Abstract
Background
Laparoscopic Sleeve Gastrectomy (LSG) is a relatively new procedure that is gaining wide acceptance. However, laparoscopic Roux-en-Y Gastric Bypass (LRYGB) remains one of the most commonly performed bariatric procedures with the best long-term results. There are few studies comparing LSG with LRYGB. The aim of this study is to compare the safety and outcome of LSG to LRYG in a single accredited center.
Methods
A retrospective analysis of data collected prospectively on patients undergoing either LSG or LRYGB between January 2009 and December 2012 was performed. LSG was performed using 36Fr bougie, while LRYGB was perfromed with a 25-mm circular stapler. The primary outcomes included length of stay (LOS), 30-day complication and readmission rates, and excess weight loss (%EWL) at 3, 6, 12, and 24 months postoperatively. LSG patients were also divided into different categories based on BMI and their %EWL compared to LRYGB.
Results
A total of 885 patients were included in our analysis. 547 patients underwent LRYGB (61.8 %) and 338 underwent LSG (38.2 %). Thirty-day complication and readmission rates for LRYGB and LSG were (1.5 and 5.1 % vs 0.6 and 0.3 %, respetively, p > 0.05). %EWL for LRYGB was significantly higher than LSG at 3, 6, 12, and 24 months. LSG with a BMI <40 achieved a similar %EWL to LRYGB in the first 12 months.
Conclusions
LSG seems to have a better safety profile in the short-term compared to LRYGB. However, at 2 years, LRYGB patients achieved a significantly higher EWL compared to LSG patients. Randomized clinical trials are needed to better elucidate our findings.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Obesity is a growing national problem affecting more than one third of the US population [29]. As of 2010, the Center for Disease Prevention and Control reported that a staggering 69.2 % of the population is considered overweight (BMI >25 kg/m2) or obese (BMI >30 kg/m2). This is a major public health concern as obesity is known to be associated with several comorbidities including type 2 diabetes, heart disease, and certain types of cancer [13]. As a result, it is one of the leading causes of preventable death [28]. In fact, obesity is cited as a contributing factor to an estimated 100,000–400,000 deaths in the USA per year [11]. Currently, the only truly effective treatment for obesity is bariatric surgery [5, 31]. While diet and lifestyle changes are recommended, these changes, on average, produce only short-term weight loss that is difficult to maintain [36, 37]. In contrast, bariatric surgery for severe obesity is associated with long-term weight loss, decreased overall mortality, and improvement in obesity-related diseases [6, 7, 25, 31]. The popularity of bariatric surgery as a treatment option has risen significantly in the USA; thus, it is important that research is continued to ensure that the most effective and safest surgical procedure is utilized [27].
The laparoscopic Roux-en-Y gastric bypass (LRYGB) is the most established and one of the most commonly performed procedures in bariatric surgery. It has a favorable safety profile and results in invariable weight loss [32]. Despite this, LRYGB is considered too invasive and complex for certain patients. On the other hand, laparoscopic adjustable gastric banding (LAGB) became very popular due to its simplicity and high safety profile. That popularity has since decreased due to the procedure’s lack of sustained weight loss [12, 16, 26] and long-term complications [4, 14, 16]. Within the past decade, however, laparoscopic sleeve gastrectomy (LSG) has emerged as a stand-alone procedure in bariatric surgery. LSG originated as stage one of the duodenal switch, but is now found to lead to substantial weight loss with minimal complications as a stand-alone procedure [3, 8, 9, 12, 30]. The procedure is less demanding than gastric bypass and is situated between LRYGB and LAGB in terms of complication rates [2, 17]. LSG has increased in popularity nationwide with the rates increasing from 9 to 36.3 %, whereas the number of LRYGB has been decreasing [26, 27]. Not surprisingly, we have received a significant increase in the number of patients requesting sleeve gastrectomy in our center. There are only a limited number of studies comparing the outcomes and safety profile of LSG vs. LRYGB, but this information is crucial for the public to make informed decisions on bariatric surgery options. Therefore, our objective is to compare LRYGB vs. LSG in terms of their safety profile, impact on weight loss, and efficacy beyond the first year.
Methods
We conducted a retrospective analysis of data collected prospectively on consecutive patients undergoing nonrevisional bariatric surgery in our institution between January 2009 and December 2012 after obtaining the approval of our institutional review board (IRB). Informed consent was obtained from all individual participants involved in the study. Patients underwent either a laparoscopic Roux-en-Y Gastric Bypass (LRYGB) or a laparoscopic Sleeve Gastrectomy (LSG) and were managed following a similar clinical pathway. Patients attend an information seminar either in person or online prior to scheduling a consultation with the bariatric surgeon. During the information seminar, the differences between laparoscopic gastric banding, LSG, and LRYGB in terms of the expected postoperative excess weight loss based on initial BMI and the rate of perioperative complications of each procedure are presented in details. In addition, all the risks and benefits of all three procedures are explained. During the consultation with the surgeon, that same information is reviewed again and the decision is ultimately made by the patient. Laparoscopic Adjustable Gastric Banding (LAGB) patients were excluded from the analysis. All surgeries were performed by (MC) and (LC), both fellowship trained bariatric surgeons. All patients underwent the same preoperative workup and followed a similar postoperative protocol. Inclusion and exclusion criteria are listed below
-
Inclusion criteria:
-
Age >18
-
Attendance at an informational seminar and support group
-
Clearance for surgery by a registered dietician and certified social worker
-
BMI >35 with at least one comorbid condition (e.g., hypertension, diabetes mellitus, sleep apnea, hypercholesterolemia) or BMI >40 without any comorbid conditions
-
Negative pregnancy test
-
American Society of Anesthesiology score 1–3
-
Ability to understand instructions and comply with all study requirements
-
Preoperative %excess weight loss (%EWL) of 5–10 %
-
No contraindication for a LRYGB or LSG based on upper endoscopy findings
-
Preoperative cardiac consultation for risk stratification
-
Evaluation by a sleep medicine specialist to identify risk factors for sleep apnea, with treatment for at least 2 weeks prior to surgery when deemed appropriate
-
Attendance of a team meeting 2 weeks prior to surgery to educate patients and review expectations following surgery (Like ambulation and use of incentive spirometry).
-
Exclusion criteria:
-
Revisional surgery
-
Conversion to open procedure
-
Laparoscopic adjustable gastric banding
-
Presence of uncontrolled mental disorder
-
Active eating disorder like bulimia nervosa (BN), binge eating disorder (BED), and compulsive overeating (COE)
-
Underlying endocrine disorder like hypothyroidism
-
Lack of the required preoperative weight loss
-
Planned pregnancy in the next 18 months
-
Schizophrenia or psychosis
-
Hospital admission to the psychiatric ward in the previous 2 years.
All patients who met the inclusion criteria between January 2009 and December 2012 and underwent primary bariatric surgery were included in our analysis and managed similarly.
Preoperative Workup
Patients completed a 3-day food log and questionnaire describing diet history and behavioral information after which they met with the registered dietitian and filled out an evaluation questionnaire. Patients were advised to lose 5–10 % of their body weight prior to surgery with weight loss encouraged through dietary changes and increased activity. Patients who were unable to lose weight in this manner were placed on a ketogenic liquid diet 2 weeks prior to surgery that includes less than 100 g of carbohydrates, 90–120 g of protein, and 64 oz of noncaloric fluid per day. In addition, patients met with our social workers for a psychological evaluation. Patients also underwent a preoperative cardiac evaluation, a sleep consultation to rule out sleep apnea and an upper endoscopy. In addition to the mandatory preoperative weight loss (5 % of the actual weight for patients with BMI <55 and 10 % for patients with a BMI of 55 or higher), our patients underwent a routine preoperative endoscopy and biopsy to rule out the presence of Helicobacter pylori. Patients who tested positive were treated with a 2-week course of amoxicillin, clarithromycin and lansoprazole. All patients were also referred for a consultation with a pulmonologist/sleep physician in order to screen for sleep apnea. Patients with suspected sleep apnea underwent a sleep study. When sleep apnea was diagnosed patients underwent a CPAP titration study preoperatively and were treated with the CPAP for at least 2 weeks prior to surgery.
Surgical Technique
All cases were performed laparoscopically. Patients received perioperative DVT prophylaxis using subcutaneous heparin (5,000 units SQ) and a single dose of antibiotic 30 min prior to the procedure. Patients voided prior to surgery and no Foley catheters were used intraoperatively. No postoperative nasogastric tubes were used. LRYGB and LSG were performed using a standardized five-trocar technique. An upper endoscopy was performed intraoperatively at the end of all bariatric procedures to check for bleeding and leak. No routine surgical drains were used.
LRYGB
The biliopancreatic limb measured around 60 cm, the roux limb measured 100–150 cm. The jejunojejunostomy was created using a linear stapling device. The gastric pouch measured 30–35 ml. The gastrojejunostomy was created using a 25-mm circular stapler with the anvil introduced transorally.
LSG
The gastrocolic ligament was transected starting 4–6 cm from the pylorus. A 36Fr bougie was used for calibration. All staple lines were reinforced with a reabsorbable buttressing material. No routine reinforcement or imbrication of the staple lines was performed unless there was a clinical concern about the formation of the staple lines.
Postoperative Protocol
Patients were started on a liquid diet 6 h following surgery. All patients were admitted to a telemetry floor to continuously monitor heart rate and oxygen saturation. Patients with sleep apnea were encouraged to use their positive airway devices when necessary. No routine radiographic studies were performed. On postoperative day 1 (POD#1) discharge criteria included tolerance of liquid diet (defined as ability to tolerate at least 1 oz of liquid diet every 15 min), absence of nausea and vomiting, stable hemoglobin and hematocrit, lack of tachycardia at rest, frequent ambulation without assistance, and oxygen saturation of at least 93 % oxygen. After a careful examination by the surgeon, and when all the discharge criteria are met, patients are discharged home to follow-up in the office in 2 weeks. Patients discharged on POD#1 are entered in our database as 24 h stay, patients discharged on POD#2 are entered as 48 h stay, etc. Patients were seen by a clinical pharmacist, the hospitalist, and the bariatric coordinator prior to discharge to review medications and discharge planning. The clinical pharmacist educated patients on medications and adjusted long-acting medications to short-acting ones. The hospitalist adjusted the dosages of the medications based on the patient clinical condition. The coordinator reviews the discharge planning and the support tools available to the patient postoperatively. On postoperative day one, every patient receives a phone call to review discharge instruction, address any question or concern, and to make sure the patient is compliant with the discharge instructions. During that phone call, we also review fluid intake, vitamin supplementation, and the support tools available such as emergency services, dietary support, and the availability of a surgeon on call 24 h a day in case of an emergency.
Data Analysis
Primary study outcomes were Length of Stay (LOS) 30 day mortality and rates of complications, readmissions, and reoperations at 30 days. Secondary outcomes were postoperative %EWL at differnet time intervals. Demographics included age, gender, race, and preoperative BMI.
LSG and LRYGB were analyzed together as well as separately. For the continous primary outcome of LOS, separate independent samples t-tests or Mann-Whitney rank sum tests were performed for normally distributed and skewed continuous variables, respectively. For the categorical primary outcomes of 30-day readmission and complication rates, separate chi-square or Fisher’s exact tests were conducted as appropriate. For the continous secondary outcomes, separate independent samples t-tests or Mann-Whitney rank sums tests were conducted depending on data distribution. Additionally, separate multivariate linear and/or logistic regression analyses were conducted to determine any predictive effects adjusted for pateint demographics. All analyses were conducted in SPSS version 21 and SAS version 9.3. For all outcomes, p ≤ 0.05 denotes statistical significance, with no adjustment for the multiple comparisons.
For the LSG groups, a mixed randomized-repeated analysis of variance (ANOVA) was conducted to determine between-group effects of BMI category and within-group effects of time on postoperative excess weight loss (%EWL). Additionally, post hoc analyses were conducted to determine which LSG BMI categories differed significantly from LRYGB patients. For all analyses, p ≤ 0.05 denotes statistical significance, with no adjustment for multiple comparisons or post hoc testing.
Results
A total of 885 patients were included in our analysis, 547 of which underwent LRYGB (61.8 %) while 338 underwent LSG (38.2 %). The breakdown of cases per surgeon was as follows: LRYG (62.7 %) and LSG (37.3 %) for MC and LRYG (61.1 %) and LSG (38.9 %) for LC. The demographics of the patients are presented in Table 1. The average age and BMI of our patient population is 45.05 and 43.17, respectively. Females constituted around 80 % of our patient population. Day of surgery BMI was 44.32 and 42.03 for LRYGB and LSG groups, respectively. Preoperative %EWL for both groups was 10.88 % on average. The primary outcomes are presented in Table 2. The mean hospital Length of Stay (LOS) in hours was similar for both groups (31.06 ± 14.80 for LRYGB vs. 29.18 ± 11.64 for LSG, p > 0.05), and each had a 24-h median and interquartile range (IQR) of 24–24 (data not shown). The complication rate for both groups was also not significantly different (1.5 % for LRYGB and 0.6 % for LSG, p > 0.05). However, the readmission rate was significantly lower for LSG than for LRYGB, with only one patient from the LSG group readmitted (0.3 %) vs. 28 for the LRYGB group (5.1 %). Furthermore, there was no significant difference in the reoperation rate between groups (1 % for LRYGB vs. .3 % for LSG, p > 0.05) (Table 2). A complete list of all complications and reoperations is presented in Table 3. We had no mortalities in our series.
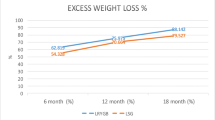
Secondary outcome measures are shown in Table 4. The mean %EWL was calculated at 3, 6, 12, and 24 months. At 3 months, the mean %EWL was 47.39 ± 10.8 for LRYGB vs. 44.67 ± 10.95 for LSG. At 12 months, the mean %EWL for LRYGB was 75.19 + 16.24 vs. 64.10 ± 17.98 for LSG. The greatest difference was seen at 24 months, where the mean %EWL was 75.43 ± 18.67 for LRYGB vs. 56.91 ± 21.31 for LSG. %EWL of LRYGB was significantly higher than LSG at all time points (p < 0.05). Our follow-up rate was 80 % at 12 months and 40 % at 24 months. The denominator for each follow-up time interval is shown in Table 4.
We also analyzed the percentage of total absolute weight loss (%TWL) and the percentage of excess BMI loss (%EBMIL) for both procedures with mean, median, and ranges represented (Table 5). On average, the %TWL for LRYGB was significantly higher than %TWL of LSG at 3, 6, 12, and 24 months (p < 0.05). Similarly, the median %EBMIL was greater for LRYGB than LSG at 12 months (73.54 and 63.95 % for LRYGB and LSG, respectively, p < 0.05) and 24 months (71.42 and 58.39 % for LRYGB and LSG, respectively, p < 0.05).
A total of 338 patients from the LSG group were further stratified into BMI groups with the following ranges: 35–39, 40–44, 45–49, and >50. Demographics of the different LSG BMI groups are shown in Table 6. Our analysis showed that there was a statistically significant negative correlation between initial BMI of LSG patients and %EWL achieved at 3, 6, 12, and 24 months (r = −0.13 to −0.41, p < 0001).
In addition, there was a significant difference in %EWL between all LSG groups at all time points (Table 6). %EWL was greatest for LSG patients in the 35–39 BMI range. The %EWL for LSG with a BMI (35–39) was 50.77, 62.62, 69.98, and 59.57 % at 3, 6, 12, and 24 months, respectively. Post hoc analyses performed to compare all the LSG BMI categories to LRYGB patients showed that at 6 months LSG with BMI = 35–39 and LSG with BMI = 40–44 both achieve the same %EWL as LRYGB patients (62.62 and 60.72 % for LSG with BMI = 35–39 and BMI = 40–44, respectively vs. 64.67 % for LRYGB). However, at 12 months, only LSG patients with BMI between 35 and 39 achieve the same %EWL as RYGB patients (69.98 % for LSG and 75.19 % for LRYGB, p > 0.05). Post hoc analysis at 24 months showed that none of the LSG patients achieved the same %EWL as LRYGB patients.
Discussion
Bariatric surgery is the only effective long treatment of obesity [6, 24]. All the other non surgical options for treatment of obesity result in modest and non lasting weight loss [36, 37]. In contrast, surgery has been shown to result in excess weight loss of 50-75% depending on the bariatric procedure [6] in addition to a high rate of resolution of co-morbidities following surgery [31]. Given the high number of obese patients seeking surgery it is crucial to provide current and evidence based information on the various weight loss surgeries [33]. Historically, LRYGB was the gold standard bariatric procedure but LSG is becoming very popular as a stand-alone procedure. Since LSG is a relatively new procedure, there are currently not many studies thoroughly investigating its outcomes compared to LRYGB. Thus, our aim was to objectively examine the outcomes of both LRYGB and LSG in our center so that our patients can make informed choices on bariatric surgery options.
Primary outcomes such as complication rate, reoperation rate, and hospital stay are important aspects of a surgical procedure. We have previously reported on our standardized and fast track protocol in managing bariatric patients at St Luke’s University [10]. Our study showed that both LRYGB and LSG are very safe as evidenced by our low complication, readmission, and reoperation rates. A recent study by Helmiö et al. [15] reported their median hospital stay to be 4 days in both groups, and others reported a median hospital stay as long as 7 days [1, 15]. Our median hospital stay using our fast track protocol was 24 h for both groups and is much shorter than the hospital stay reported by other authors [1, 15, 23]. While length of hospital stay was found to be similar, Helmiö et al. and Vidal et al. [35] both reported a greater 30-day readmission rate for LRYGB than LSG (2.2 and 6.8 % vs. 0.9 and 3.3 %). Similarly, LRYGB in our series had a higher readmission rate compared to LSG patients (5.3 vs. 0.3 %, respectively). Although readmission of LRYGB patients was mainly due to minor events like dehydration and pain control, readmission can result in increased healthcare costs and should be taken into account when a bariatric procedure is being considered.
There is substantial evidence supporting that LRYGB results in higher postoperative complications compared to LSG [21, 34]. According to a recent systematic review on randomized controlled trials over a 5-year period, LRYGB complication rates were, on average, higher than LSG (20.9 vs. 12.9 %) [34]. Leyba et al. [21] also reported that LRYGB had a higher incidence of major complications than LSG. However, this same group found a significantly higher incidence of minor complications for LSG, which included nausea and sialorrhea, than LRYGB (9.5 vs. 0 %). Helmiö et al. also reported higher minor complication rates for the LSG group. Nonetheless, minor complications are generally less difficult to resolve and are often solved without further consequence [21]. We did not report minor complications in our study but we did report serious complications as defined by Bariatric Outcome Longitudinal database (BOLD). These complications include DVT, stroke/cerebrovascular accident, heart attack, pulmonary embolus, heart failure or pulmonary edema, renal failure, liver failure, multisystem organ failure, sepsis from leak or other abdominal source, and systematic inflammatory response. Our 30-day complication and reoperation rates were not found to be significantly different, though they did trend higher for LRYGB. In counseling patients, we use that information to emphasize that both procedures are very safe with a slightly higher trend for complications in LRYGB secondary to the more invasive nature of the procedure. In regards to the overall safety of the procedure, a study conducted by Hutter et al. [17] found 30-day mortality rates as low as 0.14 % for RYGB and 0.11 % for SG. We had no mortalities in our series.
Weight loss is arguably one of the most important outcomes of bariatric surgery in addition to the resolution of comorbidities. In that regard, our study differed from other published studies comparing LSG to LRYGB. Many groups have reported comparable or only slight differences in %EWL between LRYGB and LSG. Vidal et al. reported that a %EWL over 65 % can be achieved with both techniques, and that no significant differences were observed 4 years after surgery. Leyba et al. found that %EWL was not significantly different at 1 year postsurgery and was satisfactory for both groups. Likewise, Lakdawala et al. [20] found no significant difference for %EWL at 1 year. Average %EWL across multiple studies are found to be comparable or only slightly different between LRYGB vs. LSG (62.1–94.4 % vs. 49–81 %) [34]. In addition, a group led by Karamanakos [18] found that LSG resulted in greater %EWL at 6 (55.5 ± 7.6 vs. 50.2 ± 6.5, p = 0.04) and at 12 months (69.7 ± 14.6 vs. 60.5 ± 10.7, p = 0.05). Kehagias et al. [19] similarly found that LSG resulted in greater %EWL compared to LRYGB up to 2 years, but this difference was not significant at year 3. In our series, we found that mean %EWL was significantly higher for the LRYGB group compared to LSG at 3, 6, 12, and 24 months. Furthermore, we found that %TWL was greater for LRYGB through the first 2 years. Similarly, some authors reported a higher %EWL following LRYGB compared to LSG [1, 22]. Albeladi et al. [1] reported 57 % EWL at 18 months following LSG which is comparable to the 58 % EWL at 24 months in our series. Of note, Albeladi et al.’s technique in performing LSG was similar to our technique (36Fr bougie and transection of the greater curvature starting 4–6 cm from the pylorus).
To study the issue of EWL following LSG even further, we stratified our LSG patients into BMI groups in order to evaluate the impact of preoperative BMI on postoperative weight loss. Our study showed an inverse correlation between the preoperative BMI and postoperative EWL. Of particular importance is that the %EWL for BMI group 35–39 and 40–44 was not significantly different than the %EWL for the LRYGB group at 6 months. However, at 12 months, only LSG with a BMI between 35 and 39 achieved a %EWL that is comparable to the %EWL of LRYGB. At 24 months, all the LSG BMI categories had a significantly lower %EWL compared to LRYGB. This information can be used to counsel patients with high BMI on the expected postoperative weight loss difference between LSG and LRYGB.
Our study has some advantages compared to other studies. First, unlike some of the studies comparing LSG to LRYGB, our study reports on the outcome beyond the first year. Second, the same two fellowship-trained bariatric surgeons performed all the procedures using a standardized technique thus eliminating confounding factors resulting from different treatment protocols, staff, and equipment. Additionally, we are the first study to stratify sleeve patients based on BMI. The %EWL of the different LSG categories can be used clinically in counseling patients.
However, our study has some limitations. First, our study is a retrospective study based on prospectively collected data. Although the number of patients included in our study is higher than most of the other studies, we still need large randomized trials to truly elucidate the differences between LSG and LRYGB. Second, our readmission rates did not include ER visits, and these visits were not otherwise accounted for, which could have affected the outcomes. Lastly, we did not analyze the surgical impact on comorbidities, which is arguably a very important outcome in bariatric surgery but that was beyond the scope of this study.
Conclusion
Our study showed that both LRYGB and LSG are safe and effective bariatric procedures. However, LSG seems to have a better safety profile and results in a lower readmission rate. On the other hand, LRYGB achieved a consistently higher excess weight loss up to 2 years following surgery. Long-term randomized clinical trials are needed to better define how LSG compares to LRYGB.
References
Albeladi B, Bourbao-Tournois C, Huten N. Short- and midterm results between laparoscopic Roux-en-Y gastric bypass and laparoscopic sleeve gastrectomy for the treatment of morbid obesity. J Obes. 2013;2013:934653.
Baker M. Surgical clinics of North America. Bar Met Surg. 2011;91(6):1181–201.
Baltasar A et al. Laparoscopic sleeve gastrectomy: a multi-purpose bariatric operation. Obes Surg. 2005;15(8):1124–8.
Biertho L, Steffen R, Branson R, et al. Management of failed adjustable gastric banding. Surgery. 2005;137(1):33–41.
Buchwald et al. Baratric surgery: a systematic review and meta-analysis. JAMA. 2004;292(14):1724–37.
Buchwald H. The future of bariatric surgery. Obes Surg. 2004;15:598–605.
Christou NV, Sampalis JS, Liberman M, et al. Surgery decreases long-term mortality, morbidity, and health care use in morbidly obese patients. Ann Surg. 2004;240(3):416–23. discussion 423–4.
Dakwar A et al. Case report late complication of laparoscopic sleeve gastrectomy. Case Rep Gastrointest Med. 2013;2013:1–6.
Daskalakis M, Weiner R. Sleeve Gastrectomy as a single-stage bariatric operation: indications and limitations. Obes Facts. 2009;2(1):8–10.
El Chaar M et al. Improving outcome of bariatric surgery: best practices in an accredited surgical center. Obes Surg. 2014.
Flegal KM et al. Excess Deaths associated with underweight, overweight, and obesity. JAMA. 2005;292(15):1861–7.
Franco JVA et al. A review of studies comparing three laparoscopic procedures in bariatric surgery: sleeve gastrectomy, Roux-en-Y gastric bypass and adjustable gastric banding. Obes Surg. 2011;21(9):1458–68.
Guh DP et al. The incidence of co-morbidities related to obesity and overweight: a systematic review and meta-analysis. BMC Pub Health. 2009;9(1):88.
Hady HR, Dadan J, Sołdatow M, et al. Complications after laparoscopic gastric banding in own material. Video Surg. 2012;7(3):166–74.
Helmiö M, Victorzon M, Ovaska J, et al. SLEEVEPASS: a randomized prospective multicenter study comparing laparoscopic sleeve gastrectomy and gastric bypass in the treatment of morbid obesity: preliminary results. Surg Endosc. 2012;26(9):2521–6.
Himpens J, Dapri G, Cadière GB. A prospective randomized study between laparoscopic gastric banding and laparoscopic isolated sleeve gastrectomy: results after 1 and 3 years. Obes Surg. 2006;16(11):1450–6.
Hutter MM, Schirmer BD, Jones DB, et al. First report from the American College of Surgeons Bariatric Surgery Center Network: laparoscopic sleeve gastrectomy has morbidity and effectiveness positioned between the band and the bypass. Ann Surg. 2011;254(3):410–20.
Karamanakos SN et al. Weight loss, appetite suppression, and changes in fasting and postprandial ghrelin and peptide-YY levels after Roux-en-Y gastric bypass and sleeve gastrectomy: a prospective, double blind study. Ann Surg. 2008;247(3):401–7.
Kehagias I et al. Randomized clinical trial of laparoscopic Roux-en-Y gastric bypass versus laparoscopic sleeve gastrectomy for the management of patients with BMI < 50 kg/m2. Obes Surg. 2011;21(11):1650–6.
Lakdawala MA et al. Comparison between the results of laparoscopic sleeve gastrectomy and laparoscopic Roux-en-Y gastric bypass in the Indian population: a retrospective 1 year study. Obes Surg. 2010;20(1):1–6.
Leyba JL, Aulestia SN, Llopis SN. Laparoscopic Roux-en-Y gastric bypass versus laparoscopic sleeve gastrectomy for the treatment of morbid obesity. A prospective study of 117 patients. Obes Surg. 2011;21(2):212–6.
Lim DM et al. Comparison of laparoscopic sleeve to laparoscopic roux-en-y gastric bypass for morbid obesity in a military institution. Obes Surg. 2012.
Luján JA et al. Laparoscopic versus open gastric bypass in the treatment of morbid obesity. Ann Surg. 2004;239(4):433–7.
Maggard MA et al. Meta-analysis: surgical treatment of obesity. Ann Intern Med. 2005;142:557–9.
Mann T et al. Medicare’s search for effective obesity treatments. Am Psych. 2007;63(3):220–3.
Nguyen NT et al. Changes in the makeup of bariatric surgery: a national increase in use of laparoscopic sleeve gastrectomy. J Am Coll Surg. 2013;216(2):252–7.
Nguyen NT, Root J, Zainabadi K, et al. Accelerated growth of bariatric surgery with the introduction of minimally invasive surgery. Arch Surg. 2005;140(12):1198–202.
Obesity and overweight. World Health Organization website. www.who.int/mediacentra/factsheets/fs311/en. Accessed January 24, 2014.
Overweight and obesity. Center for Disease Control and Prevention website. www.cdc.gov/obesity/resources/factsheets.html. Accessed January 22, 2014.
Peterli R et al. Early results of the Swiss Multicenter Bypass or Sleeve Study (SM-BOSS): a prospective randomized trial comparing laparoscopic sleeve gastrectomy and Roux-en-Y gastric bypass. Ann Surg. 2013;258(5):690–4.
Sjöström L et al. Lifestyle, diabetes, and cardiovascular risk factors 10 years after bariatric surgery. N Engl J Med. 2004;351(26):2683–93.
Smith BR et al. Surgical approaches to the treatment of obesity: bariatric surgery. Med Clin N Am. 2011;95:1009–30.
Sturm R, Hattori A. Morbid obesity rates continue to rise rapidly in the United States. Int J Obes (Lond). 2013;37(6):889–91.
Trastulli S, Desiderio J, Guarino S, et al. Laparoscopic sleeve gastrectomy compared with other bariatric surgical procedures: a systematic review of randomized trials. Surg Obes Relat Dis. 2013;9(5):816–29.
Vidal P et al. Laparoscopic gastric bypass versus laparoscopic sleeve gastrectomy as a definitive surgical procedure for morbid obesity. Mid-term results. Obes Surg. 2012. doi:10.1007/s11695-012-0828-4.
Wadden T et al. Efficacy of lifestyle modification for long-term weight control. Obes Res. 2012;12(S12):151S–62.
Wadden T et al. Treatment of obesity by very low calorie diet, behavior therapy, and their combination: a five-year prospective. Int J Obes. 1989;13(2):39–46.
Conflict of Interest
All authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
This study was a retrospective study and therefore for this type of study formal consent was not required.
Rights and permissions
About this article
Cite this article
El Chaar, M., Hammoud, N., Ezeji, G. et al. Laparoscopic Sleeve Gastrectomy Versus Laparoscopic Roux-en-Y Gastric Bypass: A Single Center Experience with 2 Years Follow-Up. OBES SURG 25, 254–262 (2015). https://doi.org/10.1007/s11695-014-1388-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11695-014-1388-6