Abstract
Gastric leak after sleeve gastrectomy can lead to significant morbidity and mortality. The aim of this study was to examine the safety and efficacy of endoscopic deployment of a covered esophageal stent in the management of leaks after sleeve gastrectomy. Three consecutive patients who underwent sleeve gastrectomy at outside institutions presented with leaks. All three patients underwent endoscopic placement of a covered stent. Additional procedures included laparoscopic or percutaneous drainage of abdominal collection(s). The patients were two women and one man, with a mean age of 34 years. One patient presented acutely at day 7 after the index operation and two patients presented late at 6 and 9 months, respectively. Two patients had proximal gastric leaks and one patient had a proximal gastric leak with a concomitant obstruction at the mid-aspect of the gastric sleeve. Endoscopic deployment of a covered stent was successful in all cases. There were no complications relating to the stent placement. The stent was removed at 6 weeks in two patients and at 4 months in one patient. The use of endoscopic stent was a safe and effective option in the management of leaks after sleeve gastrectomy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Sleeve gastrectomy as a primary bariatric operation has been gaining enthusiasm among surgeons and patients. Clinical advantages of the sleeve gastrectomy include good weight loss up to 5 years follow-up; no rerouting of intestine, which eliminates the risk for late bowel obstruction from internal herniation; and, unlike the gastric band, there is no foreign body present and the risk for slippage and erosion is eliminated. However, one of the most dreaded complications of the sleeve gastrectomy is a leak along the lengthy staple line. The incidence of leak was reported at an average of 2.7% from 24 studies with 1,749 patients [1]. At the current time, the leak rate after sleeve gastrectomy appears to be higher than that of Roux-en-Y gastric bypass. Leaks after sleeve gastrectomy commonly occur at the proximal aspect of the staple line immediately below the gastroesophageal junction. The treatment for leaks after sleeve gastrectomy varies and depends on the extent of the disruption, the extent of abdominal contamination, and the site of the leaks (proximal vs. distal staple line). The treatment options for postoperative leaks after sleeve gastrectomy include reoperation with closure of the defect and wide drainage or reoperation with abdominal washout and placement of a T-tube to intubate the defect and wide peritoneal drainage. Alternatively, there have been few reports of intraluminal stenting of gastric leaks after sleeve gastrectomy [2–6]. The aim of this study was to determine the safety and efficacy of endoscopic stenting in the management of leaks after sleeve gastrectomy.
Methods
Three patients who underwent sleeve gastrectomy at outside institutions presented with a diagnosis of gastric leak. Their charts were reviewed for patient characteristics, time interval between the index operation and presentation, symptoms at presentation, treatment option, and outcomes. There have been no leaks in our experience with sleeve gastrectomy performed at University of California, Irvine. This retrospective chart review was approved by the Institutional Review Board of the University of California, Irvine Medical Center.
Endoscopic Stent Technique
An endoscopy was performed to evaluate the site and extent of the leak. The scope was then passed into the gastric antrum. The site of the leak was confirmed on fluoroscopy by radiopaque markers that were placed to outline the proximal and distal extent of the stent. An ultra-stiff guide wire was placed into the gastric antrum and its placement confirmed under fluoroscopy. The endoscope was removed, leaving the remaining guide wire in place. In two patients, a 22 × 120 mm covered esophageal stent (Alimaxx-E, Alveolus® Inc., Charlotte, NC, USA) was deployed over the guide wire and positioned between the two radiopaque markers. In one patient, a 23 × 155 mm covered esophageal stent (Wallflex®, Boston Scientific Corporation, Natick, MA, USA) was used. Additionally, the endoscope was placed along the side of the stent to ensure that the proximal aspect of the stent was positioned at the optimal position. A completion endoscopy was then performed to confirm the correct position of the stent.
Results
Patient characteristics and treatment of leaks are summarized in Table 1. The patients were two women and one man with a mean age of 34 years. One patient presented with an acute leak (7 days from the index operation) and two patients presented with late leaks (6 and 9 months, respectively, from the index operation). Symptoms at presentation for the three patients included acute abdominal pain, tachycardia, and elevation of white blood cell count in the patient with an acute presentation. The two patients with late presentations had symptoms of nausea, vomiting, abdominal pain, and fever. Work-up for leaks included upper gastrointestinal (GI) contrast study or computed tomography (CT) scan of the abdomen. In the patient with an acute leak, a CT scan of the abdomen showed contrast extravasation at the proximal gastric staple line and a fluid collection was observed adjacent to the gastric sleeve (Fig. 1). Upper GI study on the two patients with late presentations showed contrast leak at the proximal staple line in both patients and a concomitant complete obstruction of the sleeve at the gastric incisura angularis in one patient.
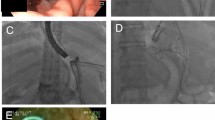
Treatment for leaks consisted of endoscopic deployment of a covered stent and either laparoscopic or percutaneous drainage of abdominal fluid collections. One patient had both gastric leak and obstruction of the gastric sleeve at the gastric incisura angularis. This patient underwent laparoscopic lysis of adhesion and endoscopic deployment of a covered stent. Endoscopic deployment of a covered stent was successful in all three cases. All patients were kept nothing per oral with nutritional supplementation given through a jejunostomy tube or total parenteral nutrition. Intravenous antibiotic was administered for a total of 2 weeks. An upper GI contrast study performed at 2 weeks after stent placement showed no leaks in all three patients (Figs. 2 and 3). A liquid diet was started and was advanced to soft diet as tolerated. Endoscopy was subsequently performed for stent removal at 6 weeks in two patients. The patient with obstruction of the gastric sleeve had the stent in place for a longer period of time and removed at 4 months post-procedure. There have been no leak recurrences at a mean follow-up period of 5.3 months.
Upper gastrointestinal contrast study showing a stent deployed for treatment of a proximal staple line leak and a partial obstruction at the mid-aspect of the gastric sleeve. Note that there is a bending of the stent at its midpoint due to the stricture in the gastric sleeve. The stent protects the leak and allows contrast to pass through the stricture into the duodenum
Discussion
Sleeve gastrectomy as a bariatric procedure is gaining popularity. The mechanism of weight loss may be related to gastric restriction, neurohormonal changes related to the gastric resection, or some other unidentified factors. A dreaded complication after sleeve gastrectomy is staple line leaks. Management of gastrointestinal leak is complex and associated with major morbidity and even mortality. In this report, we describe a small case series of postoperative gastric leaks that were managed successfully with either laparoscopic or percutaneous drainage and deployment of a covered esophageal stent.
Although the use of endoscopic esophageal stent in the management of anastomotic leak after esophagectomy is well described, there have been few reports on the outcome of endoscopic esophageal stent placement for a gastric leak after sleeve gastrectomy. Serra and colleagues [2] reported on the use of coated self-expanding stents for management of leaks after sleeve gastrectomy or duodenal switch in six patients with control of leaks in 83% of cases. Casella and colleagues reported the use of endoscopic stent for leak at the gastroesophageal junction after sleeve gastrectomy in three patients with complete healing occurring in all patients [3]. Oshiro and colleagues reported successful management of proximal gastric leak using a covered endoscopic stent in two patients who underwent prior unsuccessful laparoscopic treatment for the leak [4]. In contrast, the largest series of eight cases of endoscopic stent for leak after sleeve gastrectomy was reported by Tan et al. [5]. They reported a 50% success rate for closure of the leak, with four patients requiring premature removal of the stent due to migration, hematemesis, or obstruction from kinking at the proximal aspect of the stent. In another report, Fukumoto and colleagues reported a single case of endoscopic stent for leak after sleeve gastrectomy without success that required operative closure of the fistula [6]. Besides the use of endoscopic stent, other options in management of gastric leak after sleeve gastrectomy include placement of a T-tube within the gastric defect and peritoneal drainage or placement of a jejunal serosa patch to seal the gastric defect. Following the principles in the management of esophageal leaks after esophagectomy, direct primary closure of the defect with or without sealants should be reserved for cases that were diagnosed early (within 24–48 h) and have good tissue viability.
Several principles should be followed when an esophageal stent is considered for management of a gastric leak after sleeve gastrectomy. First, an endoscopy must be performed to evaluate the site of the leak, the size of the leak, and viability of the conduit. Gastric leaks at the proximal and mid-aspect of the gastric sleeve are the only leaks that are amenable to endoscopic treatment with stent. A leak at the distal staple line of the gastric sleeve, near the gastric antrum, will not be amenable to endoscopic stenting as the stent would be too small and would not provide appropriate sealing of the defect. Second, appropriate drainage of abdominal collection is of utmost importance using either laparoscopic or percutaneous technique in combination with nothing per oral and nutritional support using either total parenteral nutrition or jejunostomy feeding. Third, the size of the endoscopic stent should be selected erring on the larger size to prevent migration. The selection of the size of the stent is based on evaluation of the gastric sleeve diameter at the time of endoscopy. Despite using a large stent (22-mm in two patients and 23-mm in diameter in one patient) in our case series, one of three stents migrated distally into the antrum. Another strategy to minimize stent migration is to use a longer stent whereby the distal aspect of the stent is rested along the wall of the gastric antrum which preclude the stent from luminal migration.
In addition to the discussion on management of leaks after sleeve gastrectomy, it is important to discuss some technical aspects of the operation that may minimize or prevent postoperative leaks. First, it is prudent to avoid stapling close to the bougie for the last firing near the angle of His to avoid stapling on the esophagus. The esophagus lacks a serosa layer and stapling the esophageal wall may predispose to higher staple line disruption. Additionally, it is important to avoid either a functional or mechanical narrowing of the stomach at the level of the incisura angularis. A functional obstruction at this site is caused by spiraling of the anterior and posterior gastric wall resulting in a gastric fold that may act as a valve that may impede gastric flow. Mechanical obstruction at this site is often caused by stapling too close to the bougie leading to excessive narrowing of the gastric lumen. Any narrowing at the incisura angularis can lead to a partial or complete gastric outlet obstruction resulting in increased intraluminal pressure that may contribute to the risk for staple line failure. In our practice, a 32–34 Fr bougie is used for sizing of the sleeve. The greater curvature of the stomach is mobilized first. The sleeve gastrectomy begins at the antrum, 6 cm proximal to the pylorus. The green stapler cartridge is often used for the first two firings which are then switched to the blue stapler cartridge for the rest of the firings. Staple line reinforcement is used to obtain better hemostasis. An effort is made to stay off the bougie at the level of the incisura angularis. Adequate lateral retraction is performed in an effort to avoid stapling mismatch of the anterior and posterior gastric wall. Lastly, the last staple firing is angled toward the tip of the spleen in an effort to avoid stapling of the distal esophageal wall.
In summary, the use of endoscopic stent for management of gastric leak after sleeve gastrectomy should be in the armamentarium of the surgeon performing bariatric surgery. In selected cases, endoscopic stenting of gastric leaks after sleeve gastrectomy can be an effective, minimally invasive option in the management of leak complication.
References
Clinical Issues Committee of American Society for Metabolic and Bariatric Surgery. Updated position statement on sleeve gastrectomy as a bariatric procedure. Surg Obes Relat Dis. 2009. doi:10.1016/j.soard.2009.11.004.
Serra C, Baltasar A, Andreo L, et al. Treatment of gastric leaks with coated self-expanding stents after sleeve gastrectomy. Obes Surg. 2007;17(7):866–72.
Casella G, Soricelli E, Rizzello M, et al. Nonsurgical treatment of staple line leaks after laparoscopic sleeve gastrectomy. Obes Surg. 2009;19:821–6.
Oshiro T, Kasama K, Umezawa A, et al. Successful management of refractory staple line leakage at the esophagogastric junction after sleeve gastrectomy using a HANAROSTENT. Obes Surg. 2009. doi:10.1007/s11695-009-9976-6.
Tan JT, Kariyawasam S, Wijeratne T, Chandraratna HS. Diagnosis and management of gastric leaks after laparoscopic sleeve gastrectomy for morbid obesity. Obes Surg. 2009. doi:10.1007/s11695-009-0020-7.
Fukumoto R, Orlina J, McGinty J, et al. Use of Polyflex stents in the treatment of acute esophageal and gastric leaks after bariatric surgery. Surg Obes Relat Dis. 2007;3:68–72.
Conflict of interest
The authors declare that there are no conflicts of interest as related to this article.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nguyen, N.T., Nguyen, XM.T. & Dholakia, C. The Use of Endoscopic Stent in Management of Leaks After Sleeve Gastrectomy. OBES SURG 20, 1289–1292 (2010). https://doi.org/10.1007/s11695-010-0186-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11695-010-0186-z