Abstract
Background
Obesity is an independent risk factor for chronic kidney disease (CKD). Our aims were: (1) to evaluate the impact of bariatric surgery (BS) on kidney function, (2) clarify the factors determining postoperative evolution of glomerular filtration rate (ΔGFR) and urinary albumin-to-creatinine ratio (ΔUACR), and (3) access the occurrence of oxalate-mediated renal complications.
Methods
We investigated a cohort of 1448 obese patients who underwent BS. Those with baseline-estimated GFR (eGFR0) < 30 mL/min or without information about the 2-year post-surgical eGFR (eGFR2) were excluded.
Results
A total of 725 patients were included. At baseline, 38(5.2%) had hyperfiltration with eGFR0 ≥ 125 mL/min/1.73m2 (G0), 492 (67.9%) had eGFR0 90–124 mL/min/1.73m2 (G1), 178 (24.6%) had eGFR0 60–89 mL/min/1.73m2 (G2), and 17 (2.3%) had eGFR0 < 60 mL/min/1.73m2 (G3). ΔGFR significantly increased in 96.6% (ΔGFR = 23.8 (IQR 15.9–29.8)) and 82.4% (ΔGFR = 18.6 (IQR 3.6–44.0)) of the subjects with G2 and G3 CKD, respectively (p < 0.001). The variables independently associated with ΔGFR were baseline body mass index (BMI) (positively), high blood pressure (HBP) (negatively), and fasting plasma glucose (FPG) (negatively), as well as FPG variation (positively). An overall prevalence of high UACR (≥ 30 mg/g−1) of 17.9% was found, with 81.5% of these subjects presenting A2(30–300 mg/g−1) and 18.5% A3(> 300 mg/g−1) UACR. UACR significantly decreased after BS (p < 0.001). Significant predictors of ΔUACR were BMI, systolic blood pressure, and HbA1c. Urinary excretion of calcium oxalate crystals was found in 77(11.1%) patients, with only 1 presenting oxalate-mediated renal complications.
Conclusions
ΔGFR seems to be influenced by the initial kidney function, as it decreases in subjects with hyperfiltration but tends to increase in those with kidney dysfunction. These results suggest that BS is associated with improvement of kidney outcomes, without a significant increase in renal complications.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
There has been a marked increase in the prevalence of overweight/obesity in recent decades [1, 2]. Obesity has a profound impact on global morbidity and mortality, as well as on overall health-related costs, and is therefore considered a serious public health problem. There is an undeniable evidence that obesity is associated with a variety of chronic comorbidities, such as diabetes mellitus (DM), high blood pressure (HBP), cardiovascular disease, and cancer [2]. However, in addition to these major health problems, there is growing evidence that obesity is also an independent risk factor for chronic kidney disease (CKD) [3,4,5,6].
The pathophysiological mechanisms throughout which obesity leads to kidney dysfunction remain unclear. Several theories have already been proposed, suggesting the involvement, among others, of systemic inflammation [3, 5, 7, 8], oxidative stress [3], renin-angiotensin-aldosterone axis and/or sympathetic nervous system hyperactivation [3, 5], systemic and glomerular hypertension [7, 8], anomalies of glucose metabolism [7, 8], and deregulated production of adipokines [7]. In addition, there is contribution of the increased intra-abdominal pressure, caused by centripetal deposition of visceral adipose tissue, with consequent renal venous stasis [4]. Regardless of the underlying pathophysiological mechanism, kidney dysfunction in obese patients is initially characterized by glomerular hyperfiltration, followed by a progressive increase in the urinary albumin excretion (UAE), reflecting glomerular lesion [3, 9, 10].
Bariatric surgery (BS) is the most effective treatment for obesity [11]. Several authors have sought to determine the effect of sustained weight loss on kidney function of obese patients. Although the majority of studies on this matter agree on the reduction of glomerular hyperfiltration and UAE, there are still incongruent results, mainly in those regarding the evolution of the estimated glomerular filtration rate (eGFR) [12]. Furthermore, apart from the potential medical benefits of significant weight loss with BS, there are some possible renal complications that should not be neglected. Malabsorptive procedures such as Roux-en-Y gastric bypass (RYGB) decrease fat and calcium absorption, which may result in calcium oxalate nephrolithiasis and, if this is sustained, in a rare but serious form of oxalate nephropathy [7, 8].
The main objectives of our study were to evaluate the impact of BS on kidney function, trying to clarify the determinants of the evolution of both GFR and UAE. Furthermore, we also access the occurrence of oxalate-mediated kidney complications.
Material and Methods
Study Design and Participants
An observational retrospective longitudinal study was conducted in a population of obese patients evaluated by a multidisciplinary group for surgical treatment of obesity. A total of 1448 patients underwent BS between January/2010 and July/2015. Among them, those with baseline-estimated GFR (eGFR0) < 30 mL min−1 1.73 m−2 or without information about the GFR 2-year post-surgery (eGFR2) were excluded, being able to identify 725 patients that were enrolled in this study.
Demographic (age and sex), clinical and anthropometric (body mass index (BMI), waist (WC) and hip (HC) circumferences, systolic (SBP) and diastolic (DBP) blood pressures), and analytic measures (white blood cells count (WBC), C-reactive protein (CRP), lipid profile, fasting plasma glucose (FPG) and HbA1c, serum creatinine, and urinary albumin-to-creatinine ratio (UACR)) were obtained before and at 2-year follow-up after BS.
Subjects were considered having DM if fullfeed the diagnosis criteria stablished by the American Diabetes Association Standards of Medical Care in Diabetes 2017 [13]. They were considered having HBP if they were taking antihypertensive drugs. The diagnosis of dyslipidemia was based on Adult Treatment Panel III cholesterol guidelines [14].
Kidney Function Impairment Definition and Classification
The method used to determine the serum creatinine was the kinetic Jaffe method compensate to the isotope dilution mass spectrometry (ID-MS) reference method, both at baseline and at 2-year follow-up. Estimated GFR was calculated using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) formula, based on serum creatinine. Glomerular hyperfiltration was defined as an eGFR ≥ 125 mL min−1 1.73 m2. The data were analyzed collectively as a cohort and in subgroups based on eGFR ≥ 125 (G0), 125 > eGFR ≥ 90 (G1), 90 > eGFR ≥ 60 (G2), and 60 > eGFR ≥ 30 (G3) mL min−1 1.73 m2. Albuminuria was also analyzed either collectively, being classified as normal to mildly increased if UACR < 30 mg g1 (A1), moderately increased if UACR 30–300 mg g1 (A2), and severely increased if UACR > 300 mg g1 (A3), either divided in two main subgroups based on normal UACR (< 30 mg g1) or high UACR (≥ 30 mg g1). The eGFR and UACR cut-offs were selected based on Kidney Disease Improving Global Outcomes (KDIGO) that states that a normal GFR is ≥ 90 and, in young adults, is approximately 125 mL min−1 1.73 m2, and that a UACR ≥ 30 mg g1 is abnormal [15].
We also calculated the variations of the eGFR (ΔGFR) and UACR (ΔUACR) as the difference between the 2-year follow-up and baseline values of these parameters.
The presence of calcium oxalate crystals was identified by routine bright-field microscopic examination of urine sediment. The proportion of excretion reported was based on the number of crystals seen per high-power field (HPF), being classified as rare if < 5/HPF, mild if 5–10/HPF, and abundant if > 10/HPF. To evaluate the presence of oxalate-related kidney complications, we assessed all medical records, emergency department visits, as well as abdominal or renal ultrasound scans performed in the national health system during the follow-up period.
Statistical Analysis
Categorical variables were expressed as frequencies and percentages and were compared by Chi-square test. Continuous variables were presented as means or medians with their respective measures of dispersion, standard deviation, or percentiles, depending on whether they followed a normal or non-normal distribution, respectively. Normal distribution was evaluated using Shapiro-Wilk test or skewness and kurtosis. The parametric continuous variables were compared using Student’s t test and the non-parametric continuous variables using the Mann-Whitney or Wilcoxon tests, depending on whether they were independent or matched groups, respectively. The comparison between subgroups were made using one-way ANOVA or Kruskal-Wallis for continuous variables of normal or non-normal distribution, respectively, and Chi-square with z-test subanalysis for categorical variables. Independent predictors of GFR were identified by multiple linear regression. A variance inflation factor (VIF) above 3 was used to assume collinearity and to exclude variables for the regression. Reported p values were two tailed, and p < 0.05 was considered significant. Analyses were performed with the use of SPSS Statistics 20®.
Results
Baseline Characteristics
Within the 725 patients, 647 (89.2%) were women. The median age of enrolled patients was 41 years (IQR 34–51). Average preoperative weight and BMI were 112.4 kg (IQR 103.0–125.0) and 43.4 kg/m−2 (IQR 40.6–46.9), respectively. Among them, 459 (63.3%) performed RYGB, 144 (19.9%) placed an adjustable gastric band (AGB), and 122 (16.8%) underwent vertical gastrectomy (VG).
Regarding kidney function, 38 (5.2%) of subjects were classified as having G0 hyperfiltration, 492 (67.9%) had G1, 178 (24.6%) had G2, and 17 (2.3%) had G3 eGFR.
We found significant differences between these groups. Age had a significant effect on eGFR (H [3] = 114.96, p < 0.001). Subjects in G0 eGFR category were significantly younger (p < 0.001) than the patients in all other groups, as were those in G1 as compared with subjects in G2 and G3 groups. On the other hand, despite SBP did not show a significant effect on eGFR (H [3] = 7.07, p = 0.070), we found that the median values of this variable were significantly different (p = 0.043) between G1 and G2 groups, with higher values in the last one. The effect of HBP on eGFR was significant (χ2 [3] = 13.85, p = 0.003), with a higher (p = 0.003) proportion of patients with this diagnosis in G3 (64.7%) than in G0 (21.1%) categories. HbA1c also demonstrated a significant effect on eGFR (H [3] = 20.96, p < 0.001), showing significantly different (p = 0.042) median values between G0 and G3 groups. Furthermore, the presence of DM also had a significant effect on eGFR (χ2 [3] = 22.80, p < 0.001), with a significantly higher (p < 0.001) proportion of patients with this diagnosis in the higher categories of eGFR (G2 and G3). The comparison of demographic and metabolic features at baseline between eGFR categories is shown on Table 1.
Concerning UACR, we only had data for both baseline and 2-year follow-up in a total of 301 patients, of whom 10 (3.3%) had A3, 44 (14.6%) had A2, and 247 (82.1%) had A1 albuminuria. We found significant differences between the two main subgroups of normal and high UACR (Table 2). Males presented increased UACR (χ2 [1] = 9.305, p = 0.002). Subjects in high UACR group had significantly higher weight (U = 4977, p = 0.003) and BMI (U = 5275, p = 0.016) than those in the normal UACR group. Not only the median values for SBP (U = 2964.5, p < 0.001) and DBP (U = 3668, p = 0.007) were significantly higher in high UACR group, as well as the proportion of subjects with the diagnosis of HBP was also higher in this subgroup of patients (χ2 [1] = 7.324, p = 0.007). Similar results were found in the evaluation of DM, with higher values of FPG (U = 4876, p = 0.002) and HbA1c (U = 3878.5, p < 0.001) in high UACR group, as well as a higher proportion of subjects with baseline DM diagnosis in this subgroup of patients (χ2 [1] = 8.715, p = 0.003). Furthermore, there were also differences regarding the lipid profile, with lower high-density lipoprotein cholesterol (HDL-C) (U = 5132, p = 0.021) and higher triglycerides (TG) (U = 4916.5, p = 0.008) in high UACR subgroup.
Regarding the possible oxalate-related kidney complications, 7 of 329 (2.1%) patients already presented calcium oxalate crystals urinary excretion at baseline, being rare in 2, mild in 4, and abundant in 1 patient.
Postoperative Follow-up
BS provided a statistically significant (p < 0.001) decrease in weight, BMI, WC, and HC, with an excess weight loss (EWL) of 60.1% (IQR 43.7–72.3) at 2-year follow-up. Surgery was associated with a significant reduction (p < 0.001) of SBP and DBP. An improved lipid profile was observed, with significant reduction (p < 0.001) in total cholesterol (TC), low density lipoprotein cholesterol (LDL-C) and TG, as well as a significant increase (p < 0.001) in HDL-C. Regarding glycemic control, there was a significant reduction (p < 0.001) of FPG and HbA1c after surgery. We found a significant decrease (p < 0.001) in the proportion of patients with the diagnosis of HBP, dyslipidemia, and DM. Surgery was also associated with improvement of systemic inflammatory status, showing a significant reduction (p < 0.001) in total WBC count and CRP. The comparison of cohort clinical and metabolic features between baseline and 2-year follow-up is shown in Table 3.
The Impact on Kidney Function
The kidney function parameters also improved after surgery. There was a significant reduction (p < 0.001) in serum creatinine at the second post-operative year. At 2-year follow-up, 64 (8.8%) of subjects were classified as having G0 hyperfiltration, 611 (84.3%) as G1, 42 (5.8%) as G2, and only 8 (1.1%) as G3 eGFR.
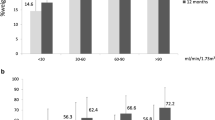
Figure 1 shows the evolution of eGFR throughout the 2-year follow-up after BS, according to initial level of kidney function. The variation of eGFR was not significant (p = 0.057) for patients with baseline hyperfiltration, however, the majority (63.2%) of these subjects showed a tendency for a decrease on eGFR median value. On the other hand, eGFR significantly increased (p < 0.001) on subjects with baseline G2 eGFR, of whom 96.6% showed a positive change in their GFR values (ΔGFR = 23.8 (IQR 15.9–29.8)), as well as on those with baseline G3 eGFR (p = 0.004), with a positive variation in 82.4% of these subjects (ΔGFR = 18.6 (IQR 3.6–44.0)). However, despite an overall improvement in kidney function, 57.9% of subjects with baseline hyperfiltration maintained values of eGFR2 > 125 mL/min/1.73 m2, as well as 41.2% of subjects with baseline G3 eGFR still presented values of eGFR2 < 60 mL/min/1.73 m2. The major benefit was found for subjects with baseline G2 eGFR, of whom 86.0% showed a normal kidney function at 2 years after surgery.
Regarding UACR, we found that the median value of this variable also significantly decreased (p < 0.001) after surgery, and at 2-year follow-up, only 1 (0.3%) patient had A3, 17 (5.6%) had A2, and 283 (94%) had A1 (p < 0.001). Of the 44 subjects with baseline A2 UACR, 37 (84.1%) presented normal UACR (A1) at 2-year follow-up and, of the 10 patients with baseline A3 UACR, only 1 maintained this level of excretion, with 7 (70%) subjects presenting A2 and 2 (20.0%) presenting A1 UACR at 2-year follow-up.
Of the seven patients that already presented at baseline urinary excretion of calcium oxalate crystals, only one maintained positive excretion during all follow-up period, with an increase in the proportion of excretion from mild at baseline to abundant either at 1- and 2-year follow-up. In general, 77/694 (11.1%) patients presented calcium oxalate crystals excretion at some point during the 2-year follow-up, 51 (7.7%) patients at 1-year reassessment (rare in 6 (0.9%), mild in 19 (2.9%), and abundant in 26 (3.9%) patients) and 37 (5.5%) at 2-year revaluation (rare in 4 (0.6%), mild in 14 (2.1%), and abundant in 19 (2.8%) patients). In 11 of these patients, crystals excretion was sustained from the first to the second year after surgery, with maintained proportion of excretion in the majority (9) of them, increase from mild to abundant in only 1 and decrease from abundant to mild in 2 patients. In most cases, calcium oxalate crystals urinary excretion was only temporary and not sustained in time. Oxalate-mediated renal complications were found in only one of these patients, who presented de novo calcium oxalate crystals excretion at 1-year follow-up, maintained over the second year, with records of nephrolithiasis and proven nephrocalcinosis. No association was found between calcium oxalate crystals urinary excretion either at baseline or during follow-up and baseline kidney function. On the other hand, we found a significant association between crystals excretion and the type of BS performed (χ2 [2] = 7.755, p = 0.021). Subjects submitted to AGB showed a lower likelihood of crystals urinary excretion and, in contrast, patients submitted to RYGB were more likely to present positive excretion. In fact, 60 of the 77 (77.9%) patients that presented crystals urinary excretion were submitted to RYGB.
Multiple Linear Regression Models
The baseline characteristics that had a statistical significant difference between the four categories of baseline eGFR in the univariate analysis were included in a multiple linear regression model as potential predictors of eGFR before and after surgery. Only age was a significant predictor of eGFR, either at baseline (F [5589] = 35.80, p < 0.001; R2 = 0.233), as well as 2 years after surgery (F [5589] = 109.0, p < 0.001; R2 = 0.481). These results are summarized in Table 4.
We also studied if any of the measured variables at baseline made an important contribution to ΔGFR. A Spearman’s rank-order correlation was run to determine the relationship between ΔGFR and those clinical and metabolic parameters. There was a week correlation between ΔGFR and BMI (rs = 0.098, p = 0.009), FPG (rs = −0.159, p < 0.001), and HBP diagnosis (rs = −0.131, p = 0.001). Applying a multiple linear regression, these correlations were maintained and a significant regression equation was found (F [3683] = 10.29, p < 0.001; R2 = 0.043). These results are shown in Table 5. Furthermore, we also found that ΔGFR correlates positively with ΔFPG (Fig. 2). We have showed that FPG significantly decreased (p < 0.001) 2 years after surgery, with a median ΔFPG of − 6.0 (IQR − 19.0–2.0). When applied the linear regression model, we found a significant regression equation (F [1708] = 12.26, p < 0.001; R2 = 0.017).
We also tried to identify potential predictors of baseline UACR. Weight was excluded due to its collinearity with BMI, and FPG was excluded due its collinearity with HbA1c. Being male, as well as higher SBP and A1c were significantly correlated with higher baseline UACR (F [9306] = 3.78, p < 0.001; R2 = 0.074). No predictors of 2-year follow-up UACR were found (F [10, 419] = 1.12, p = 0.349). A Spearman’s rank-order correlation was run to determine possible associations between ΔUACR and the clinical and metabolic parameters analyzed. Week correlations were found with sex (rs = 0.161, p = 0.005), weight (rs = − 0.198, p = 0.001), BMI (rs = − 0.208, p < 0.001), SBP (rs = − 0.279, p < 0.001), DBP (rs = − 0.202, p = 0.001), HBP (rs = − 0.120, p = 0.040), TG (rs = − 0.155, p = 0.008), dyslipidemia (rs = − 0.129, p = 0.029), FPG (rs = − 0.159, p = 0.006), HbA1c (rs = − 0.198, p = 0.001), and DM (rs = − 0.218, p < 0.001). Weight and FPG were excluded due to its collinearity with BMI and HbA1c, respectively. Higher BMI, SBP, and A1c were significantly correlated with lower ΔUACR (F [9233] = 3.631, p < 0.001; R2 = 0.089).
Conclusions
We found that ΔGFR depends on the initial category of renal function in patients that underwent bariatric surgery. In patients with baseline hyperfiltration, ΔGFR showed a downward trend and, in contrast, in those with baseline kidney dysfunction, ΔGFR significantly increased. ΔGFR was minimal in patients with normal baseline kidney function. However, we did not obtain statistical significance in patients with baseline glomerular hyperfiltration, probably due to the small sample size of this subpopulation. Coupaye et al. [3] found exactly the same trend for ΔGFR during 1-year follow-up period following BS in 323 obese patients. However, population distribution by baseline kidney function categories in that publication was quite different from ours. Before surgery, the authors presented that 59% of patients had glomerular hyperfiltration (contrasting with only 5.2% of our patients) and only 22% had normal kidney function (vs. 67.9% of our patients). The different threshold for hyperfiltration, 120 mL/min1 for Coupaye and 125 mL/min1 in our study, is insufficient to justify such considerable differences. In addition, Coupaye achieved more significant reductions in GFR in patients with baseline hyperfiltration, of whom only 45% maintained the same GFR category, compared with 57.9% in our study.
Similarly, in Coupaye et al. [3], the main factors associated with baseline kidney dysfunction, especially with the most advanced stage of dysfunction (G3), were older age, higher baseline HbA1c, and previous diagnosis of HBP and DM. However, in our study, only age showed to be an independent predictor of eGFR, both before and 2 years after BS. In contrast, Friedman et al. showed that not only age but also BMI and HbA1c, could be significantly and independently associated with baseline CKD [11].
We found that the major benefit of BS on improving GFR in obese patients was in those with underlying kidney dysfunction. These findings are in concordance with those reported by Holcomb et al. [6] and Chun-Cheng Hou et al. [16].
Regarding UACR, we found an overall prevalence of high UACR of 17.9%, 81.5% presenting microalbuminuria (A2 equivalent) and 18.5% macroalbuminuria (A3 equivalent). These results were similar to the ones described by Rosenstock et al. [17]. We found a significant decrease in UACR after BS, with the majority (84.1%) of our subjects with baseline A2 UACR experiencing resolution by the second year of follow-up, as described by Reid et al. [18]. Our results showed that the main factors associated to higher baseline UACR were male sex, higher baseline SBP and HbA1c. For Rosenstock et al., DM diagnosis was the only significant factor associated with albuminuria [17]. Coupaye et al., in its turn, found that baseline microalbuminuria was higher in patients taking drugs for DM and HBP [3]. Combining our results with those of the aforementioned studies, we can presume that not only the diagnosis of DM and HBP may be relevant for the development of high UACR but also the inadequate control of these comorbidities. Furthermore, we found that obese males have a higher likelihood of presenting high UACR before surgery. This difference between genders can be explained by the differences we found in HBP and DM prevalence according to gender. Analyzing the subgroup of 301 patients with results for UACR, we found a higher prevalence of DM in males (53.1%), comparing with females (32.3%) (χ2 [1] = 5.46, p = 0.019). Furthermore, median baseline values of SBP were significantly higher in males (140.0 (125.0–146.0) vs. 130.0 (120.0–140.0), U = 1409.0, p = 0.037).
Analyzing the whole cohort, it is possible to find determinants of ΔGFR, namely, BMI, FPG (either its baseline value and its variation), and the baseline diagnosis of HBP. Baseline BMI showed a positive correlation with ΔGFR, i.e., the higher the BMI value before surgery, the greater the ΔGFR. On the other hand, baseline FPG exhibited a negative correlation with ΔGFR, and the opposite (positive) correlation was found for ΔFPG. This means that the greater the decrease in mean FPG values after surgery, the greater the improvement in GFR over the same time period. In addition, the presence of HBP prior to surgery was associated with a lower ΔGFR. For ΔUACR, we found negative correlations with baseline BMI, SBP, and HbA1c, showing that the resolution of high UACR were less likely for higher baseline values of these variables. Coupaye et al. showed similar results, stating that the delta of microalbuminuria was linked to the decrease in SBP and FPG [3].
Regarding the urinary excretion of calcium oxalate crystals, we found a significant correlation with the type of BS performed, with patients submitted to RYGP presenting a higher likelihood of positive excretion. These differences are already described in literature, with evidence of the highest risk for malabsorptive procedures and the lowest for purely restrictive ones [19]. In procedures with a malabsorptive component like RYGB, non-absorbed fatty acids bind to calcium, making it unavailable to precipitate as calcium oxalate and to be excreted in stools. This results in hyperoxaluria and increased intestinal absorption of oxalate, which is excreted unchanged in urine [19, 20]. Only one of the patients with positive crystals excretion developed nephrolithiasis during follow-up. This low incidence of nephrolithiasis can be related with the short period of follow-up since the mean interval from BS to the diagnosis of new-onset nephrolithiasis range from 1.5 to 3.6 years [19].
Our study has some limitations. First, non-weight-based formulas normalized to body surface area, such as the MDRD and CDK-EPI equations, may underestimate renal function in morbidly obese individuals [21,22,23]. On the other hand, weight-based eGFR formulas like Cockcroft equation often overestimate eGFR in obese subjects. In fact, most of the formulas commonly used to calculate eGFR already have an inherent bias when applied to individuals undergoing BS, since surgery induces changes in muscle mass which in turn influence plasma creatinine values [3]. Hence, using CKD-EPI equation to estimate GFR may have resulted in eGFR false elevations after BS, since this formula assume that muscle mass is constant for sex and age. However, Friedman et al. accounting for this fact, performed an additional analysis using year 1 after BS as baseline, when weight loss plateaued in most patients. These authors presented results qualitatively similar to the main analysis, with improvement of CKD risk by year 2 in 51, 58, and 30% of patients with moderate, high, and very high baseline CKD risk, respectively [11]. This proves the likely beneficial effects of BS on kidney health, reducing the potential bias introduced by using a formula based on serum creatinine. We chose to exclude from our evaluation patients with severe renal impairment with eGFR < 30 mL/min/1.73 m2, so our results cannot be extrapolated to this subpopulation. In addition, the number of males included was small, representing only 10.8% of our sample, as it happens in other studies on bariatric population. Furthermore, as we only have included patients with information about the GFR before and 2 years after BS, only half of our whole initial population was enrolled in this study. Regarding the subanalysis of UACR and crystal urinary excretion, we cannot eliminate the potential effect of drugs and diet, since these parameters were not considered in our evaluation.
In conclusion, besides the global improvement in metabolic and inflammatory status, BS also seems to be associated with improvement of renal outcomes, without significant increase of renal complications.
European [24] and American [2] recommendations for the surgical treatment of obesity agree on the treatment of patients with BMI ≥ 40 kg/m2 as well as those with BMI 35–40 kg/m2 with obesity-related comorbidities. In both recommendations are listed several of these possible comorbidities, without reference to CKD. Our results raise the question whether non-dialysis-dependent CKD should be considered for the selection of patients as candidates for BS.
Abbreviations
- DM:
-
Diabetes mellitus
- HBP:
-
High blood pressure
- CKD:
-
Chronic kidney disease
- UAE:
-
Urinary albumin excretion
- BS:
-
Bariatric surgery
- eGFR:
-
Estimated glomerular filtration rate
- RYGB:
-
Roux-en-Y gastric bypass
- eGFR0:
-
Baseline-estimated glomerular filtration rate
- eGFR2:
-
2-Year post-surgery estimated glomerular filtration rate
- BMI:
-
Body mass index
- WC:
-
Waist circumference
- HC:
-
Hip circumference
- SBP:
-
Systolic blood pressure
- DBP:
-
Diastolic blood pressure
- WBC:
-
White blood cells count
- CRP:
-
C-reactive protein
- FPG:
-
Fasting plasma glucose
- UACR:
-
Urinary albumin-to-creatinine ratio
- ID-MS:
-
Isotope dilution mass spectrometry
- CKD-EPI:
-
Chronic kidney disease epidemiology collaboration formula
- KDIGO:
-
Kidney disease improving global outcomes
- ΔGFR:
-
Glomerular filtration rate variation
- ΔUACR:
-
Urinary albumin-to-creatinine ratio
- HPF:
-
High-power field
- VIF:
-
Variance inflation factor
- AGB:
-
Adjustable gastric band
- VG:
-
Vertical gastrectomy
- EWL:
-
Excess weight loss
- HDL-C:
-
High-density lipoprotein cholesterol
- TG:
-
Triglycerides
- TC:
-
Total cholesterol
- LDL-C:
-
Low-density lipoprotein cholesterol
References
Ng M, Fleming T, Robinson M, et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2014;384(9945):766–8.
Garvey WT, Mechanick J, Brett EM, et al. American Association of Clinical Endocrinologists and American College of Endocrinology comprehensive clinical practice guidelines for medical care of patients with obesity. Endocr Pract. 2016;22(Suppl 3):1–203.
Coupaye M, Flamant M, Sami O, et al. Determinants of evolution of glomerular filtration rate after bariatric surgery: a 1-year observational study. Obes Surg. 2017;27(1):126–33.
Carlsson LM, Romeo S, Jacobson P, et al. The incidence of albuminuria after bariatric surgery and usual care in Swedish Obese Subjects (SOS): a prospective controlled intervention trial. Int J Obes. 2015;39(1):169–75.
Hunley TE, Ma L, Kon V. Scope and mechanisms of obesity-related renal disease. Curr Opin Nephrol Hypertens. 2010;19(3):227–34.
Holcomb CN, Goss L, Almehmi A, et al. Bariatric surgery is associated with renal function improvement. Surg Endosc. 2018;32(1):276–81.
Chang AR, Chen Y, Still C, et al. Bariatric surgery is associated with improvement in kidney outcomes. Kidney Int. 2016;90(1):164–71.
Agrawal V, Navaneethan S. Bariatric surgery for obesity-associated decline in kidney function: filling the knowledge gap? Kidney Int. 2016;90(1):28–30.
Kim EY, Kim Y. Does bariatric surgery really prevent deterioration of renal function? Surg Obes Relat Dis. 2016;12(4):856–61.
Chagnac A, Weinstein T, Korzets A, et al. Glomerular hemodynamics in severe obesity. Am J Physiol Ren Physiol. 2000;278(5):F817–22.
Friedman AN, Wahed A, Wang J, et al. Effect of bariatric surgery on CKD risk. J Am Soc Nephrol. 2018:15.
Bolignano D, Zoccali C. Effects of weight loss on renal function in obese CKD patients: a systematic review. Nephrol Dial Transplant. 2013;28(Suppl 4):iv82–98.
Marathe PH, Gao HX, Close KL. American Diabetes Association standards of medical care in diabetes 2017. J Diabetes. 2017;9(4):320–4.
National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation. 2002;106(25):3143–421.
Eknoyad G, Lameire N. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Inter Suppl. 2013;3:1–150.
Hou CC, Shyu R, Lee WJ, et al. Improved renal function 12 months after bariatric surgery. Surg Obes Relat Dis. 2013;9(2):202–6.
Rosenstock JL, Pommier M, Stoffels G, et al. Prevalence of proteinuria and albuminuria in an obese population and associated risk factors. Front Med (Lausanne). 2018;5:122.
Reid TJ, Saeed S, McCoy S, et al. The effect of bariatric surgery on renal function. Surg Obes Relat Dis. 2014;10(5):808–13.
Bhatti UH, Duffy AJ, Roberts KE, et al. Nephrolithiasis after bariatric surgery: a review of pathophysiologic mechanisms and procedural risk. Int J Surg. 2016;36(Pt D):618–23.
Gonzalez RD, Canales BK. Kidney stone risk following modern bariatric surgery. Curr Urol Rep. 2014;15(5):401.
Getty JL, Hamdallah I, Shamseddeen HN, et al. Changes in renal function following Roux-en-Y gastric bypass: a prospective study. Obes Surg. 2012;22(7):1055–9.
Friedman AN, Moe S, Fadel WF, et al. Predicting the glomerular filtration rate in bariatric surgery patients. Am J Nephrol. 2014;39(1):8–15.
Lovell A, Game P, Wittert G, et al. Estimating renal function in morbidly. Obes Surg. 2013;23(9):1427–30.
Fried M, Yumuk V, Oppert JM, et al. Interdisciplinary European guidelines on metabolic and bariatric surgery. Obes Facts. 2013;6(5):449–68.
Funding
This research did not receive any specific grant from any funding agency in the public, commercial, or not-for-profit sector.
Author information
Authors and Affiliations
Contributions
All authors participated in the collection of data. DM interpreted the data and wrote the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Ethical Approval
All procedures performed in this study involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed Consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Magalhães, D.S.C., Pedro, J.M.P., Souteiro, P.E.B. et al. Analyzing the Impact of Bariatric Surgery in Kidney Function: a 2-Year Observational Study. OBES SURG 29, 197–206 (2019). https://doi.org/10.1007/s11695-018-3508-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11695-018-3508-1