Abstract
Background
The purpose of this study is to determine the effects of posture and drink volume on gastric/pouch emptying (G/PE), intestinal transit, hormones, absorption, glycaemia, blood pressure and gastrointestinal (GI) symptoms after gastric bypass (Roux-en-Y gastric bypass (RYGB)).
Methods
Ten RYGB subjects were studied on four occasions in randomized order (sitting vs. supine posture; 50 vs. 150 ml of labelled water mixed with 3 g 3-O-methyl-d-glucose (3-OMG) and 50 g glucose). G/PE, caecal arrival time (CAT), blood glucose, plasma insulin, glucagon-like peptide-1 (GLP-1), glucose-dependent insulinotropic polypeptide (GIP), peptide YY (PYY), 3-OMG, blood pressure, heart rate and GI symptoms were assessed over 240 min. Controls were ten volunteers with no medical condition or previous abdominal surgery, who were studied with the 150-ml drink in the sitting position.
Results
Compared to controls, PE (P < 0.001) and CAT (P < 0.001) were substantially more rapid in RYGB subjects. In RYGB, PE was more rapid in the sitting position (2.5 ± 0.7 vs. 16.6 ± 5.3 min, P = 0.02) and tends to be faster after 150 ml than the 50-ml drinks (9.5 ± 2.9 vs. 14.0 ± 3.5 min, P = 0.16). The sitting position and larger volume drinks were associated with greater releases of insulin, GLP-1 and PYY, as well as more hypotension (P < 0.01), tachycardia (P < 0.01) and postprandial symptoms (P < 0.001).
Conclusions
Pouch emptying, blood pressure and GI symptoms after RYGB are dependent on both posture and meal volume.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In health, both posture and meal volume exert little effect on gastric emptying (GE) of either solids or high-nutrient liquids [1–4], whereas GE of low nutrient liquids is relatively faster with greater volume [4] and in the erect posture [1–4], reflecting the primary regulation of GE by inhibitory feedback arising from the interaction of nutrients with the small intestine. The rate of nutrient delivery to the small intestine (overall between 1 and 4 kcal/min) and small intestinal transit also affects the release of the two incretin hormones, glucose-dependent insulinotropic polypeptide (GIP) and glucagon-like peptide-1 (GLP-1) [5] as well as both blood pressure [6] and gastrointestinal (GI) symptoms [1–4]. Elevations of GIP, GLP-1 and other gut hormones also result in slowing of GE, suppression of appetite [7–9] and the induction of postprandial GI symptoms. In patients with early dumping syndrome after gastrectomy, it has been proposed that the exaggerated early releases of GLP-1 [10], GIP and glucagon [11] result in sympathetic activation, leading to transient early onset of anxiety and nausea.
Roux-en-Y gastric bypass (RYGB) is one of the most commonly performed bariatric procedures for morbid obesity [12, 13]. The alterations in GI anatomy after RYGB allow rapid transit of nutrient into the small intestine which may account for changes in gut hormone secretion and, potentially, “dumping syndrome” [14–16]. We hypothesized that, in contrast to subjects with an intact stomach, both posture and meal volume will have a substantial impact on the rate of nutrient transit, gut hormonal responses, postprandial blood pressure and GI symptoms in RYGB subjects. The current study, therefore, aimed to determine the effects of posture and drink volume on pouch emptying (PE), small intestinal transit, glycaemia, gut hormone concentrations, glucose absorption, blood pressure and GI symptoms after RYGB.
Subjects and Methods
Subjects
Ten subjects who underwent RYGB for morbid obesity at least 12 months previously were recruited by advertisements placed on hospital notice boards and the Obesity Clinic of the Royal Adelaide Hospital. The RYGB was performed 5.7 ± 0.3 years prior to the study, and the decrease in BMI was 12.9 ± 3.4 kg/m [2]. All subjects had open RYGB with a ∼25-ml pouch created by stapling off the proximal stomach with a TA 90B four-row stapler (Autosuture®, Covidien®) or dividing the stomach using a linear stapler (GIATM, Covidien®, or TLCTM, Ethicon®). The excluded stomach was not resected. Exclusion criteria included a history of significant respiratory, renal or hepatic disease; diabetes; chronic alcohol abuse; smoking; or the use of medication known to influence GI motility, appetite or glycaemia. The presence of dumping syndrome was assessed using Sigstad’s clinical diagnostic index (positive if score >7) [15]. “Controls” were ten age- and gender-matched volunteers who were not known to have any medication condition, were not known to have a history of abdominal surgery or were not taking medication. The study was approved by the local research ethics committee, and each subject provided written informed consent.
Outline of Study Protocol
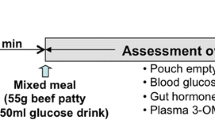
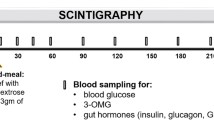
The study protocol is summarized in Fig. 1. The RYGB subjects were studied on four occasions in randomized order for both posture (sitting vs. supine) and drink volume (50 vs. 150 ml), i.e. (i) sitting and 50-ml drink, (ii) sitting and 150-ml drink, (iii) supine and 50-ml drink and (iv) supine and 150-ml drink. All drinks contained 50 g glucose, 3 g 3-O-methyl-d-glucose (3-OMG) and 20 MBq 99mTc-sulphur colloid (total calories = 200 kcal) and were consumed within 3 min. The controls were studied on one occasion in the sitting position when they consumed a 150 ml of glucose drink.
Concurrent measurements of (i) gastric emptying (GE) or pouch emptying (PE; 50 % emptying time) and caecal arrival time (CAT); (ii) blood glucose, gut hormone concentrations (GLP-1, insulin, GIP and PYY) and glucose absorption (3-OMG); and (iii) GI symptoms (visual analogue scale (VAS)) over 240 min were obtained. In all subjects, venous blood samples were taken via an intravenous cannula immediately prior (t = 0 min) to the ingestion of the drink and then at regular intervals for measurement of blood glucose, 3-OMG, plasma insulin, GIP, GLP-1 and peptide YY (PYY) [16, 17] (Fig. 1). Blood samples for plasma insulin, GIP and GLP-1 were collected in ice-chilled EDTA-treated tubes containing 400 kIU aprotinin/ml blood (Trasylol, Bayer Australia Ltd., Pymble, Australia).
Mean blood pressure (MAP) and heart rate (HR) were quantified with an automated oscillometric BP monitor (DINAMAP ProCare 100) at ∼3-min intervals for the first 2 h and every 10 min thereafter [18]. Perceptions of appetite (hunger, fullness, desire to eat), preprandial and postprandial GI symptoms (nausea and bloating) and symptoms of dumping (nausea, vomiting, diarrhoea, tachycardia, salivation and dizziness) were assessed using validated VASs [17] at baseline (t = 0 min) and 15-min intervals thereafter for 240 min. Subjects indicated the strength of sensation by placing a vertical line along a 100-mm scale [16, 17].
Measurements
GE, PE and CAT
The transit of labelled glucose drink through the stomach or gastric pouch and the small intestine was quantified using scintigraphy [16]. Data were corrected for subject movement, radionuclide decay and γ-ray attenuation.
Blood Glucose and Plasma 3-OMG, Insulin, GIP, GLP-1 and PYY
Blood glucose concentrations were determined immediately using a portable blood glucose meter (Medisense Companion 2 Meter, Medisense, Inc., Waltham, MA) [16]. Plasma 3-OMG concentrations were measured using high-performance exchange chromatography [16]. As previously described [16, 17], total plasma GIP, GLP-1 and PYY were measured by radioimmunological assay (RIA), and plasma insulin was measured by ELISA.
Statistical Analysis
The sample size was based on power calculations of primary endpoints, which are the known differences in PE, caecal arrive time and GI symptoms between RYGB subjects and controls [19, 20], and provided 80 % power to detect a 20 % difference between the modes of nutrient delivery amongst RYGB subjects, accepting an α error of 0.05. Data were evaluated using repeated measures analysis of variance (ANOVA), with “treatment” and “time” as factors, and are presented as mean ± SEM, unless stated otherwise. To examine relationships between variables by linear regression analysis, data from the two studies were pooled. A P value <0.05 was considered significant in all analyses.
Results
Although none of the RYGB subjects had dumping syndrome based on Sigstad’s score, two subjects experienced dumping symptoms with hypotension and dizziness after ingesting the glucose drink in the sitting position, requiring them to lie down. Differences in the characteristics of the RYGB and control subjects are summarized in Table 1.
PE and Caecal Arrival Time
Compared to controls, independent of posture or drink volume, both PE (P < 0.01) and CAT (P < 0.001) were faster in RYGB subjects than the GE of controls (Fig. 2). In RYGB subjects, although there were no differences in CAT amongst various postures and meal volumes, PE in the sitting posture was faster (∼twice as rapid) than that in the supine posture (P < 0.001). In both postures, there was a trend for the 150-ml drinks to empty more rapidly than the 50-ml drinks (Fig. 2a).
Blood Glucose and Gut Hormonal Responses
There were no differences in baseline blood glucose between RYGB and control subjects. After the drink, blood glucose concentrations were higher in RYGB subjects than those in controls (P < 0.01; Fig. 3a). Amongst the RYGB, there were no significant differences in peak blood glucose concentrations between the postures and drink volumes (Fig. 3a).
Baseline plasma concentrations of PYY, GLP-1, insulin and GIP were comparable in RYGB and control subjects. When compared to controls, after the drink, plasma GLP-1 (P = 0.03) and PYY (P < 0.01) were higher and peaked earlier in RYGB subjects, irrespective of posture and drink volume (Fig. 3c, e). With the exception of the 50-ml drink consumed supine, plasma concentrations of insulin (P = 0.02) were higher in RYGB subjects than those in controls (Fig. 3b). In contrast, after the drink, concentrations of GIP (P < 0.01) were lower in the RYGB subjects than those in the controls, irrespective of meal or posture (Fig. 3d). In RYGB subjects, consumption of the 150-ml drink in the supine, but not sitting, position was associated with larger increases in plasma insulin (P < 0.01), GLP-1 (P = 0.02) and PYY (P < 0.001) than that of the 50-ml drink.
Intestinal Glucose Absorption
Although integrated plasma concentrations (AUC 0–240 min) were comparable between RYGB subjects and controls, plasma 3-OMG concentrations peaked much earlier in the RYGB subjects, independent of posture or meal volume (76 ± 5 vs. 114 ± 6 min, P < 0.001; Fig. 3f). In RYGB subjects, there was a trend for the rise in plasma 3-OMG to be slower after consumption of the 50-ml glucose drink in the supine position (P = 0.08).
Haemodynamics
Although ingestion of the glucose drink had no effect on BP or heart rate in the controls, it led to sustained changes in BP and heart rate of RYGB subjects. These were most pronounced in the sitting posture with a 10 ± 4-mmHg reduction in systolic BP and a 14 ± 5-bpm elevation in heart rate (Fig. 4). Drink volume had no impact of the changes in BP. When RYGB subjects consumed the drink while supine, the 150-ml drink induced a greater, and earlier, increase in heart rate than the 50-ml drink (P < 0.001). (See Fig. 4.)
GI Symptoms and Appetite
Irrespective of drink volume and posture, RYGB subjects experienced more bloating (P < 0.001), nausea (P < 0.001), anxiety (P < 0.001) and discomfort (P < 0.001) and less desire to eat (P < 0.001) after the glucose drink than controls. In RYGB subjects, after the 150-ml drink consumed in sitting position, bloating, nausea,discomfort and anxiety were greater and the desire to eat was less than that in supine position (P < 0.01) (Fig. 5).
Discussion
This “proof-of-concept” study highlights the importance of intact anatomy and small intestinal feedback regulation to the regulation of GE and small intestinal transit [21]. Our hypothesis was that in RYGB subjects, the emptying of content through the upper GI tract would be more rapid and be markedly influenced by gravity. Specific observations are that, after RYGB, PE of a glucose drink in sitting posture is much more rapid and associated with greater increases in insulin, GLP-1 and PYY, as well as more hypotension, tachycardia and postprandial symptoms. In contrast, the effects of drink volume on these parameters are less pronounced and observed mainly in the supine position.
The more rapid small intestinal transit of a nutrient liquid observed in our RYGB subjects was to be expected given the substantially greater rate of meal delivery to the intestine. Our findings are in keeping with a recent study [14], reporting more rapid orocaecal transit of liquids after RYGB. Small intestinal transit in these subjects, however, appears to be dependent on the consistency of a meal, as orocaecal transit of mixed or solid meals has been reported to be more prolonged than that of liquids [19, 20]. This contrasts with controls, in whom intestinal transit of solids and liquids has been reported to be comparable [22]. The reasons for the discrepancy remain unknown.
In the current study, the more rapid transit of glucose to the distal intestine accounts for the observed differences in the glycaemic and gut hormonal profiles between the RYGB subjects and controls. Given that PYY and GLP-1 are secreted predominantly from the distal intestine, their plasma concentrations were significantly greater in the RYGB subjects, particularly after consumptions of the drinks in the sitting position. This contrasts with the GIP response, a hormone release from the proximal gut, where plasma levels were lower in the RYGB subjects than those in the controls, presumably reflecting the “bypass” of nutrients from the proximal intestine. The substantial elevation of GLP-1 and, to a lesser extent, the early rise of GIP are likely to contribute to the greater plasma insulin concentrations in the RYGB subjects, which were highest after consumptions of the drink in the sitting position. Despite the greater GLP-1 response in RYGB subjects, their postprandial blood glucose levels were higher, at least for the first 60 min, which may potentiate insulin secretion. Hyperglycaemia is likely to reflect the more rapid intestinal glucose absorption consequent to the rapid transit of glucose from the pouch, as well as insulin resistance as reflected by the higher HOMA-IR score.
The significant postprandial hypotension and tachycardia in RYGB subjects, especially after ingesting the drink in sitting position, are also likely to be related to the substantially higher rate of nutrient delivery to the small intestine, which we have estimated to vary from 38 kcal/min (supine posture) to 104 kcal/min (sitting position). This is compared to a duodenal glucose load of 1 to 4 kcal/min in healthy subjects with an intact stomach, where a rate >2 kcal/min has been reported to elicit maximal postprandial hypotension and tachycardia in healthy older subjects [23]. In contrast to an intact stomach, where gastric distention can contribute to perceptions of hunger and fullness, postprandial symptoms after RYGB are likely to be triggered by the rapid arrival of nutrients to the distal intestine, leading to augmented releases of gut hormones, such as GLP-1, PYY and CCK, which are well known to suppress appetite via both peripheral and central mechanisms [25]. In patients with early dumping syndrome after gastrectomy, the exaggerated early releases of GLP-1 [10], GIP and glucagon [11] have been proposed to result in sympathetic activation and thus leading to more anxiety and nausea. In addition to these neurohumoral changes, the haemodynamic effects may also potentially result in direct central effects on appetite reduction. The intensity of these nutrient-induced haemodynamic and neurohumoral changes is likely to determine whether the dumping syndrome will be therapeutic or problematic. In the majority, the responses are mild to moderate and, accordingly, likely to be “therapeutic” by inducing early appetite suppression and a consequent reduction in oral intake. In a lesser proportion of subjects, however, the responses are more intense and lead to “problematic” dumping syndrome. In these subjects, ingestion of small-volume meals in the supine or semi-supine posture, therefore, may represent a useful approach to minimize symptoms. It is intriguing, however, that in the RYGB group, the degree of haemodynamic disturbances was not proportional to the marked increased intestinal glucose load, which may relate to an adaptation of the small intestine after RYGB [24] to avoid problematic dumping syndrome.
There are a number of limitations of the current study which should be recognized. While the number of subjects were relatively small, the studies were randomized, repeated over four occasions and technically demanding. Most importantly, major and highly significant differences were observed. Given the known effects of posture and meal volume on GE in the intact stomach, only a single posture and drink volume was chosen, based on the expectation that most, if not all, liquids that are consumed are at least 150 ml in volume and consumed in the sitting position. Whilst it would be of interest to study RYGB subjects with active dumping syndrome, this may not be ethically justifiable given that postprandial symptoms induced by a glucose drink would be anticipated to be substantial and distressing. Our RYGB subjects did not report clinical dumping syndrome, but 2 out 10 had problematic dumping symptoms after the glucose drinks, suggesting that these subjects had modified their diet and meal size to minimize the symptoms. For logistical reasons, we evaluated the effects of oral glucose only and it would be of interest to determine the responses to other macronutrients and mixed solid/liquid meals. None of our RYGB subjects had type 2 diabetes; given our observation, further studies in type 2 diabetes would be of interest.
In conclusion, after RYGB, gravity and, to a lesser extent, meal volume can influence the rate of PE, which, in turn, modifies the release of gut hormones, glycaemia, blood pressure, heart rate and GI symptoms. Given that consumption of large volume drinks consumed in the upright position is associated with the most rapid PE, ingestion of small-volume meals in the supine or semi-supine posture may minimize problematic dumping syndrome; this concept warrants further evaluation.
References
Anvari M, Horowitz M, Fraser R, Maddox A, Myers J, Dent J, et al. Effects of posture on gastric emptying of nonnutrient liquids and antropyloroduodenal motility. Am J Physiol. 1995;268:G868–71.
Jones KL, O’Donovan D, Horowitz M, Russo A, Lei Y, Hausken T. Effects of posture on gastric emptying, transpyloric flow, and hunger after a glucose drink in healthy humans. Dig Dis Sci. 2006;51:1331–8.
Horowitz M, Jones K, Edelbroek MA, Smout AJ, Read NW. The effect of posture on gastric emptying and intragastric distribution of oil and aqueous meal components and appetite. Gastroenterology. 1993;105:382–90.
Doran S, Jones KL, Andrews JM, Horowitz M. Effects of meal volume and posture on gastric emptying of solids and appetite. Am J Physiol. 1998;275:R1712–8.
Hellstrom PM, Gryback P, Jacobsson H. The physiology of gastric emptying. Best Pract Res Clin Anaesthesiol. 2006;20:397–407.
Jones KL, O’Donovan D, Russo A, Meyer JH, Stevens JE, Lei Y, et al. Effects of drink volume and glucose load on gastric emptying and postprandial blood pressure in healthy older subjects. Am J Physiol Gastrointest Liver Physiol. 2005;289:G240–8.
Asmar M. New physiological effects of the incretin hormones GLP-1 and GIP. Dan Med Bull. 2011;58:B4248.
Lin HC, Elashoff JD, Gu YG, Meyer JH. Nutrient feedback inhibition of gastric emptying plays a larger role than osmotically dependent duodenal resistance. Am J Physiol. 1993;265:G672–6.
Lin HC, Doty JE, Reedy TJ, Meyer JH. Inhibition of gastric emptying by sodium oleate depends on length of intestine exposed to nutrient. Am J Physiol. 1990;259:G1031–6.
Yamamoto H, Mori T, Tsuchihashi H, Akabori H, Naito H, Tani T. A possible role of GLP-1 in the pathophysiology of early dumping syndrome. Dig Dis Sci. 2005;50:2263–7.
Gebhard B, Holst JJ, Biegelmayer C, Miholic J. Postprandial GLP-1, norepinephrine, and reactive hypoglycemia in dumping syndrome. Dig Dis Sci. 2001;46:1915–23.
Tice JA, Karliner L, Walsh J, Petersen AJ, Feldman MD. Gastric banding or bypass? A systematic review comparing the two most popular bariatric procedures. Am J Med. 2008;121:885–93.
Nguyen NQ, Game P, Bessell J, Debreceni TL, Neo M, Burgstad CM, et al. Outcomes of Roux-en-Y gastric bypass and laparoscopic adjustable gastric banding. World J Gastroenterol. 2013;19:6035–43.
Dirksen C, Damgaard M, Bojsen-Moller KN et al. Fast pouch emptying, delayed small intestinal transit, and exaggerated gut hormone responses after Roux-en-Y gastric bypass. Neurogastroenterol Motil. 2013;25:346–e255. doi:10.1111/nmo.12087
Laurenius A, Olbers T, Naslund I, Karlsson J. Dumping syndrome following gastric bypass: validation of the dumping symptom rating scale. Obes Surg. 2013;23:740–55.
Nguyen NQ, Debreceni TL, Bambrick JE et al. Rapid gastric and intestinal transit is a major determinant of changes in blood glucose, intestinal hormones, glucose absorption, and postprandial symptoms after gastric bypass. Obesity (Silver Spring). 2014;22:2003–9. doi:10.1002/oby.20791
Pilichiewicz AN, Little TJ, Brennan IM, Meyer JH, Wishart JM, Otto B, et al. Effects of load, and duration, of duodenal lipid on antropyloroduodenal motility, plasma CCK and PYY, and energy intake in healthy men. Am J Physiol Regul Integr Comp Physiol. 2006;290:R668–77.
Gentilcore D, Doran S, Meyer JH, Horowitz M, Jones KL. Effects of intraduodenal glucose concentration on blood pressure and heart rate in healthy older subjects. Dig Dis Sci. 2006;51:652–6.
Dirksen C, Damgaard M, Bojsen-Moller KN, Jorgensen NB, Kielgast U, Jacobsen SH, et al. Fast pouch emptying, delayed small intestinal transit, and exaggerated gut hormone responses after Roux-en-Y gastric bypass. Neurogastroenterol Motil. 2013;25:346–e255.
Pellegrini CA, Deveney CW, Patti MG, Lewin M, Way LW. Intestinal transit of food after total gastrectomy and Roux-Y esophagojejunostomy. Am J Surg. 1986;151:117–25.
Anderwald CH, Tura A, Promintzer-Schifferl M, Prager G, Stadler M, Ludvik B, et al. Alterations in gastrointestinal, endocrine, and metabolic processes after bariatric Roux-en-Y gastric bypass surgery. Diabetes Care. 2012;35:2580–7.
Bennink R, Peeters M, Van den Maegdenbergh V, Geypens B, Rutgeerts P, De Roo M, et al. Evaluation of small-bowel transit for solid and liquid test meal in healthy men and women. Eur J Nucl Med. 1999;26:1560–6.
Vanis L, Gentilcore D, Rayner CK, Wishart JM, Horowitz M, Feinle-Bisset C, et al. Effects of small intestinal glucose load on blood pressure, splanchnic blood flow, glycemia, and GLP-1 release in healthy older subjects. Am J Physiol Regul Integr Comp Physiol. 2011;300:R1524–31.
Carswell KA, Vincent RP, Belgaumkar AP et al. The effect of bariatric surgery on intestinal absorption and transit time. Obes Surg. 2014;24:796–805. doi:10.1007/s11695-013-1166-x.
Lawaetz O, Blackburn AM, Bloom SR, Aritas Y, Ralphs DN. Gut hormone profile and gastric emptying in the dumping syndrome. A hypothesis concerning the pathogenesis. Scand J Gastroenterol. 1983;18:73–80.
Author Contribution
NN, CR, GW and MH designed the research. TD and CB conducted the research. MB provided the essential material. JW and NN analyzed the data. NN wrote the paper and has the primary responsibility for the final content. All authors read and approved the final manuscript.
Funding
This study was supported by funding from a National Health and Medical Research Council grant.
Conflict of Interest
No authors have any conflict of interest to declare
Author information
Authors and Affiliations
Corresponding author
Additional information
What is already known about the subject?
• In health, posture and meal volume exert little effect on gastric emptying (GE) of either solids or high-nutrient liquids.
• Roux-en-Y gastric bypass (RYGB) allows rapid transit of nutrients into the small intestine which may account for changes in gut hormone secretion and, potentially, “dumping syndrome”.
• The impact of posture and meal volume on GI transit, absorption and symptoms in these patients has not been investigated.
What does this research add?
• In RYGB subjects, the sitting posture was associated with more rapid pouch emptying, increased hypotension and postprandial symptoms.
• Compared to the effects of posture, those of drink volume were relatively minor.
• In RYGB subjects with “problematic dumping syndrome”, ingestion of small-volume meals in the supine or semi-supine posture may potentially minimize adverse haemodynamic changes and GI symptoms.
Rights and permissions
About this article
Cite this article
Nguyen, N.Q., Debreceni, T.L., Burgstad, C.M. et al. Effects of Posture and Meal Volume on Gastric Emptying, Intestinal Transit, Oral Glucose Tolerance, Blood Pressure and Gastrointestinal Symptoms After Roux-en-Y Gastric Bypass. OBES SURG 25, 1392–1400 (2015). https://doi.org/10.1007/s11695-014-1531-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11695-014-1531-4