Abstract
Bariatric surgery is considered the most effective current treatment for morbid obesity. Since the first publication of an article by Kremen, Linner, and Nelson, many experiments have been performed using animal models. The initial experiments used only malabsorptive procedures like intestinal bypass which have largely been abandoned now. These experimental models have been used to assess feasibility and safety as well as to refine techniques particular to each procedure. We will discuss the surgical techniques and the postsurgical physiology of the four major current bariatric procedures (namely, Roux-en-Y gastric bypass, gastric banding, sleeve gastrectomy, and biliopancreatic diversion). We have also reviewed the anatomy and physiology of animal models. We have reviewed the literature and presented it such that it would be a reference to an investigator interested in animal experiments in bariatric surgery. Experimental animal models are further divided into two categories: large mammals that include dogs, cats, rabbits, and pig and small mammals that include rats and mice.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Obesity is a rapidly growing worldwide problem. Bariatric surgery is the most effective method of obtaining weight loss in a morbidly obese person (NIH Consensus 1991). A remarkable advantage of these operations is that they can induce resolution of associated metabolic disorders especially type 2 diabetes. The use of animal models is an excellent way of developing and learning bariatric surgical techniques as well as understanding the postsurgical physiology. We are presenting a review of the anatomy and physiology of the animal models and also the surgical techniques and postsurgical physiology of the four main bariatric surgical procedures in various animal models (Table 1).
We have included only those articles which have significantly contributed to the development of modern bariatric surgery in terms of development of techniques as well as understanding the postsurgical physiology.
Overview of Animal Models: Anatomy and Physiology
Pigs
Anatomy
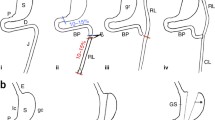
Due to their large body structure, surgical techniques in pigs can be performed to simulate those done in humans. Although the pig stomach is similar to the human stomach, there are a few differences—the cardia is exaggerated [1], the fundus is large [2], the diverticulum ventriculi is a small partially isolated pocket present within the fundus 4 cm distal to the cardia [3], thickened perigastric membranes, and a small lesser sac is present [1, 4]. Unlike the human small intestine, the pig counterpart has a variable length with an intraperitoneal duodenum [5], smaller caliber, fragile nature [5], and mesenteric vascular arcades in the subserosa [6]. The colon is the same caliber as the small bowel, is supported to the dorsal abdominal wall by the mesocolon, has a spiral course, has a left-sided cecum [2], and lacks a proper transverse mesocolon and appendix [3] (Fig. 1 and 2).
Anatomy of pig stomach [3] (reproduced with permission from Bluedoor, LLC)
Physiology
Electrical Activity of Intestine
In the gastrointestinal tract, the tissue continuity allows coordinated propagation of pacemaker potentials generated by the interstitial cells of Cajal (ICC) network on the neighboring smooth muscle cells, which enables them to generate spike potentials and contract in an organized manner. A natural intestinal pacemaker is located in the duodenum, which controls the intestinal motility [7]. Groner et al. [8] and Zabielski [9] reported that in young pigs, the antral and duodenal electrical control activity frequencies were similar to values in human adults and that the migrating motor complex cycle duration was only slightly shorter.
Dogs
Anatomy
The stomach lies distal to the liver. It is C-shaped with the lesser curvature facing the liver and the greater curvature facing the rest of the abdominal wall. The stomach is very similar to the human stomach having four parts: cardia, around entry of the esophagus; fundus, adjacent to the left abdominal wall; body, the largest part; and pylorus, adjacent to the right abdominal wall which continues as the small intestine (Fig. 3).
The duodenum has a cranial and a caudal duodenal flexure with an interposed descending duodenum. The caudal flexure is followed by the ascending duodenum which in turn continues as the jejunum, the longest part of dog intestine (Fig. 4). Unlike in humans, the dog ileum is only 15–20 cm long [10].
The rat stomach (reproduced with permission from Elsevier) [15]
There is a 5 × 2-cm-sized [11] short spiral bent [12] tortuous cecum [10] which is puckered [12]. It has no appendix [11]. The ileum does not communicate with the cecum [11]. The large intestine does not differ much in caliber from the small intestine [12] (Fig. 4). The entire colon except the cecum has a mesentry [12].
Rats
Anatomy
In spite of the small size of the organs of the rat, the anatomy of the rat GI tract is strikingly similar to humans (see Figs. 5 and 6).
Rat gastrointestinal tract (reproduced with permission from Elsevier) [15]. *Please note that the limiting ridge in the stomach is shown delimiting the forestomach from hindstomach
Gastric bypass with Roux-en-Y operation in the rat. a Staple line; b pouch; c gastroesophageal junction; d gastrojejunostomy; e stomach; f Roux-en-Y limb; g jejunojejunostomy; h afferent limb; i efferent limb [32] (reproduced with pemission from Elsevier)
The esophagus opens into the lesser curvature of the stomach. The rat stomach has a cardia or forestomach which is continuous with the esophagus and is lined by squamous epithelium. It is thinner and relatively non-motile. The fundus and pylorus (glandular stomach) have a similar anatomy as humans [13]. The forestomach and the pylorus are separated by a band of tissue called margo plicatus (limiting ridge) [14]. The greater and lesser omentum and mesentery have a similar anatomy as humans. The liver has four lobes [13].
Unlike the human intestine, the jejunum has the greatest length but cannot be easily distinguished from the other two segments. The ileum is recognized by the presence of large lymph nodes which appear as small bumps on its surface [13].
Given the small organs of the rat, one needs to use microsurgical instruments and techniques.
Physiology
The physiological characteristics of the rat models used to study bariatric surgery have been summarized in Table 2:
Given that 10–13 days in a rat’s life is equal to one human year, it is possible to learn about long-term physiological changes of bariatric surgery not currently studied in humans.
Two kinds of controls are used: a sham-operated group where the bowels are just exposed after a laparotomy for the same time as the standard operation. The sham-operated group can also be pair fed.
Roux-en-Y Gastric Bypass
Pigs
Pigs have played a leading role as an experimental model in the development of the surgical technique of Roux-en-Y gastric bypass (RYGB). In the early 1990s, with the advent of advanced laparoscopy, several investigators used these animals to test the safety and feasibility of laparoscopic RYGB.
The technique of RYGB in pigs is very similar to the technique in humans, with a few differences due to anatomical differences (described earlier).
Surgical Technique
A 10- to 30-ml gastric pouch is created using a linear cutting stapler. The pouch can be created using a Baker’s tube [5]. A V-shaped pouch can also be created taking advantage of the small lesser sac [1]. Since it is difficult to identify the ligament of Treitz, the duodenum can be followed to the point where it passes behind the colon. The Roux limb can be positioned either antegastric or retrogastric, but it cannot be retrocolic as there is no proper transverse mesocolon [5]. The gastrojejunostomy can be hand-sewn or stapled. Due to the variable length of the intestine, it is better to calculate the length of the Roux and afferent limbs as a fraction of the total intestinal length [1]. The jejunojejunostomy can be done intracorporeally or extracorporeally and can be either hand-sewn or stapled. Gastrostomy, to avoid gastric dilation, and jejunostomy, for postoperative feeding, have been used by some investigators [28]. Also, the staple lines frequently need to be reinforced with sutures due to the thick stomach wall and perigastric membranes. The fragile nature of intestine causes frequent complications due to leakage—this has to be taken into account when using the pig as a survival model. Awareness of the liver and spleen anatomy is also necessary to avoid complications during retraction [5].
Postsurgical Physiology
Flum et al. [1] have studied postsurgical physiology of pigs after RYGB. They found that after RYGB, there was an increase in both the fasting and postprandial ghrelin levels, but no change in fasting and postprandial peptide YY (PYY) levels. They also evaluated food intake and reported that the “limited feeding time” approach produced more weight loss than ad libitum feeding in their pig model.
Kiciak et al. [29] compared “uncut” Roux procedure and RYGB. They reported that there were more ICC in upper jejunum and there was an earlier return of the migrating motor complex in the uncut group than in the RYGB group. They attributed the decrease in postoperative adhesions in the uncut group to this. There is preservation of the natural pacemaker function in the duodenum in an “uncut Roux” technique, whereas ectopic pacemakers develop in the Roux limb, causing retrograde peristalsis and thus slowing intestinal transit.
Rats
Surgical Technique
A 20% residual volume stapled gastric pouch is created. The stomach is better divided to avoid gastro-gastric fistulas. The lengths of the biliopancreatic limb, alimentary limb, and common channel used by Meguid et al. are 16, 10, and 34 cm, respectively. Very similar lengths were also used by Aprahamian et al. Increasing the biliopancreatic channel to 30 cm and reducing the common channel to 18 cm produces more sustained weight loss [30, 31].
Postsurgical Physiology
Rat models of RYGB have been used to study mainly the metabolic aspects of the surgery. Various parameters are affected by RYGB, all of which ultimately contribute to weight loss and resolution of type 2 diabetes mellitus (DM). A review of those parameters is presented in Table 3:
Dogs
Surgical Technique
Dogs have mainly been used to study the electrical activity of the stomach and its role in producing weight loss. The operation on the control group consists of implantation of three pairs of electrodes of the cardiac pacemaker type in the serous membrane along the greater curvature of the stomach at the level of fundus, body, and antrum. The electrodes are fixed with sutures to prevent dislocation. The operation done on the experimental group consists of an RYGB; the width of both the gastrojejunostomy and jejunojejunostomy being 5 cm, the latter was placed 25 cm from the former. Paired gastric electrodes are then implanted on the greater curvature of the excluded stomach at the fundus, body, and antrum and fixed with sutures. The electrodes are brought to the exterior and fixed to the abdominal wall. The records of the electrical signals are processed by a hardware unit and appropriate software [44].
Postsurgical Physiology
Electrical control activity (fasting and postprandial) and electrical response activity were studied to detect any difference in the electrical activity of controls and the experimental groups for each gastric segment on one hand and the different gastric segments of each group on the other [44].
Gastric Banding
Pigs
Surgical Technique
Coelho et al. [45] first investigated the technique of placing gastric bands in pigs by laparotomy. With the advent of laparoscopic surgery, there was a need to develop a gastric band which could be placed laparoscopically. Belachew et al. [46], through a series of experiments on their pig model, developed a band that could be placed laparoscopically. They also worked out the method and appropriate instrumentation required for a safe retrogastric dissection.
Postsurgical physiology after gastric banding has not been studied in pigs.
Rats
Surgical Technique
Due to anatomical differences, it is very difficult to perform a gastric banding operation equivalent to that in humans [47]. Many techniques of placing bands in rats have been successfully tested. One of them is to place the band between the upper and the lower parts of the stomach after incising the stomach at the boundary between the forestomach and the pylorus (see Fig. 6) [47]. To facilitate appropriate positioning of the pouch, a balloon embolectomy catheter can be inserted into the stomach and pulled against the cardia after inflating the balloon. The band is placed around the lower part of the stomach just below the gastroesophageal junction. To prevent band slippage, the separated stomach is fixed just above the band. The band has also been placed around the glandular stomach without the incision mentioned above [48] by just developing an opening in the greater and lesser curvatures [49]. The forestomach can also be inverted [50]. Different types of bands have been used, but it is relevant here to only mention about adjustable bands which are widely used in humans. The adjustable gastric band used in the rat model is a commercially available (In Vivo Metric, CA, USA) vascular occluding device consisting of a silicone ring (8-mm width, 14-mm diameter) with an inflatable inner lining and a tube which can be directed out of the peritoneum, threaded subcutaneously, and externalized in the dorsal neck region [50] (Figs. 7 and 8).
Rat model of gastric band (reproduced with permission from Springer) [47]
Postsurgical Physiology
Rat models have been designed by Endo et al., Kanno et al., Monteiro et al., Rockiki et al., and Wang et al. who reported decreased weight gain in their rat models [49, 51–53]. The parameters studied have been summarized in Table 4.
Dogs
The idea of gastric bands was initiated by Wilkinson and Peloso who did their first experiments on dogs before moving onto humans. This is only of historical interest.
Sleeve Gastrectomy
Pigs
Surgical Technique
The greater curvature of the stomach is denuded with ultrasonic shears including the short gastric vessels and the posterior attachments of the fundus. This is begun 6–8 cm proximal to the pylorus. A gastric tube is created over a 38- to 60-F bougie by repeated firing of linear cutting staples from the distal antrum (6 cm from the pylorus) to the His angle with complete removal of the greater curvature and the fundus [55, 56]. The stomach wall is very thick, and only thick tissue staples should be used (green load).
Postsurgical Physiology
An experiment comparing the efficacy of sleeve gastrectomy with ileal transposition (SGIT), ileal transposition, and RYGB was done by Boza et al. [55] where they proved that SGIT is as effective as RYGB in terms of weight loss and food intake, at least in the short term. Sleeve gastrectomy has been combined with duodeno-jejunal bypass and found to be feasible in causing weight loss in a pig model. A low complication rate of the procedure and lack of histological changes in the duodenum has been reported by the same authors [56].
Rats
Surgical Technique
The technique has been well described by Lopez et al. [14] and Wang and Lu [57]. The gastric omentum, the greater curvature, and the entire fundus are removed (70–80% of total stomach, which includes 90% of the forestomach and 70% of the glandular stomach). A gastric tube is created with vascular clamps from the antrum to the fundus across both the forestomach and the glandular stomach and closed in three layers with non absorbable suture, starting from the distal antrum (1.5–2 mm from the pylorus) to the angle of His (Fig. 9).
Top figure intact rat stomach, bottom figure stomach after sleeve gastrectomy (reproduced with permission from Elsevier [14])
Postsurgical Physiology
The changes in the postsurgical parameters have been summarized in Table 5. Pereferrer et al. [58] reported that physiological changes depend on the rat model chosen.
Biliopancreatic Diversion
Pigs
Surgical Technique: Biliopancreatic Diversion with Duodenal Switch (BPD-DS)
After attachments of the stomach to the greater curvature are transected, a sleeve gastrectomy is performed. The duodenum is divided just distal to the pylorus, and the anvil of the circular stapler is passed along with an orogastric tube and brought out of the proximal duodenum. After the bowel is transacted 250 cm (or 40% of the length of intestine from ileocecal valve to the ligament of Treitz) from the ileocecal valve using a linear cutting stapler, an end-to-side duodenoenterostomy is created between the gastroduodenal pouch and upper segment of the Roux limb. Then, a side-to-side enteroenterostomy is fashioned between the end of the biliopancreatic limb and a point on the small bowel 100 cm from ileocecal junction. A methylene blue testing of the sleeve gastrectomy and duodenoenterostomy is done. The authors reported that frequent leakage at the duodenoenterostomy site was due to the thin-walled nature of the swine duodenum and that the thickness of the pig stomach requires staples of greater height [59].
Postsurgical physiology after biliopancreatic diversion has not been studied in pigs.
Dogs
Surgical Technique
Scopinaro et al. [60] evaluated the feasibility of biliopancreatic diversion in dogs in 1979. The procedure consisted of a three quarters gastric resection, closure of distal duodenum, division of the jejunum 20 cm distal to the ligament of Treitz, an antecolic anti-peristalitic, a gastroentero anastomosis using the distal end of the transected jejunum, and an end-to-side jejuno-ileal anastomosis using the proximal end of the jejunum. The distance of the latter anastomosis from the ileocecal valve was varied in the original experiment by different fractions of the total intestinal length (footnote of Table 6) and was found that a common channel of one sixth of the intestinal length was optimal for allowing adequate weight loss without medical complications.
Postsurgical Physiology
Rats
Surgical Technique: BPD-DS
The bowel is transacted 50 cm from the ileocecal valve, and the distal part of the transected intestine is anastomosed end to side with the duodenum 1–1.5 cm from the pylorus. The duodenum is closed distal to this anastomosis with a titanium clip. Though the limb lengths are representative of the human operation, the duodenum is not divided due to technical reasons. A vertical gastrectomy is performed by excising the entire fibrous membrane, thus removing two thirds of the gastric capacity. Then, a side-to-side enteroenterostomy is fashioned between the end of the biliopancreatic limb and a point on the small bowel 20 cm from ileocecal junction (the alimentary limb being 30 cm and the common limb 20 cm) [61]. But different investigators have used different limb lengths. For example, a common limb of only 5 and 10 cm were created by Zeron et al. and Borg et al., respectively [62, 63] (Fig. 10).
Rat model of biliopancreatic diversion—duodenal switch (reproduced with permission from Springer) [63]. Upper and lower arrows indicate gastrojejunostomy and enteroenterostomy, respectively
Postsurgical Physiology
Various investigators like Jiminez et al., Zeron et al., Borg et al., Nadreau et al., and Evrard et al., have designed rat models for biliopancreatic diversion. All of them have reported reduced weight in their respective animal models [61–65]. The changes in other physiological parameters have been summarized in Table 7.
The cat and rabbit models have not contributed a great deal to our understanding of modern bariatric surgery [66, 67].
Other large animals including non-human primates have not been used to experiment bariatric surgical techniques.
Mouse
Though they are not widely used to study bariatric surgery, these animals have been extensively used as models for obesity and may be useful to study postsurgical physiology of bariatric surgeries. Liu et al. [68] designed a mouse model of RYGB and type 2 DM using the C57BL/6 strain. They used three groups: duodeno-jejunal bypass (DJB), long limb DJB, and sham-operated. They were the first to design a mouse model for bariatric surgery and reported that mouse had several advantages over rats, including being more cost-effective, having quicker postoperative recovery time, and providing greater opportunities for genomic and proteomic manipulations in future research. Also, the C57BL/6 strain of mouse has already been widely used for diabetes research. In the future, application of the bariatric surgical techniques in knockout or transgenic mice may help in the understanding of the physiological mechanisms of type 2 DM resolution.
Troy et al. [69] studied the physiological effect of gastric banding and RYGB in mice and reported that increased intestinal gluconeogenesis acting through GLUT-2 receptors was responsible for decreased food intake and resolution of glucose intolerance in RYGB-treated rats.
This strain has been used to create genetically modified mouse strains, e.g., ob/ob mouse. C57BL/6J mice fed a high-fat diet develop obesity, mild to moderate hyperglycemia [70], hyperinsulinemia, and hyperlipedemia [71]. Also, obesity developed after high-fat diet tends to be maintained even after switching to regular chow [72]. C57BL/6J mice fed an atherogenic diet (1.25% cholesterol, 0.5% cholic acid, and 15% fat) for 14 weeks develop atherosclerotic lesions. The cytokines and adipokines associated with obesity have been studied in this mouse model [73].
A new mouse model C57BL/6J-Nmf15/+with a dominant mutation has been characterized which develops complications related to the metabolic syndrome (MS) and would be an excellent model for the same and especially for MS-related cardiomyopathy.
Transgenic mice can be maintained by breeding them with C57BL/6 mice. For example, transgenic ob/ob mouse strains with human islet amyloid polypeptide (hIAPP) overexpression have been created and have been proven to develop extensive islet amyloid and higher glucose concentrations [74, 75]. On pathological examination of C57BL/6 mice with diet-induced obesity, islets show no extracellular amyloid, but there is aggregation of amyloid fibrils in the β-cell secretory granules and no reported decrease in β cell mass. Extracellular amyloid is found only in the hIAPP transgenic mice [76]. They are the most widely used lab mouse strain due to the availability of congenic strains, easy breeding, robustness, and their relationship to genetically modified mouse models, making them ideal controls. C57BL/6J was the DNA source for the international collaboration that generated the first high-quality draft sequence of the mouse genome.
Choice of Animal Model
Obviously, surgical techniques are better developed and learnt with the help of large animals. Pigs have been extensively used for this purpose. The physiological changes after these surgeries are better studied using small animals, especially rats. Rats are also useful in developing and learning the surgical techniques to some extent. The choice of the rat model again depends on whether the investigator needs to study the anatomical aspects or the postsurgical physiology. The effects of bariatric surgeries on diabetes are obviously better studied using Zucker diabetic fatty or the Goto–Kakizaki rats. The Sprague–Dawley and Wistar rats have been used extensively to study RYGB and gastric banding, respectively. With a few mouse models being developed more recently, even these could be useful in the future for the study of physiology of bariatric surgeries and especially metabolic surgery.
Conclusion
The efficacy and safety of most novel techniques are better studied in animal models even before pilot studies are performed in humans. The choice of the animal model depends on whether the surgical technique or the postsurgical physiology needs to be studied. Pigs and rats have extensively been used to study various aspects of bariatric surgery. The mouse model for bariatric surgery has been developed recently. Dogs have been widely used to study the electrophysiological aspects of bariatric procedures. Other large mammals have not been used in the recent past to study bariatric procedures. This article is meant to provide a reference and guidance for researchers in bariatric surgery.
References
Flum DR, Devlin A, Wright AS, et al. Development of a porcine Roux-en-Y gastric bypass survival model for the study of post-surgical physiology. Obes Surg. 2007;17:1332–9.
Bollen PJ, Hansen AK. Important biological features. In: Suckow MA, editor. The laboratory swine. Boca Raton: CRC; 2000. p. 11–2.
Wingerd BD. The digestive system. Pig anatomy and dissection guide. Eden Prairie: Bluedoor; 2006.
Swindle MM. Endoscopic, laparosscopic and minimally invasive surgery swine in the laboratory. Boca Raton: CRC; 2007.
Scott DJ, Provost DA, Tesfay ST, et al. Laparoscopic Roux-en-Y gastric bypass using the porcine model. Obes Surg. 2001;11:46–53.
Swindle MM, Smith AC. Swine in biomedical research. In: Conn PM, editor. Sourcebook of models for biomedical research. New York: Springer; 2009. p. 235.
Suzuki N, Prosser CL, Dahms V. Boundary cells between longitudinal and circular layers: essential for electrical slow waves in cat intestine. Am J Physiol Gastrointest Liver Physiol. 1986;250:G287–94.
Groner JI, Altschuler SM, Ziegler MM. The newborn piglet: a model of neonatal gastrointestinal motility. J Pediatr Surg. 2009;25:315–8.
Zabielski R, Naruse S. Neurohormonal regulation of the exocrine pancreas during postnatal development. In: Pierzynowski SG, Zabielski R, editors. Biology of the pancreas in growing aimals. Amsterdam: Elsevier Science BV; 1999. p. 151–91.
Shively MJ. The digestive system dissection of the dog and cat. Ames: Iowa State University Press; 1985.
Evans HE, De Lahunta A. The abdomen, pelvis and pelvic limb. In: Miller ME, editor. Guide to the dissection of the dog. Ithaca: Saunders; 1952. p. 195–8.
Bradley OC, Grahame T. Thorax anatomy of the dog. 6th ed. New York: Macmillan; 1959. p. 45–6.
Wingerd BD. The digestive system. In Rat dissection manual. 2008; p. 522 41–48.
Lopez PP, Nicholson SE, Burkhardt GE, et al. Development of a sleeve gastrectomy weight loss model in obese Zucker rats. J Surg Res. 2009;157:243–50.
Hofstetter J, Suckow MA, Hickmann DL. Morphophysiology. In: Suckow MA, Weisbroth SS, Franklin CL, editors. The laboratory rat. 2nd ed. Burlington: Elsevier; 2006. p. 103–4.
Estadella D, Oyama LM, Dâmaso AR, et al. Effect of palatable hyperlipidic diet on lipid metabolism of sedentary and exercised rats. Nutrition. 2004;20:218–24.
Prada PO, Zecchin HG, Gasparetti AL, et al. Western diet modulates insulin signaling, c-Jun N-terminal kinase activity, and insulin receptor substrate-1ser307 phosphorylation in a tissue-specific fashion. Endocrinology. 2005;146:1576–87.
Weksler-Zangen S, Yagil C, Zangen DH. The newly inbred cohen diabetic rat: a nonobese normolipidemic genetic model of diet-induced type 2 diabetes expressing sex differences. Diabetes. 2001;50:2521–9.
Patriti A, Aisa MC, Annetti C, et al. How the hindgut can cure type 2 diabetes. Ileal transposition improves glucose metabolism and beta-cell function in Goto–Kakizaki rats through an enhanced Proglucagon gene expression and L-cell number. Surgery. 2007;142:74–85.
Kobatake T, Matsuzawa Y, Tokunaga K, et al. Metabolic improvements associated with a reduction of abdominal visceral fat caused by a new alpha-glucosidase inhibitor, AO-128, in Zucker fatty rats. Int J Obes. 1988;13:147–54.
Shimoike T, Yanase T, Umeda F. Subcutaneous or visceral adipose tissue expression of PPAR gamma gene is not altered in fatty (fa/fa) Zucker Rat. Metabolism. 1998;47:1494–8.
Pick A, Clark J, Kubstrup C. Role of apoptosis in failure of beta-cell mass compensation for insulin resistance and beta-cell defects in the male Zucker diabetic fatty rat. Diabetes. 1998;47:358–64.
Corsetti JP, Sparks JD, Peterson RG, et al. Effect of dietary fat on the development of non-insulin dependent diabetes mellitus in obese Zucker diabetic fatty male and female rats. Atherosclerosis. 2000;148:231–41.
Portha B, Giroix MH, Serradas P. Beta-cell function and viability in the spontaneously diabetic GK rat: information from the GK/Par colony. Diabetes. 2001;50:S89–93.
Picarel-Blanchoft F, Berthelier C, Bailbe D. Impaired glucose secretion and excessive hepatic glucose production are both early events in diabetic GK rat. Am J Physiol. 1996;27:E755–62.
Héliès JM, Diane A, Langlois A, et al. Comparison of fat storage between Fischer 344 and obesity-resistant Lou/C rats fed different diets. Obes Surg. 2005;13:3–10.
Levy JR, Davenport B, Clore JN, et al. Lipid metabolism and resistin gene expression in insulin-resistant Fischer 344 rats. Am J Physiol Endocrinol Metab. 2001;282:E626–33.
Potvin M, Gagner M, Pomp A. Laparoscopic Roux-en-Y gastric bypass for morbid obesity—a feasibility study in pigs. Surg Lap And Endo. 1997;7:294–7.
Kiciak A, Woliñski J, Borycka K, et al. GI and epithelial: Roux-en-Y or ‘uncut’ Roux procedure? Relation of intestinal migrating motor complex recovery to the preservation of the network of interstitial cells of Cajal in pigs. Exp Physiol. 2007;92:399–408.
Aprahamian CJ, Tekant G, Chen M, et al. A rat model of childhood diet-induced obesity: Roux-en-Y gastric bypass induced changes in metabolic parameters and gastric peptide ghrelin. Pediatr Surg Int. 2009;23:653–7.
Meguid MM, Glade MJ, Middleton FA. Weight regain after Roux-en-Y: a significant 20% complication related to PYY. Nutrition. 2008;24:832–42.
Xu Y, Ohinata K, Meguid MM, et al. Gastric bypass model in the obese rat to study metabolic mechanisms of weight loss. J Surg Res. 2002;107:56–63.
Meguid MM, Ramos EJB, Suzuki S, et al. A surgical rat model of human Roux-en-Y gastric bypass. J Gastrointest Surg. 2009;8:621–30.
Stenstrom B, Furnes MW, Tømmeras K, et al. Mechanism of gastric bypass-induced body weight loss: one-year follow-up after micro-gastric bypass in rats. J Gastrointest Surg. 2006;10:1384–91.
Rubino F, Zizzari P, Tomasetto C, et al. The role of the small bowel in the regulation of circulating ghrelin levels and food intake in the obese Zucker rat. Endocrinology. 2005;146:1745–51.
Tichansky DS, Boughter Jr JD, Harper J, et al. Gastric bypass surgery in rats produces weight loss modeling after human gastric bypass. Obes Surg. 2008;18:1246–50.
Guijarro A, Suzuki S, Chen C, et al. Characterization of weight loss and weight regain mechanisms after Roux-en-Y gastric bypass in rats. Am J Physiol Regul Integr Comp Physiol. 2007;293:1474–89.
Ramos EJB, Xu Y, Romanova I, et al. Is obesity an inflammatory disease? Surgery. 2009;134:329–35.
Suzuki S, Ramos EJB, Goncalves CG, et al. Changes in GI hormones and their effect on gastric emptying and transit times after Roux-en-Y gastric bypass in rat model. Surgery. 2005;138:283–90.
Pacheco D, de Luis DA, Romero A, et al. The effects of duodenal-jejunal exclusion on hormonal regulation of glucose metabolism in Goto–Kakizaki rats. Am J Surg. 2007;194:221–4.
Furnes MW, Stenstrom B, Tømmerås K, et al. Feeding behavior in rats subjected to gastrectomy or gastric bypass surgery. Eur Surg Res. 2008;40:279–88.
Romanova IV, Ramos EJB, Xu Y, et al. Neurobiologic changes in the hypothalamus associated with weight loss after gastric bypass. J Am Coll Surg. 2004;199:887–95.
Xu Y, Ramos EJB, Middleton F, et al. Gene expression profiles post Roux-en-Y gastric bypass. Surgery. 2004;136:246–52.
Ferraz ÁAB, Leão CS, Campos JM, et al. An experimental study of the electrical activity of the bypassed stomach in the Roux-en-Y gastric bypass. Arq Gastroenterol. 2007;44:162–7.
Coelho JCU, Soihaug JH, Soihaug JH, et al. Experimental evaluation of gastric banding for treatment of morbid obesity in pigs. Am J Surg. 1987;149:228–31.
Belachew M, Legrand M, Vincent V, et al. Laparoscopic adjustable gastric banding. World J Surg. 1998;22:955–63.
Endo Y, Ohta M, Kai S, et al. An obese rat model of bariatric surgery with gastric banding. Obes Surg. 2007;17:815–9.
Monteiro MP, Monteiro JD, Águas AP, et al. A rat model of restrictive bariatric surgery with gastric banding. Obes Surg. 2006;16:48–51.
Rokicki W, Rokicki M, Rokicki Jr W. The original experimental model in rats to study gastric banding. Surgery Obes Surg. 2008;18:686–9.
Kampe J, Brown WA, Stefanidis A, Dixon JB, et al. A rodent model of adjustable gastric band surgery—implications for the understanding of underlying mechanisms. Obes Surg. 2009;19:625–31.
Wang Y, Liu J. Plasma ghrelin modulation in gastric band operation and sleeve gastrectomy. Obes Surg. 2009;19:357–62.
Monteiro MP, Monteiro JD, Águas AP, et al. Rats submitted to gastric banding are leaner and show distinctive feeding patterns. Obes Surg. 2006;16:597–602.
Monteiro MP, Ribeiro AH, Nunes AF, et al. Increase in ghrelin levels after weight loss in obese Zucker rats is prevented by gastric banding. Obes Surg. 2009;17:1599–607.
Kanno H, Kiyama T, Fujita I, et al. A rat gastric banding model for bariatric surgery. J Nippon Med Sch. 2009;75:202–6.
Boza C, Gagner M, Devaud N, et al. Laparoscopic sleeve gastrectomy with ileal transposition (SGIT): a new surgical procedure as effective as gastric bypass for weight control in a porcine model. Obes Surg. 2008;22:1029–34.
del Genio G, Gagner M, Cuenca-Abente F, et al. Laparoscopic sleeve gastrectomy with duodeno-jejunal bypass: a new surgical procedure for weight control. Feasibility and safety study in a porcine model. Obes Surg. 2008;18:1263–7.
Wang Y, Liu J. Sleeve gastrectomy relieves steatohepatitis in high-fat-diet-induced. Obese Rats Obes Surg. 2009;19:921–5.
Pereferrer FS, Gonzàlez MH, Rovira AF, et al. Influence of sleeve gastrectomy on several experimental models of obesity: metabolic and hormonal implications. Obes Surg. 2008;18:97–108.
De Csepel J, Burpee S, Jossart G, et al. Laparoscopic biliopancreatic diversion with a duodenal switch for morbid obesity: a feasibility study in pigs. J Laparoendosc Adv Surg Tech. 2001;11:79–83.
Scopinaro N, Gianetta E, Bonalumi U, et al. Bilio-pancreatic bypass for obesity: 1. An experimental study in dogs. Br J Surg. 1979;66:613–7.
Nadreau E, Baraboi E-D, Samson P, et al. Effects of the biliopancreatic diversion on energy balance in the rat. Int J Obes. 2006;30:419–29.
Borg CM, le Roux CW, Ghatei MA, et al. Biliopancreatic diversion in rats is associated with intestinal hypertrophy and with increased GLP-1, GLP-2 and PYY levels. Obes Surg. 2009;17:1193–8.
Mendieta-Zerón H, Larrad-Jiménez Á, Frühbeck G, et al. Larrad biliopancreatic diversion in Sprague–Dawley rats. Analysis of weight loss related to food intake. Obes Surg. 2009;19:484–9.
Jiméneza ÁL, del Pilar ÁM, del Pilar FM, et al. Derivación biliopancreática de Larrad. Descripción de un modelo experimental en la rata. Cir Esp. 2008;83:89–92.
Evrard S, Aprahamian M, Loza E, et al. Malnutrition and body weight loss after biliopancreatic bypass in the rat. Int J Obes. 1991;15:51–8.
Skarstein A, Lekven J. Influence of gastric banding on stomach blood supply with or without concurrent splenectomy. Am J Surg. 1985;149:351–6.
Chung RS, Anderson BP. Gastric adaptive changes following experimental gastroplasty. J Surg Res. 1987;43:264–70.
Liu W, Zassoko R, Mele T, et al. Establishment of duodenojejunal bypass surgery in mice: a model designed for diabetic research. Microsurgery. 2008;28:197–202.
Troy S, Soty M, Ribeiro L, et al. Intestinal gluconeogenesis is a key factor for early metabolic changes after gastric bypass but not after gastric lap-band in mice cell. Metabolism. 2008;8:201–11.
Winters WD, Huo YS, Yao DL. Inhibition of the progression of type 2 diabetes in the C57BL/6J mouse model by an anti-diabetes herbal formula. Phytother Res. 2009;17:591–8.
Shen KP, Lin HL, Hsieh SL, et al. Eugenosedin-A prevents hyperglycaemia, hyperlipidaemia and lipid peroxidation in C57BL/6J mice fed a high-fat diet. J Pharm Pharmacol. 2009;57:517–25.
Guo J, Jou W, Gavrilova O, et al. Persistent diet-induced obesity in male C57BL/6 mice resulting from temporary obesigenic diets. Plos One. 2009;4:e5370.
Li L, Yang G, Shi S, et al. The adipose triglyceride lipase, adiponectin and visfatin are downregulated by tumor necrosis factor-alpha (TNF-alpha) in vivo. Cytokine. 2009;45:12–9.
Ahrén B, Oosterwijk C, Lips CJ, et al. Transgenic overexpression of human islet amyloid polypeptide inhibits insulin secretion and glucose elimination after gastric glucose gavage in mice. Diabetologia. 1998;41:1374–80.
Höppener JW, Oosterwijk C, Nieuwenhuis MG, et al. Extensive islet amyloid formation is induced by development of type II diabetes mellitus and contributes to its progression: pathogenesis of diabetes in a mouse model. Diabetologia. 1999;42:427–34.
de Koning EJ, Höppener JW, Verbeek JS, et al. Human islet amyloid polypeptide accumulates at similar sites in islets of transgenic mice and humans. Diabetes. 1994;43:640–4.
Meirelles K, Ahmed T, Culnan DM. Mechanisms of glucose homeostasis after Roux-en-Y gastric bypass surgery in the obese, insulin-resistant Zucker rat. Ann Surg. 2009;249:277–28.
Disclaimer
Authors certify no financial or commercial conflict of interest related to this publication.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rao, R.S., Rao, V. & Kini, S. Animal Models in Bariatric Surgery—A Review of the Surgical Techniques and Postsurgical Physiology. OBES SURG 20, 1293–1305 (2010). https://doi.org/10.1007/s11695-010-0135-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11695-010-0135-x