Abstract
Background
Laparoscopic sleeve gastrectomy (LSG) is one of the most commonly performed bariatric procedures for treatment of morbid obesity. Despite its popularity, it is not without risks, the most serious of which is the staple line leak. Staple line leaks are difficult to manage and require significant resources in the form of surgical, radiological and endoscopic interventions; long hospital and intensive care stay and significant morbidity. International experience is slowly emerging, but there are still no clear guidelines regarding optimal management of leaks. This study aims to describe the experience of endoscopic management of these leaks by the authors and the development of a customised stent for this condition.
Methods
Middlemore Hospital is the largest bariatric surgery centre in New Zealand. Since June 2007, a total of 21 patients have received endotherapy for post-LSG leak management. Treatment included the deployment of primary self-expanding metal stents (SEMS) across the leak site, combined with complementary endoscopic modalities. Persistent leaks were treated with follow-up stenting. This study aimed to evaluate the effectiveness of post-LSG staple line leak management at Middlemore Hospital.
Results
A total of 20/21 (95 %) patients now have resolved leaks following a mean of 75 days of treatment (median 47, range 9–187). The mean number of endoscopic procedures required was five. Inpatient stay and average duration till leak resolution has been notably reduced since the addition of customised stents. Clinically significant stent migration occurred in 19 % of primary stents.
Conclusion
The use of SEMS in conjunction with complementary endotherapy has shown to be both safe and effective in treating sleeve leaks; however, migration is the limiting factor for optimal management. Recent improvements in stent design, such as the one proposed in this paper, show promise in addressing this problem. Earlier use of SEMS seems to reduce the time till closure as well as the total hospital stay, as is apparent from our data.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The worldwide prevalence of obesity has more than doubled since 1980, with now more than 600 million obese and 1.9 billion overweight adults [1]. The 2012/2013 New Zealand Ministry of Health Survey found that almost one in three adults (31 %) and one in nine children (11 %) were obese [2]. This epidemic poses significant population health implications as obesity is one of the most important modifiable risk factors for a number of major diseases, including type 2 diabetes mellitus, ischaemic heart disease and ischaemic stroke.
Considering these implications on our health system, the necessity for bariatric interventions has become apparent. A rapidly emergent technique for treatment of morbid obesity is the laparoscopic sleeve gastrectomy (LSG). It is quickly gaining popularity as a stand-alone treatment due to its promising results and perceived technical simplicity relative to other bariatric procedures [3, 4]. The procedure is not without serious risks and has been associated with severe complications such as sleeve leaks, stenosis and multi-organ failure [4, 5].
With a reported overall incidence of 1.9–2.4 % [6, 7], the sleeve leak remains the most important complication causing significant morbidity and mortality [5]. The management of such leaks has been constantly evolving; however, currently, there are no clear guidelines regarding optimal leak management. Over the last decade, there has been increasing use and development of self-expanding metal stents (SEMS) for the treatment of anastomotic leaks [8–13]. Alternative management strategies to have included use of glue injection and metal clipping with a combination of percutaneous drainage and the other endoscopic therapies [10, 17]. More recently, treatment options include the insertion of endobiliary stents [24–26]. This study aimed to evaluate the effectiveness of post-LSG staple line leak management at Middlemore Hospital.
Methods
Middlemore Hospital is the largest bariatric surgery centre in New Zealand. Our gastroenterology department maintains a prospectively collected database of all post-operative gastric sleeve leak cases. We have conducted a retrospective review of the endoscopic management of patients who presented with post-operative gastric sleeve leaks between the period of June 2007 and December 2014. Institutional and local ethics committee approval was obtained for the study.
In accordance with the 2011 International Sleeve Gasterctomy Expert Panel [14], post-operative leaks were defined as acute (post-operative days 1–7), early (post-operative weeks 1–6), late (post-operative weeks 6–12) or chronic (post-operative week 12+). Sleeve leak was diagnosed based on clinical symptoms of pain and sepsis combined with leak evidence on a Gastrografin® swallow, abdominal CT scan and oesophagogastroscopy. Peri-gastric collections were drained by either laparoscopic, or CT guided drain placement based on the clinical stability of the patient. Drain placement usually preceded the endoscopic management.
Fistula treatment consisted of primary stent deployment across the leak site. Additionally, at the discretion of the endoscopist, a range of complementary endoscopic treatments were utilised. Those treatments included cyanoacrylate glue and metal clips [Resolution clip (Boston Scientific Corporation, USA), Over-the-Scope Clip® (OTSC®, Ovesco Endoscopy, Germany)] which were implemented to assist in fistula closure; however, in some cases, the use of OTSC® clips was technically difficult due to tissue friability and immobility around the fistulae. Additionally, distal sleeve dilations with balloon dilators [CRE balloons 15–20 mm and Rigiflex II achalasia dilators 30–35 mm (both Boston Scientific Corporation, USA)] and pyloric Botox injections were utilised in some cases to reduce distal pressure and improve clearance of food. For well-epithelised fistulae, either Gold Probe cauterisation or Argon Plasma Coagulation (APC) were used to create raw surfaces and induced granulation, thereby promoting closure. In one case, a 5 cm long 10 F biliary stent with RX delivery system (Boston Scientific Corporation, USA) was placed into a small paragastric collection to close the fistula.
If the primary treatment failed to adequately close the fistula or the stent positioning was suboptimal, a second stent was deployed. These stents were used either as a replacement or overlapping the primary stent. The mean time till stent removal was 6 weeks and was accomplished using rat tooth forceps. All stents were inserted under endoscopic and fluoroscopic guidance using conscious sedation (combination of intravenous Fentanyl and Midazolam), with the majority of the procedures undertaken by the most experienced interventional endoscopist of the department (Ogra, R). Patients who were treated with stents were put on a gastric diet protocol, but those with unsatisfactory oral intake were administered temporary nasojejunal feeding. Some patients were receiving total parenteral nutrition.
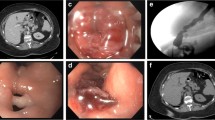
Due to lack of international experience regarding ideal stent choices earlier on in the series, a variety of stents types and sizes were used (Table 1). Initially, the stent of choice was the fully covered Taewoong Niti-S™ oesophageal stent (18 mm wide with 24-mm flared ends). As institutional experience grew, it was felt that a tight proximal mucosal seal was important to prevent ongoing contamination of the fistula tract and also to prevent stent migration. Following this principle, Ultraflex partially covered stents (Boston Scientific Corporation, USA) were inserted for acute leaks. To facilitate easy removal of these uncovered stents, overlapping stents were placed on the ingrowth aspects [(Alimaxx (Merit Medical Endotek, USA) or Niti-S™ Oesophageal stents (Taewoong Medical, Korea)]. The overlapping stents were removed 2–3 weeks after insertion allowing disimpaction of the mucosal ingrowth. With the international development of wider stents, including additional anti-migratory mechanisms, our stent repertoire also included the Taewoong Niti-S™ Beta stent (24 mm wide, 150–200-mm long double anti-migratory cuffed stent with a 32 mm wide proximal flared end [Fig. 1]) and Taewoong Niti-S™ Megastent (24 mm wide, 230 mm long with two 32 mm wide flared ends). In constant search for the ideal stent and following our experiences with the partially uncovered Ultraflex stent, our department has developed a customised stent. Our development is a modification of Taewoong Niti-S™ Beta II stent leaving 10 mm of the proximal flare uncovered (Figs. 2 and 3).
Treatment success was defined as closure of the leak (confirmed by Gastrografin® studies, endoscopic surveillance and resolution of clinical symptoms) following primary stent removal (primary resolution) or after follow-up stent(s) removal (secondary resolution).
Results
Patients
Between the period of June 2007 and December 2014, a total of 1050 LSG were performed at our institution. During this time, 16 patients presented with staple line leaks (1.52 %). An additional five leak patients were referred to our institution for management from other institutions, adding to a total of 21 patients. Seven (33 %) of the patients were male and 14 (67 %) were female, ranging in ages from 32 to 58 years old, with a mean age of 44.7 years. The mean pre-OP BMI was 44.4 kg/m2 with a range of 31.2–58.1 kg/m2. A summary of the relevant clinical demographics is outlined in Table 2.
Of the 21 patients presenting with clinical leaks, 6 were acute (29 %), 12 were early (57 %), 1 was late (5 %) and 2 were chronic (9 %). Leaks were diagnosed at an average interval of 31 days (median 10) post-LSG. The earliest leak presentation was on post-operative day 2, and the latest was on post-operative day 286 that followed the development of severe mid and distal sleeve stenosis. The majority of leaks occurred proximally, at the gastro-oesophageal junction (71 %) with less occurring in mid (21 %) and distal (8 %) sleeve aspects. Multiple staple-line leaks occurred in three patients, two of which were proximal and mid, with the third proximal and distal. Gastrobronchial fistulae occurred in three patients (14 %), and one patient developed a delayed gastro-colic fistula (5 %). There was no mortality in our study.
Five patients (24 %) achieved leak resolution using endoscopic treatments alone without requiring surgical or radiological drain placement. Before referral to our department, six patients (29 %) had radiological drainage, and nine patients (43 %) had surgical interventions, including a combination of washout, drain-placement and additional suturing techniques. One patient (5 %) had both radiological and surgical interventions.
Outcomes
An average of 1.6 therapeutic stents were used per patient (median 1, range 1–4), requiring on average five oesophagogastroscopic procedures (median 3, range 2–13). Five (24 %) patients required planned overlapping stents to assist in disimpaction of mucosal ingrowth from the uncovered parts of the stents. This required an average of two additional stents (range 1–3) per patient.
The majority of patients receiving stents also had complementary endoscopic treatment (90 %). A median of two additional endoscopic modalities were used per patient (mean 1.6, range 0–4). A more detailed per patient summary is included on Table 1.
Following endoscopic management, primary resolution was observed in 15/21 patients (71 %) (Fig. 4). In this group, leak closure was confirmed after a mean duration of 55 days and a mean inpatient stay of 33 days. Of the remaining six patients with persistent fistulae, five achieved secondary resolution during follow-up treatment. In these patients leak closure was confirmed after a mean duration of 128 days and a mean inpatient stay of 82 days. Overall, 20/21 patients (95 %) had resolved leaks following a mean of 75 days (median 47, range 9–187) and 3 years follow-up. The remaining unresolved patient experienced a persistent leak despite extensive endoscopic interventions, thus requiring a RYGB conversion. Unfortunately, the patient later developed multiple anastomotic site leaks requiring further surgery, extensive inpatient stay and to date extensive fistulae persist.
There was a significant delay between leak identification and referral for endotherapy, with some cases delayed up to 14 weeks. This delay was a manifestation of attempts at leak closure via re-operation, radiologic drainage and a lack of international consensus regarding optimal management. The mean time to leak resolution for patients who were stented within 2 weeks of diagnosis (n = 14) was 61 ± 58.1 days versus 105 ± 64.3 days for patients later than 2 weeks (n = 7) (p = 0.077). Similarly, there was a statistically significant shorter total inpatient stay for patient’s stented within 2 weeks of diagnosis. Quantitatively, this was shown by a total inpatient stay of 58.6 ± 42.3 days for early stent intervention, compared to 123.9 ± 32.4 days for those with delayed stent intervention (p = 0.001).
Stent Complications
The most prominent complication associated with stent use was migration which occurred in four of six (67 %) patients who failed primary endoscopic treatment. An additional six instances of minor migration occurred, three requiring manual repositioning of the stent, while the other three still provided adequate fistula cover. In all six of these migrations, primary closure was still achieved. Overall, we reported a primary stent migration rate of 48 %; however, after adjusting for minor migrations easily corrected with repositioning of the stents, this rate is reduced to 19 %. Notably, no instances of migration occurred while using partially uncovered stents [Ultraflex (n = 3) or custom-made leak stent (Ogra) (n = 1)].
Two patients developed new staple line leaks distal to the primary stent. In the first patient, this leak development followed distal erosion caused by partially impacted end of the uncovered Ultraflex stent. The other patient had significant mid-sleeve stenosis which we suspect created a sufficiently high pressure intra-gastric environment to establish this secondary leak.
Common consequences of stent usage were retrosternal pain, nausea and vomiting. Most of these settled within a few days or were managed conservatively with pharmacological treatment. Severe patient intolerance necessitated early therapeutic stent removal in 5/34 (15 %) patients. Of these, three (9 %) were due to proximal migration causing significant nausea and vomiting, one (3 %) was due to stent kink causing symptomatic outflow obstruction and one (3 %) due to just stent intolerance; all but the last patient were able to tolerate follow-up stenting.
Two patients (10 %) developed a benign oesophageal stricture secondary to tissue hyperplasia at the proximal end of the stents. These were both endoscopically managed by serial dilations.
Discussion
Sleeve leak management options are varied and dependent on their timing and clinical presentation. Patients who present with clinical instability justify prompt laparoscopic re-intervention for washout and drainage. If the tissue is not severely inflamed and friable, this may be coupled with isolation techniques such as omental patching or primary closure of defect [15]. With regard to stable patients and leaks presenting later in the post-operative course, guidelines for optimal management remain unclear [16–19].
This study has shown an overall success rate of 95 %, with 71 % via primary closure, through the use of SEMS and complementary endoscopic modalities. Stenting has the immediate benefit of reducing intra-gastric pressure as well as providing a temporary bypass of the leak, favouring its healing and enabling oral nutrition. However, stent placement for post-LSG sleeve leak is usually limited by the high rate of migration; these rates have been reported to be as high as one third of all cases. Galloro et al. [20] has suggested that these migration events are likely due to the inappropriate use of covered oesophageal stents within the gastric sleeve, where a proper containment is lacking. Furthermore, the silicon coating prevents mucosal ingrowth and thus inhibits integration into the stomach remnant wall.
Various novel attempts to reduce migration have been tried in other studies, including wider stents (40 mm) [21], intraoperative suturing to the sleeve and use of transnasally externalised threads [21], use of partially covered Ultraflex stents (Ultraflex Boston Scientific, USA), and use of stents with double layer anti-migratory cuffs [Beta stent (Taewoong Medical, Korea)]. While our experience with the partially covered Ultraflex stents has been effective at reducing migration (0 % migration rate), we have noted that often a single overlapping stent is insufficient to cause disimpaction of mucosal ingrowth due to a tissue seal at both ends. This is problematic as it has the potential to require further endoscopic procedures and multiple overlapping stents.
The Taewoong Niti-S™ Megastent (24 mm wide, 230 mm long with two 32 mm wide flared ends) has been recently developed for the treatment of LSG staple line leaks. This stent combines the anti-migratory qualities of a wide profile with a longer body to provide effective drainage of the entire sleeve. It appears that the wider diameter also provides sufficient radial force to cause dilation of possible stenosis, which seems to be an important factor in delaying leak closure [22]. To date, this novel stent design has shown good efficacy in promoting primary closure of fistulae [21, 23]. Our experience with this stent has been successful, but the wide distal flare seems to cause significant mucosal ulceration and is not tolerated as well as the other stents.
We hypothesise that the ideal stent should be wider than normal, it should include anti-migratory mechanisms, it should be long enough to cover incisura/distal end of sleeve and it should also have the proximal part uncovered to allow tissue seal. Following these principles, the author (Ogra, R) has modified the Taewoong Niti-S™ Beta II [24 mm × 200 mm double anti-migratory cuffed stent] to keep the proximal flare (10 mm length) uncovered. The rationale behind this modification is to allow the uncovered portion to be integrated into oesophageal mucosa, thus creating a seal. This seal diverts oral contents away from the fistula as well as providing an additional anti-migratory mechanism. Only a small portion of the stent is left uncovered to ensure easy removal without the need for overlapping stent(s) for disimpaction.
To date, this novel stent has been deployed in one of our most recent patients, resulting in primary success following 25 days of endoscopic treatment (case no. 20, Table 1). We acknowledge that a single patient represents a limited experience; however, it demonstrates a new single-stage endoscopic management option. The new modified stent is now on order, and we plan to produce a follow-up paper with a more comprehensive experience as more staple line leak patients present to our department.
Some authors suggest the use of endoscopically placed endobiliary catheters (plastic biliary or pancreatic endoprosthesis) as an alternative to SEMS. These stents are placed through the fistula tract into the collection with the aim of allowing drainage of the area and reduction of the cavity size. This appears to be an attractive alternative modality of treatment which has been reported with good success [24–26]. The most recent of which is a study by Donatelli et al. [26] which describes the use of endoscopic internal drainage of leaks using pigtail stents coupled with enteral nutrition (EDEN). This treatment modality yielded a 95 % success rate following a mean of 55.5 days till resolution, 2.95 endoscopic procedures and a relatively long period of enteral feeding. Despite an identical success rate, their treatment results are apparently superior to our study that required a mean of 75 days before resolution and five endoscopic procedures. However, when comparing outcomes, it is important to note that our study included patients from as early as 2007, a time when there was little international experience regarding endoscopic management, and purpose-built LSG stents did not exist. If only considering patients treated since the introduction of second-generation purpose-built LSG-leak stents (Megastent and custom-made Ogra stent) in early 2014 (three patients), the data shows a 100 % success rate following an average of 34 days of treatment, 2.6 endoscopic procedures and 24 days of inpatient stay following referral.
In our experience, the use of endobiliary catheters is better suited for cases with relatively small fistulae and a freely draining gastric remnant. These observations align with a recent study by Nedeleu et al. [27], showing good results following a new algorithm which is guided by the diameter of the fistulous site and circumstantial use of endobiliary catheters. They suggest that endobiliary catheters (PIGTAIL double drain) should be reserved for freely draining fistulae smaller than 10 mm and that leaks larger than 10 mm or of any size in the presence of sleeve stenosis should be managed with SEMS. The authors accept the limitations of this retrospective study, and the future direction in this condition would be to attempt a randomised trial of the new stents and endobiliary catheter placement in order to arrive at an effective management plan for these leaks.
The algorithm followed by the authors includes assessment of the size of the fistula and presence of any stenosis at the incisura. Stent placement is primary option for large leaks and more so if these are associated with sleeve stenosis. For the smaller leaks not accompanied by stenosis, we have started using double pigtail catheters. Complementary endoscopic therapies such as metal clips, OTSC clips, Glue injection and APC are determined case by case determined by the endoscopic appearance of the fistula.
Conclusion
With a 95 % success rate and no mortality, the data suggests that the authors approach in using SEMS in combination with complementary endoscopic modalities can be safe and effective in the treatment of staple line leaks following LSG. Stent migration remains to be the main limiting factor in optimising treatment success. Recent improvements in stent design, such as the one proposed in this paper, shows promise in addressing this problem. According to the current paper earlier, use of SEMS seems to reduce the time till leak closure as well as the total hospital stay.
References
World Health Organization. Obesity and overweight factsheet [WHO website]. Jan 2015. http://www.who.int/mediacentre/factsheets/fs311/en/. Accessed 22 Feb 2015.
New Zealand Ministry of Health. Obesity data and stats [NZ Ministry of Health website]. 2015. http://www.health.govt.nz/nz-health-statistics/health-statistics-and-data-sets/obesity-data-and-stats. Accessed 22 Feb 2015
Brethauer SA. Sleeve gastrectomy. Surg Clin North Am. 2011;91:1265–79.
Buchwald H, Oien DM. Metabolic/bariatric surgery worldwide 2011. Obes Surg. 2013;23(4):427–36.
Sarkhosh K, Birch DW, Sharma A, et al. Complications associated with laparoscopic sleeve gastrectomy for morbid obesity: a surgeon’s guide. Can J Surg. 2013;56(5):347–52.
Aurora AR, Khaitan L, Saber AA. Sleeve gastrectomy and the risk of leak: a systematic analysis of 4,888 patients. Surg Endosc. 2012;26(6):1509–15.
Benedix F, Benedix DD, Knoll C, et al. Are there risk factors that increase the rate of staple line leakage in patients undergoing primary sleeve gastrectomy for morbid obesity? Obes Surg. 2014;24(10):1610–6.
Eisendrath P, Cremer M, et al. Endotherapy including temporary stenting of fistulas of the upper gastrointestinal tract after laparoscopic bariatric surgery. Endoscopy. 2007;39:625–30.
Eubanks S, Edwards CA, Fearing NM, et al. Use of endoscopic stents to treat anastomotic complications after bariatric surgery. J Am Coll Surg. 2008;206:935–9.
Casella G, Soricelli E, Rizzello M, et al. Non surgical treatment of staple line leaks after laparoscopic sleeve gastrectomy. Obes Surg. 2009;19:821–6.
Nguyen NT, Nguyen XMT, Dholakia C. The use of endoscopic stent in management of leaks after sleeve gastrectomy. Obes Surg. 2010;20(9):1289–92.
Tan JT, Kariyawasam S, Wijeratne T, et al. Diagnosis and management of gastric leaks after laparoscopic sleeve gastrectomy for morbid obesity. Obes Surg. 2010;20(4):403–9.
Abou Rached A, Basile M, El Masri H. Gastric leaks post sleeve gastrectomy: review of its prevention and management. World J Gastroenterol. 2014;20(38):13904–10.
Rosenthal RJ, International Sleeve Gastrectomy Expert Panel, Diaz AA, et al. International Sleeve Gastrectomy Expert Panel Consensus Statement best practice guidelines based on experience of 12,000 cases. Surg Obes Relat Dis. 2012;8(1):8–19.
Burgos AM, Braghetto I, Csendes A, et al. Gastric leak after laparoscopic-sleeve gastrectomy for obesity. Obes Surg. 2009;19(12):1672–7.
de Aretxabala X, Leon J, Wiedmaier G, et al. Gastric leak after sleeve gastrectomy: analysis of its management. Obes Surg. 2011;21(8):1232–7.
Corona M, Zini C, Allegritti M, et al. Minimally invasive treatment of gastric leak after sleeve gastrectomy. Radiol Med. 2013;118(6):962–70.
Donatelli G, Ferretti S, Vergeau BM, et al. Endoscopic Internal Drainage with Enteral Nutrition (EDEN) for treatment of leaks following sleeve gastrectomy. Obes Surg. 2014;24(8):1400–7.
Alazmi W, Al-Sabah S, Ali DA, et al. Treating sleeve gastrectomy leak with endoscopic stenting: the Kuwaiti experience and review of recent literature. Surg Endosc. 2014;28(12):3425–8.
Galloro G, Magno L, Musella M, et al. A novel dedicated endoscopic stent for staple-line leaks after laparoscopic sleeve gastrectomy: a case series. Surg Obes Relat Dis. 2014;10(4):607–11.
Fischer A, Bausch D, Richter-Schrag HJ. Use of a specially designed partially covered self-expandable metal stent (PSEMS) with a 40-mm diameter for the treatment of upper gastrointestinal suture or staple line leaks in 11 cases. Surg Endosc. 2013;27(2):642–7.
Márquez MF, Ayza MF, Lozano RB, et al. Gastric leak after laparoscopic sleeve gastrectomy. Obes Surg. 2010;20(9):1306–11.
Basha J, Appasani S, Sinha SK, et al. Mega stents: a new option for management of leaks following laparoscopic sleeve gastrectomy. Endoscopy. 2014;46(Suppl 1 UCTN):E49–50.
Pequignot A, Fuks D, Verhaeghe P, et al. Is there a place for pigtail drains in the management of gastric leaks after laparoscopic sleeve gastrectomy? Obes Surg. 2012;22(5):712–20.
Slim R, Smayra T, Chakhtoura G, et al. Endoscopic stenting of gastric staple line leak following sleeve gastrectomy. Obes Surg. 2013;23(11):1942–5.
Donatelli G, Ferretti S, Vergeau BM, et al. Endoscopic Internal Drainage with Enteral Nutrition (EDEN) for treatment of leaks following sleeve gastrectomy. Obes Surg. 2014;24(8):1400–7.
Nedelcu M, Manos T, Cotirlet A, et al. Outcome of leaks after sleeve gastrectomy based on a new algorithm addressing leak size and gastric stenosis. Obes Surg. 2015.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The study was approved by the Institutional research ethics committee and has been performed in accordance with the ethical standards as laid down in the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards.
Conflict of Interest
Dr. Ravinder Ogra declares an Honorarium received for an ANZ medical advisory meeting for Boston Scientific Corporation Australia in 2013. Dr. Tien Huey Lim and Thomas Southwell do not have any conflict of interest or declaration.
Informed Consent
Informed consent was obtained from all individual participants included in this study.
Rights and permissions
About this article
Cite this article
Southwell, T., Lim, T.H. & Ogra, R. Endoscopic Therapy for Treatment of Staple Line Leaks Post-Laparoscopic Sleeve Gastrectomy (LSG): Experience from a Large Bariatric Surgery Centre in New Zealand. OBES SURG 26, 1155–1162 (2016). https://doi.org/10.1007/s11695-015-1931-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11695-015-1931-0