Abstract
Purpose
Obesity and diabetes mellitus are now leading causes of chronic kidney disease. Our study investigated the effects of bariatric surgery on estimated glomerular filtration (eGFR) and urinary microalbumin/creatinine ratio (ACR) in morbidly obese patients.
Materials and Methods
The clinical materials for patients who underwent bariatric surgery were retrospectively analyzed with a 6-month follow-up period between January 1, 2018, and June 1, 2020. The eGFR (ml/min) was calculated using the Cockcroft-Gault formula equation. The urinary ACR was measured during the follow-up. Body mass index (BMI, kg/m2), percent weight loss (%WL), systolic blood pressure (SBP), and diastolic blood pressure (DBP) were recorded during the follow-up.
Results
Sixty-one patients who underwent bariatric surgery—laparoscopic Roux-en-Y gastric bypass (LRYGB; n = 22) and laparoscopic sleeve gastrectomy (LSG; n = 39)—were included in this study. The eGFR of both groups decreased at the follow-up outpatient visits (p < 0.001), although eGFR did not differ between the two groups. Unexpectedly, the ACR increased in the first month after LSG (p < 0.01) but decreased with a descending trend with no significant difference (p > 0.05) throughout the remaining follow-up period. Interestingly, ACR showed a descending trend with no significant difference during the follow-up in the LRYGB group (p > 0.05). The SBP and DBP decreased after bariatric surgery, with no significant difference between the two groups (p > 0.05).
Conclusion
Bariatric surgery is associated with improvements in postoperative renal function 6 months following surgery. The different alterations in ACR following LSG and LRYGB procedures demonstrate the underlying mechanism.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Worldwide, the prevalence of chronic kidney disease (CKD) in the general population is increasing, which is one to two orders of magnitude higher than the prevalence of end-stage renal disease (ESRD) [1, 2]. Separate from it being a precursor to ESRD, CKD epidemiology has been incorporated into programs of nationwide health-promotion and disease-prevention goals [3]. The prevalence of obesity (body mass index [BMI] 30 kg/m2) in the general population has risen dramatically over the past few decades [4]. Obesity is associated with glomerular hypertension, glomerulomegaly, and microvascular stretching, eventually leading to podocyte loss and the appearance of focal segmental glomerular sclerosis (FSGS) lesions [5]. The obesity epidemic has led to an increased incidence of obesity-related glomerulopathy (ORG), a distinct entity featuring proteinuria, glomerulomegaly, progressive glomerulosclerosis, and renal functional decline. Potential mechanisms explaining the increased risk for chronic kidney disease (CKD) and ESRD include obesity-mediated hypertension, insulin resistance, glomerular hyperfiltration, activation of the renin-angiotensin-aldosterone system, inflammation, and adipocytokine dysregulation [6, 7]. The risk associated with obesity may be reversible by weight loss.
Bariatric surgery has been found to be superior to lifestyle modifications for treating patients with morbid obesity [8]. Several studies have reported the effects of bariatric surgery on kidney function in obese patients with or without diabetes [9,10,11,12,13]. Nevertheless, the results were inconsistent. Thus, more evidence for the potential benefits and risks of bariatric surgery in regard to kidney function is needed in different ethnic groups.
Laparoscopic Roux-en-Y gastric bypass (LRYGB) and laparoscopic sleeve gastrectomy (LSG) procedures are both effective in terms of weight loss and improvement or remission of hyperglycemia or hypertension; however, LRYGB is associated with a greater amount of weight loss than LSG [14]. Regarding renal function, LRYGB and LSG may also act in different ways.
We anticipated that the effect on renal function would be different for each procedure. Our study investigated the effects of bariatric surgery on estimated glomerular filtration (eGFR) and microalbumin/creatinine ratio (ACR) in obese patients with or without ORG.
Methods
Study Design and Settings
This was a retrospective study of 61 patients who underwent bariatric surgery at Dalian Municipal Central Hospital Affiliated of Dalian Medical University between January 1, 2018, and June 1, 2020. The exclusion criteria were incomplete medical records, BMI < 27.5 kg/m2, and loss to follow-up. All of the patients provided informed consent for the study, which was approved by the local ethics committee.
Demographic and clinical anthropometric data were recorded. Renal function was evaluated by the urinary microalbumin/creatinine ratio and eGFR, which was calculated using the Cockcroft-Gault formula. All the variables were obtained four times: the day before surgery (preoperative), the 1st-month postoperation, the 3rd-month postoperation, and the 6th-month postoperation. To account for variation in follow-up at each point, 15 days prior and 15 days following the expected time of visit were considered at each time point. If a patient presented more than once during the same interval, only the outcome data from the visit nearest to the target date were included in analysis.
Operative Techniques
For all patients, standardized operation techniques were used, and the procedures were performed by the surgeons at the same center. In the LSG procedure, a longitudinal resection from the angle of His to approximately 4–6 cm proximal to the pylorus was performed using a 38-French bougie inserted along the lesser curve. Each procedure was designed to achieve 60 to 80% loss of stomach volume and restrict the pouch to an 80- to 120-ml postresection volume with multiple consecutive applications of an endo-GIA stapler. A methylene blue leak test was performed to confirm the integrity of the staple line. LRYGB was performed with a 90~100-cm-long biliopancreatic limb and a 100~110-cm antecolic Roux limb using endo-GIA stapled gastrojejunostomy, in which the volume of the small gastric pouch was limited to 30–50 ml. Peterson and mesenteric defects were sutured with nonabsorbable sutures.
Statistical Analysis
Descriptive statistics were used to compare patient characteristics and compliance. The continuous variables are shown as the mean and standard deviation; the categorical variables are shown as the number and percentage. Paired Student’s t tests were used to compare the pre- and postoperative data. Wilcoxon tests were used for nonnormal distributions. Two-way ANOVA was used to compare data with LSG and LRYGB data. Statistical analysis was performed using SPSS version 23.0 (IBM Corporation, New Orchard Road Armonk, NY 10504, produced in the USA). The graphics were generated using GraphPad Prism 5 (GraphPad Software, Inc., La Jolla, CA, USA). A p value < 0.05 was considered statistically significant.
Results
The study cohort consisted of 61 patients (21 men, 40 women) with a mean age of 33.59 ± 9.88 years. Table 1 provides an overview of the included patient characteristics. The LRYGB procedure was performed in 22 patients (37%), and 63% of patients (63%) underwent LSG. The mean age of patients who underwent LRYGB was 38.18, and that of the LSG group was 31 (p < 0.01). Patients with diabetes were more likely to receive the LRYGB procedure than the LSG procedure (91% vs 21%, p < 0.001).
Postoperatively, all patients had notably reduced HbA1c measurements, reaching the desirable target values and a notably reduced need for insulin treatment. Eighteen (86%) patients stopped insulin treatments or oral hypoglycemic drug therapy, and 3 (14%) patients reduced the need for insulin treatments in the LRYGB group. Six (86%) patients stopped insulin treatments or oral hypoglycemic therapy, and 1 patient (14%) halved the amount of insulin following LSG procedures. There was no significant difference between the two procedures (p > 0.05). This result suggests that more studies are required to demonstrate the effectiveness of different surgical approaches in treating obesity-related diabetes. Only one patient stopped antihypertensive medication with normal blood pressure following LRYGB.
The eGFR of the two groups decreased at the time of follow-up (p < 0.001; Fig. 1, a1–a3, b1–b3). Only one patient who underwent LSG suffered a low glomerular filtration rate (< 90 ml/min) at the 3rd month after the operation and gradually recovered during the follow-up period. Interestingly, the ACR increased in the first month after LSG surgery and decreased during the follow-up period with no significant difference (Fig. 1, c1–c3). However, there was no significant difference during the follow-up in the LRYGB group, with a descending trend in ACR (Fig. 1, d1–d3). Two of the patients with positive ACR (> 30 mg/g) obtained negative ACR after bariatric surgery, and 5 patients obtained decreased ACR following the procedures. No patient suffered oxalate nephropathy following bariatric surgery during the follow-up period. Only two patients who underwent LSG reported less than 1000 ml of urine at the 1st-month postoperative outpatient visit, among whom one patient still reported less than 1000 ml of urine at the 3rd-month postoperative outpatient visit.
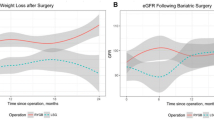
Changes in percent weight loss and eGFR were plotted over time from surgery for both types of operations (Fig. 2). However, there was no difference in the improvement in postoperative eGFR and %WL, which was different from a previous report [11].
The mean systolic blood pressure (SBP) decreased from 140.86 ± 15.79 to 126.00 ± 12.22 mmHg in the LSG group (p < 0.05) and 134.50 ± 12.37 to 110.40 ± 11.20 mmHg in the LRYGB group (p < 0.05) between preoperation and 6 months postoperation. The mean diastolic blood pressure (DBP) decreased from 89.55 ± 11.52 to 80.92 ± 8.23 mmHg (p > 0.05) and from 81.64 ± 9.92 to 70.60±5.78 mmHg (p < 0.05) in the LRYGB group preoperatively and 6 months postoperatively. However, there was no significant difference between the LSG and LRYGB groups during the follow-up (Fig. 3).
One patient who underwent LSG suffered cerebral hemorrhage 1 year after the procedure as a result of intracerebral aneurysm. One patient who underwent LSG suffered cerebral infarction 5 months after the operation. One patient who underwent LRYGB suffered intestinal obstruction as a result of mesangial hemorrhage near enterointestinal anastomosis, which was relieved by conservative treatment. Two patients who suffered refractory hypoferric anemia following LRYGB procedures received oral iron therapy during the follow-up.
Discussion
Obesity has been regarded as an independent risk factor for chronic kidney disease [15, 16], which was associated with a 6-fold increased risk of developing end-stage kidney disease (ESKD) and a 19-fold risk of ESKD related to diabetic kidney disease (DKD) [17]. In asymptomatic patients with obesity undergoing bariatric surgery, pathologic changes, including glomerulomegaly, glomerulosclerosis, podocyte hypertrophy, and foot process effacement, are commonly present [18, 19]. Weight loss after bariatric surgery has been reported to decrease glomerular hyperfiltration and stop the cascade of events caused by hyperfiltration [20, 21]. However, it is unclear how metabolic bariatric surgery affects the dramatic attenuation of kidney disease in those with obesity and/or type 2 diabetes mellitus (T2DM).
Hyperfiltration is thought to represent a precursor to single-nephron increases in glomerular pressure, which eventually leads to podocyte injury, proteinuria, and glomerulosclerosis [22]. Currently, there is no validated method of measuring GFR in patients with rapid weight loss, as lean muscle mass and creatinine also decrease during this period regardless of overall renal function [11]. A previous study [23] suggested that CCG is superior to the Modification of Diet in Renal Disease equation (MDRD) when both are compared to 24-h urinary creatinine clearance in estimating GFR in an obese population. In our study, we used the CCG-GFR formula to calculate the estimated GFR pre- and postoperatively, which was convenient at the outpatient visit during the follow-up. The eGFR decreased distinctly following bariatric surgery (Fig. 1, a1–a3, b1–b3), which was consistent with previous reports [11, 24]. Previous data indicate that patients who underwent LRYGB procedures exhibited improved eGFR compared with LSG [25, 26]. However, there was no difference between the LRYGB and LSG groups (Fig. 2a) in our study.
Hypertension and hyperfiltration may contribute to glomerulomegaly and podocyte stress, eventually leading to glomerulosclerosis [27]. However, a meta-analysis demonstrated that there was no significant correlation between changes in systolic blood pressure and uACR [28]. In our study, decreased SBP and DBP were demonstrated at the time series plot of outpatient visits between the two groups (Fig. 3), and there was no significant difference between the LSG and LRYGB groups during the follow-up. According to the trend of blood pressure and uACR, our results seem to be consistent with the results of the meta-analysis. More studies on the relationship between blood pressure and uACR are required.
ACR is recommended by The National Kidney Foundation Disease Outcomes Quality Initiative (NKFK/DOQI) to assess proteinuria status instead of 24-h urinary microalbuminuria (UmAlb), which is used comprehensively in the current staging criteria for albuminuria and chronic kidney disease and is convenient for outpatient follow-up [29]. Statistically significant reduction in uACR after surgery was demonstrated in previous reports [30]. The reduction in proteinuria and albuminuria could be caused by reduced glomerular filtration, which is commonly seen after bariatric surgery [31]. Still, report demonstrated that new-onset albuminuria and microalbuminuria occurred in 9.8% (95% CI 4.7 to 16.5%) and 9.7% (95% CI 0.6 to 25.4%) of patients [30]. Notably, the ACR in our studies increased at the first-month postoperative visit following the LSG procedure (Fig. 1, c1, p < 0.01) and decreased at the following visits (Fig. 1, c3, p > 0.05). However, the ACR decreased after LRYGB procedures, although the difference was not statistically significant (Fig. 1, d1–d3). The trend of ACR was not completely parallel to the eGFR in our study, which was inconsistent with previous report [32].
There seems to be no reasonable explanation for this unexpected phenomenon. The incidence of early postoperative acute kidney injury (AKI) after bariatric surgery is approximately 1%, and the most common causes are dehydration and infectious complications [33]. There was no previous evidence demonstrating that LSG causes increased AKI compared with LRYGB.
Differential changes in lipid profiles may offer a plausible explanation. Patients who underwent hybrid malabsorptive/restrictive bariatric procedures (RYGB and biliopancreatic diversion, BPD) experienced an overall marked reduction in serum sphingolipids, including many ceramides and sphingomyelin species, compared to those undergoing restrictive bariatric procedures (LSG and adjustable gastric banding, AGB) [22, 34]. Changes in gut microbiota and gut barrier function may also affect the progression of chronic kidney disease by generating endotoxins or bacterial metabolites that cause systemic inflammation [35]. Alternatively, there may be some underlying mechanism in the fundus-kidney axis for xanthoderm race, for which more clinical and laboratory data are required.
Previous studies report that the presence of preoperative kidney impairment and diabetes are associated with a greater risk of AKI after bariatric surgery [36]. Postoperative kidney stone formation is also a concern, particularly with procedures (Roux-en-Y gastric bypass and biliopancreatic diversion with duodenal switch) that affect oxalate and citrate excretion [37,38,39]. A more severe manifestation of this process is oxalate nephropathy, which has even been associated with rapid progression to ESRD [40]. In our study, two of the patients with positive ACR (> 30 mg/g) obtained negative ACR after bariatric surgery, and 5 patients obtained decreased ACR following the procedures. Only two patients who underwent LSG reported less than 1000 ml of urine at the 1st-month postoperative outpatient visit, among whom one patient still reported less than 1000 ml of urine at the 3rd-month postoperative outpatient visit. No patient suffered oxalate nephropathy following bariatric surgery during the follow-up period in our study.
The increase in ACR in patients who underwent LSG procedures was demonstrated in our study, and protective effects on renal function (eGFR, ACR, SBP, and DBP) were recognized during the short-term follow-up.
Our study is limited by the retrospective observational design and the short follow-up time, which was based on a small sample size in a single center. Currently, there is no gold standard method for measuring GFR in morbidly obese patients with rapid weight loss, as lean muscle mass and creatinine also decrease during this period regardless of overall renal function. Thus, our ability to draw conclusions on the precise improvement in AKI or renal function is limited. More clinical data, kidney biopsy results, and animal and cellular biological experiments are required.
Conclusion
Bariatric surgery is associated with improvements in postoperative renal function during the 6 months following surgery, especially in patients with underlying kidney disease. The different alterations in ACR following LSG and LRYGB procedures suggest an underlying mechanism driving the improvement in renal function by bariatric surgery rather than weight loss alone.
Abbreviations
- LSG:
-
Laparoscopic sleeve gastrectomy
- LRYGB:
-
Laparoscopic Roux-en-Y gastric bypass
- eGFR:
-
Estimated glomerular filtration rate
- %WL:
-
Percent weight loss
- ACR:
-
Microalbumin/creatinine ratio
- CCG:
-
Cockcroft-Gault formula
- BMI:
-
Body mass index
- ORG:
-
Obesity-related glomerulopathy
- ESRD:
-
End-stage renal disease
- FSGS:
-
Focal segmental glomerular sclerosis
- MDRD:
-
Modification of Diet in Renal Disease equation
- BPD:
-
Biliopancreatic diversion
- AGB:
-
Adjustable gastric banding
- SBP:
-
Systolic blood pressure
- DBP:
-
Diastolic blood pressure
- NKFK/DOQI:
-
The National Kidney Foundation Disease Outcomes Quality Initiative
- AKI:
-
Acute kidney injury
- UmAlb:
-
Urinary microalbuminuria
- ESKD:
-
End-stage kidney disease
- DKD:
-
Diabetic kidney disease
- T2DM:
-
Type 2 diabetes mellitus
References
Glassock RJ, Warnock DG, Delanaye P. The global burden of chronic kidney disease: estimates, variability and pitfalls. Nat Rev Nephrol. 2017;13(2):104–14.
Brück K, Stel VS, Gambaro G, et al. CKD prevalence varies across the European general population. J Am Soc Nephrol. 2016;27(7):2135–47.
Murphy D, McCulloch CE, Lin F, et al. Trends in prevalence of chronic kidney disease in the United States. Ann Intern Med. 2016;165(7):473–81.
Ng M, Fleming T, Robinson M, et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2014;30, 384(9945):766–881.
Praga M, Morales E. The fatty kidney: obesity and renal disease. Nephron. 2017;136(4):273–6.
Bagby SP. Obesity-initiated metabolic syndrome and the kidney: a recipe for chronic kidney disease? J Am Soc Nephrol. 2004;15:2775–91.
Griffin KA, Kramer H, Bidani AK. Adverse renal consequences of obesity. Am J Physiol Ren Physiol. 2008;294:685–96.
Gloy VL, Briel M, Bhatt DL, et al. Bariatric surgery versus non-surgical treatment for obesity: a systematic review and meta-analysis of randomized controlled trials. BMJ. 2013;347:f5934.
Billeter AT, Kopf S, Zeier M, et al. Renal function in type 2 diabetes following gastric bypass. Medicine. 2016;113:827–33.
Faraaz AI. Kassam, Ahmad Mirza, Young Kim, et al. Long-term outcomes in patients with obesity and renal disease after sleeve gastrectomy. Am J Transplant. 2020;20:422–9.
Holcomb CN, Goss LE, Almehmi A, et al. Bariatric surgery is associated with renal function improvement. Surg Endosc. 2018;32(1):276–81.
Koppe U, Nitsch D, Mansfield KE, et al. Long-term effects of bariatric surgery on acute kidney injury: a propensity matched cohort in the UK Clinical Practice Research Datalink. BMJ Open. 2018;8:e020371.
Hatch M. Intestinal adaptations in chronic kidney disease and the influence of gastric bypass surgery. Exp Physiol. 2014;99(9):1163–7.
Peterli R, Wolnerhanssen BK, Vetter D, et al. Laparoscopic sleeve gastrectomy versus roux-Y-gastric bypass for morbid obesity-3-year outcomes of the prospective randomized swiss multicenter bypass or sleeve study (SM-BOSS). Ann Surg. 2017;265(3):466–73.
Declèves A-E, Sharma K. Obesity and kidney disease: differential effects of obesity on adipose tissue and kidney inflammation and fibrosis. Curr Opin Nephrol Hypertens. 2015;24:28–36.
Wickman C, Kramer H. Obesity and kidney disease: potential mechanisms. Semin Nephrol. 2013;33(1):14–22.
Vivante A, Golan E, Tzur D, et al. Body mass index in 1.2 million adolescents and risk for end-stage renal disease. Arch Intern Med. 2012;172(21):1644–50.
Goumenos DS, Kawar B, El Nahas M, et al. Early histological changes in the kidney of people with morbid obesity. Nephrol Dial Transplant. 2009;24(12):3732–8.
Serra A, Romero R, Lopez D, et al. Renal injury in the extremely obese patients with normal renal function. Kidney Int. 2008;73(8):947–55.
Chagnac A, Weinstein T, Herman M, et al. The effects of weight loss on renal function in patients with severe obesity. J Am Soc Nephrol. 2003;14(6):1480–6.
Navarro-Dı ´az M, Serra A, Romero R, et al. Effect of drastic weight loss after bariatric surgery on renal parameters in extremely obese patients: long-term follow-up. J Am Soc Nephrol. 2006;17: S213–217.
Ramos-Molina B, Castellano-Castillo D, Alcaide-Torres J, et al. Differential effects of restrictive and malabsorptive bariatric surgery procedures on the serum lipidome in obese subjects. J Clin Lipidol. 2018;12(6):1502–12.
Drion I, Joosten H, Santing L, et al. The Cockcroft–Gault: a better predictor of renal function in an overweight and obese diabetic population. Obes Facts. 2011;4(5):393–9.
Hou CC, Shyu RS, Lee WJ, et al. Improved renal function 12 months after bariatric surgery. Surg Obes Relat Dis. 2013;9(2):202–6.
Nehus EJ, Khoury JC, Inge TH, et al. Kidney outcomes three years after bariatric surgery in severely obese adolescents. Kidney Int. 2017;91(2):451–8.
Imam TH, Fischer H, Jing B, et al. Estimated GFR before and after bariatric surgery in CKD. Am J Kidney Dis. 2017;69(3):380–8.
Praga M. Synergy of low nephron number and obesity: a new focus on hyperfiltration nephropathy. Nephrol Dial Transplant. 2005;20:2594–7.
Scheurlen KM, Probst P, Kopf S, et al. Metabolic surgery improves renal injury independent of weight loss: a meta-analysis. Surg Obes Relat Dis. 2019;15(6):1006–20.
Andrassy KM. Comments on ‘KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease’. Kidney Int. 2013;84(3):622–3.
Xu Z, Li L, Joey S.W. Kwong, et al. Impact of bariatric surgery on renal functions in patients with type 2 diabetes mellitus: systematic review of randomized trials and observational studies. Surg Obes Relat Dis. 2016;12(10):1873–82.
Friedman AN, Moe S, Fadel WF, et al. Predicting the glomerular filtration rate in bariatric surgery patients. Am J Nephrol. 2014;39:8–15.
Fenske WK, Dubb S, Bueter M, et al. Effect of bariatric surgery-induced weight loss on renal and systemic inflammation and blood pressure: a 12-month prospective study. Surg Obes Relat Dis. 2013;9(4):559–68.
Hanipah ZN, Punchai S, Augustin T, et al. Impact of early postbariatric surgery acute kidney injury on long-term renal function. Obes Surg. 2018;28(11):3580–5.
Kayser BD, Lhomme M, Dao MC, et al. Serum lipidomics reveals early differential effects of gastric bypass compared with banding on phospholipids and sphingolipids independent of differences in weight loss. Int J Obes. 2017;41(6):917–25.
Ramezani A, Raj DS. The gut microbiome, kidney disease, and targeted interventions. J Am Soc Nephrol. 2014;25(4):657–70.
Weingarten TN, Gurrieri C, McCaffrey JM, et al. Acute kidney injury following bariatric surgery. Obes Surg. 2013;23(1):64–70.
Lieske JC, Mehta RA, Milliner DS, et al. Kidney stones are common after bariatric surgery. Kidney Int. 2015;87:839–45.
Maalouf NM, Tondapu P, Guth ES, et al. Hypocitraturia and hyperoxaluria after Roux-en-Y gastric bypass surgery. J Urol. 2010;183(3):1026–30.
Sinha MK, Collazo-Clavell ML, Rule A, et al. Hyperoxaluric nephrolithiasis is a complication of Roux-en-Y gastric bypass surgery. Kidney Int. 2007;72(1):100–7.
Nasr SH, D’Agati VD, Said SM, et al. Oxalate nephropathy complicating Roux-en-Y gastric bypass: an underrecognized cause of irreversible renal failure. Clin J Am Soc Nephrol. 2008;3(6):1676–83.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical Approval Statement
For this type of study, formal consent is not required.
Statement of Informed Consent
Informed consent was obtained from all individual participants included in the study.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zhang, T., Wang, Y., Zhang, X. et al. The Impact of Bariatric Surgery on Renal Function: a Retrospective Analysis of Short-Term Outcomes. OBES SURG 31, 3476–3482 (2021). https://doi.org/10.1007/s11695-021-05366-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11695-021-05366-1