tissue to verify proper contact and stimulation ability. Each device was checked and adjusted at every follow-up. The device was activated 4 weeks after implantation.
Explantation Procedure After 12 Months of Follow-Up
After completion of the 12-months follow-up visit, device and leads were explanted laparoscopically.
Replacement Surgery
The device contains a sealed battery inside the IPG which was calculated to support the device in self activation mode for the whole study. Due to incompliance of the first two patients, who missed activating the device several times, activation mode was changed into an automated continuous mode. This resulted in a shorter battery life. As a consequence, battery had to be replaced in all participating patients once throughout the study. Replacement was performed in an outpatient setting and under local anesthesia.
Statistical Analysis
The mean values were tested for changes over time by using a Wilcoxon-signed-rank test. A significance level of α = 0.05 was used for all tests.
Results
Implantation
The implantation procedure took about 30–45 min in all of the 12 patients. There were no intraoperative complications. One patient developed a postoperative wound infection. Secondary wound closure was performed under local anesthesia.
Safety and Performance
Device-related severe adverse events did not occur. All patients described an intraabdominal prickle during the first activation of the BALANCE system. However, these symptoms were not reported again during the course of the study. Nine patients reported a reduced maximum meal size and a softer stool under stimulation. Sporadic episodes of diarrhea and vomiting during the follow-up period occurred in six patients.
Intestinal electrical activity was recorded at all required follow-up visits indicating normal functionality of the device.
Stimulation mode and stimulation parameters (frequency and amplitude) were checked and adapted by BetaStim technicians.
According to the protocol, the first two patients were trained to activate IES manually prior to eating. Battery life was calculated expecting short prandial activation periods. However, read out of the device in the first two patients revealed that manual activation was not performed reliably. Reasons for non-adherence were not related to pain or negative sensations associated with the device Therefore, activation mode was changed into an automatic continuous mode for patients 3–12 which resulted in a shorter battery life. As a consequence in all nine patients included in the analysis, the battery had to be replaced once during the study.
Effectiveness
Effects on glycemic parameters were assessed by monitoring HbA1C, fasting blood glucose (FBG), and C-peptide without any change in nutritional or physical exercise behavior and also without any change in diabetes medications. Cardiovascular parameters and weight were also investigated.
The device was implanted in 12 patients. The first two patients were excluded from analysis due to lack of adherence in activating the system prior to meals. One patient withdrew consent from the study in order to undergo bariatric surgery. Therefore, nine patients were included in statistical analysis.
Changes in Glycemic, Metabolic, and Cardiovascular Parameters
Results are summarized in Table 5 and Fig. 3. HbA1c decreased from 8 % (±1.12) to 7.2 % (±0.97) at the end of the study (p = 0.02).
Average fasting blood glucose reduced from of 173 mg/dl (±40.61) at screening to 140 mg/dl (±28.33) at month 12 (p = 0.038). Mean reduction in fasting blood glucose was 33 mg/dl. Initial C-peptide level was 4.3 mg/dl (±2.74) ruling out type 1 diabetes. At month 12, C-peptide level had dropped to 3.67 mg/dl (±1.87) (p = 0.336).
Mean LDL and triglyceride levels did not change significantly. HDL increased by 4 mg/dl (from 43.78 mg/dl (±10.54) at screening to 47.89 mg/dl (±10.71) at month 12) (p = 0.033). Mean body weight loss was 4 kg. Body weight decreased from 131.56 to 127.56 kg (±17.39, p = 0.084).
Mean systolic blood pressure at the beginning of the study was 149.56 mmHg (±25.67) and reduced to 139.56 mmHg (±23.35) (p = 0.213).
Discussion
In this first-in-man study, safety and feasibility of a laparoscopically implantable duodenal electric pacer was demonstrated. The implantation procedure is short, i.e., around 30–45 min, and safe and can be performed at an outpatient setting. No device-related major adverse events occurred during the follow-up period.
The main adverse events were episodes of transient diarrhea and vomiting and some patients described fullness and limited meal sizes. Reduced meal sizes occurring with the BALANCE system could be the result of a decreased gastric emptying as shown in the preclinical rat model [9]. Also, Liu et al. demonstrated delayed gastric emptying and decreased water intake through an endoscopically placed feeding tube in healthy volunteers under IES conditions [10]. Or findings are in line with results from retrograde gastric electric stimulation studies, which have shown a delayed gastric emptying, lower food intake, and weight loss in animal models and in humans [6, 11–14].
A softer stool under EDS conditions might be caused by intestinal malabsorption. In rats, malabsorption of fatty acids under IES has been described [15]. This may result in diarrhea in humans. However, since our patients did not receive standardized test meals or stool analyses, this remains speculative [16–18].
Unlike bariatric procedures in which adaption to the new gastrointestinal anatomy may take several weeks or months, our patients did not experience major side effects and a postoperative modification in eating patterns is not required. The majority of patients were not aware of the system except of a slight prickling during the first activation. The physician has the ability to change the stimulation parameters (intensity, frequency, and duration) by the wireless remote control noninvasively, which may improve putative symptoms or metabolic effects.
However, we experienced that a manual control (“on-off mode”) of the system was not as effective as an automatic continuous mode. The reasons for non-adherence were not related to discomfort associated with the device. Likely, patients simply forgot to activate the system. One problem we did not expect when we changed to the automatic mode was the shorter battery life which resulted in the necessity for battery change in all nine patients.
In our study, the effectiveness of the BALANCE system in improving glycemic parameters was demonstrated. The underlying cause however is speculative. A combination of a slower gastric emptying with a longer perception of satiety and a decrease of duodenal transit time can be assumed. This may be related to a suppression of ghrelin levels or other intestinal hormones [19–22]. As in RYGB, undigested food reaches distal parts of the intestine which triggers increased incretin production. Unfortunately, glucagon-like peptide 1 (GLP-1) levels were not measured in our trial. Proof of this conception is therefore missing.
The mean HbA1c at baseline was 8 % and reduced to 7.2 % after 12 months. At the same time, the average weight loss was 4 kg. According to one study, a weight loss of 5 kg results in a HbA1c reduction of 0.4 % in 1 year [23]. It is therefore likely that further effects of duodenal stimulation are responsible for the metabolic effect. The glucose-lowering effect is comparable to commonly used antidiabetic medications such as DPP4 inhibitors or SGLT2 inhibitors. Glycemic control was not monitored after explantation of the device. Therefore, the durability of the effect remains unclear. Invasive therapeutic procedures in patients with a BMI < 35 kg/m2 are third- or forth-line antidiabetic treatment and should only be considered if conservative options fail to reach glycemic goals. However, in patients with high demand for insulin, ineffective treatment, or intolerance to oral antidiabetic therapies, minimal invasive procedure like the BALANCE system of the Endobarrier® device can be a therapeutic option. However, while being less traumatic, none of the systems currently under investigation reach the metabolic efficacy of bariatric surgery.
While mean LDL level was not affected during the study, HDL increased by 4 mg/dl (from 44 mg/dl at screening to 48 mg/dl at month 12). However, it is unclear whether this translates into a measurable clinical benefit. Weight loss occurred in eight out of the nine patients during the study. Even if weight loss was not significant in this small study group, this effect is of importance since many other therapy options in diabetes (i.e., insulin, sulfonylureas) lead to an increase of body weight.
The major limitation of our study is the small number of study participants. Therefore, metabolic effects are very preliminary and need to be further investigated. In addition and with regard to the small effect on body weight, the system might be more sufficient in patients with a BMI < 35. However, when conducting the study protocol in 2009, local ethical authorities did not approve the inclusion of patients not eligible for bariatric procedures according to national standards.
In summary, our preliminary results represent the first human trial of duodenal electrical stimulation in patients suffering from T2DM and obesity. The system is safe and surgical handling is well feasible. A statistically significant effect of the BALANCE therapy was found for glycemic control and HDL. As this study was designed as a feasibility study, it only shows very preliminary data. Further prospective randomized studies are needed to investigate underlying mechanisms, the clinical relevance, and long-term effectiveness.
References
Chan JC et al. Diabetes in Asia: epidemiology, risk factors, and pathophysiology. JAMA. 2009;301:2129.
Pories WJ, Caro JF, Flickinger EG, et al. The control of diabetes mellitus (NIDDM) in the morbidly obese with the Greenville Gastric Bypass. Ann Surg. 1987;206:316.
Buchwald H et al. Weight and type 2 diabetes after bariatric surgery: systematic review and meta-analysis. Am J Med. 2009;122:248.
Rubino F, Gagner M. Potential of surgery for curing type 2 diabetes mellitus. Ann Surg. 2002;236:554.
Laferrere B et al. Effect of weight loss by gastric bypass surgery versus hypocaloric diet on glucose and incretin levels in patients with type 2 diabetes. J Clin Endocrinol Metab. 2008;93:2479.
Zhang J, Chen JD. Systematic review: applications and future of gastric electrical stimulation. Aliment Pharmacol Ther. 2006;24:991.
Xing JH, Lei Y, Ancha HR, et al. Effect of acute gastric electrical stimulation on the systemic release of hormones and plasma glucose in dogs. Dig Dis Sci. 2007;52:495.
Cigaina V. Long-term follow-up of gastric stimulation for obesity: the Mestre 8-year experience. Obes Surg. 2004;14 Suppl 1:S14.
Khawaled R, Blumen G, Fabricant G, et al. Intestinal electrical stimulation decreases postprandial blood glucose levels in rats. Surg Obes Relat Dis. 2009;5:692.
Liu S, Hou X, Chen JD. Therapeutic potential of duodenal electrical stimulation for obesity: acute effects on gastric emptying and water intake. Am J Gastroenterol. 2005;100:792.
Shikora SA. Implantable gastric stimulation—the surgical procedure: combining safety with simplicity. Obes Surg. 2004;14 Suppl 1:S9.
Bohdjalian A et al. One-year experience with Tantalus: a new surgical approach to treat morbid obesity. Obes Surg. 2006;16:627.
Chen JZ, Ueno T, Xu X, et al. Reverse gastric pacing reduces food intake without inducing symptoms in dogs. Scand J Gastroenterol. 2006;41:30.
Liu J, Qiao X, Hou X, et al. Effect of intestinal pacing on small bowel transit and nutrient absorption in healthy volunteers. Obes Surg. 2009;19:196.
Sun Y, Chen J. Intestinal electric stimulation decreases fat absorption in rats: therapeutic potential for obesity. Obes Res. 2004;12:1235.
Layzell T, Collin J. Retrograde electrical pacing of the small intestine—a new treatment for the short bowel syndrome? Br J Surg. 1981;68:711.
Cullen JJ, Doty RC, Ephgrave KS, et al. Changes in intestinal transit and absorption during endotoxemia are dose dependent. J Surg Res. 1999;81:81.
Huge A, Weber E, Ehrlein HJ. Effects of enteral feedback inhibition on motility, luminal flow, and absorption of nutrients in proximal gut of minipigs. Dig Dis Sci. 1995;40:1024.
Gibbs J, Young RC, Smith GP. Cholecystokinin decreases food intake in rats. J Comp Physiol Psychol. 1973;84:488.
Liddle RA, Carter JD, McDonald AR. Dietary regulation of rat intestinal cholecystokinin gene expression. J Clin Investig. 1988;81:2015.
Xu J, McNearney TA, Chen JD. Gastric/intestinal electrical stimulation modulates appetite regulatory peptide hormones in the stomach and duodenum in rats. Obes Surg. 2007;17:406.
Asakawa A et al. Antagonism of ghrelin receptor reduces food intake and body weight gain in mice. Gut. 2003;52:947.
Redmon JB et al. One-year outcome of a combination of weight loss therapies for subjects with type 2 diabetes: a randomized trial. Diabetes Care. 2003;26:2505–11.
Acknowledgments
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all individual participants included in the study.
Conflict of Interest
Jens Aberle, Philipp Busch, Jochen Veigel, Anna Duprée, Thomas Roesch, Christine zu Eulenburg, Björn Paschen, Bernd M. Scholz, Stefan Wolter, Kaja Ludwig, Jakob Izbicki, and Oliver Mann declare no conflict of interest. The study itself was financed by BetaStim Ltd®, 2 Hatohen St., Caesarea Industrial Park (North), P.O.B. 3143, Caesarea 38900, Israel.
Author information
Authors and Affiliations
Corresponding author
Additional information
Jens Aberle, Philipp Busch and Jochen Veigel contributed equally to this work.
Rights and permissions
About this article
Cite this article
Aberle, J., Busch, P., Veigel, J. et al. Duodenal Electric Stimulation. OBES SURG 26, 369–375 (2016). https://doi.org/10.1007/s11695-015-1774-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11695-015-1774-8