Abstract
Background
The enzyme 11-β-hydroxysteroid dehydrogenase type 1 (11β-HSD1) catalyzes intracellular glucocorticoid reactivation by conversion of cortisone to cortisol in different tissues and have been implicated in several metabolic disorders associated with obesity. The aim of this study was to evaluate the 11β-HSD1 expression in liver, visceral adipose tissue (VAT), and subcutaneous adipose tissue (SAT) in morbidly obese patients undergoing bariatric surgery and its correlations with clinical, anthropometric, and biochemical variables.
Methods
A prospective study was conducted over a 27-month period. Hepatic, VAT, and SAT samples were obtained at the time of surgery. 11β-HSD1 and 18S gene expression was measured using real-time quantitative reverse transcriptase-polymerase chain reaction.
Results
Forty nine patients met the inclusion criteria [mean age: 42.2 ± 10 years, body mass index (BMI): 42 ± 6 kg/m2, 71% women and 63% with metabolic syndrome (MS)]. 11β-HSD1 mRNA levels were higher in liver than fat tissue (p < 0.001), being higher in SAT than in VAT (p < 0.001) without gender-specific differences. Hepatic expression of 11β-HSD1 correlated positively with SAT and VAT, alanine aminotransferase (ALT), and serum glucose and was inversely associated with BMI. 11β-HSD1 mRNA in VAT correlated positively with insulinemia, ALT, and LDL cholesterol. There were no associations between 11β-HSD1 mRNA in SAT and the variables analyzed.
Conclusions
11β-HSD1 expression is higher in liver in comparison to adipose tissue in obese patients. The observed correlations between hepatic and VAT 11β-HSD1 expression with dyslipidemia and insulin resistance suggest that this enzyme might have a pathogenic role in obesity and related metabolic disorders.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
In the past few decades, there has been a worldwide increase in the prevalence of obesity and associated metabolic disorders including glucose intolerance, insulin resistance [1], dyslipidemia, hypertension, and visceral adiposity [2, 3]. In clinical practice, the presence of these conditions defines the metabolic syndrome (MS), which, independently of the criteria used for its diagnosis (WHO, NCEP: ATP III, EGIR) [4], is associated with an increased risk of cardiovascular events, a proinflammatory and prothrombotic state, and the occurrence of nonalcoholic fatty liver disease [2, 5–7].
Several authors have noticed clinical similarities between hypercortisolism effects seen in the Cushing syndrome and the group of disorders related to MS and central obesity, postulating that MS could be conceived as a subclinical form of Cushing's syndrome with normal plasma glucocorticoid levels [8, 9]. In fact, a decade ago, Bujalska et al. suggested that central obesity might be a “Cushing's disease of the omentum” [8]. In this view, visceral adipose cells would produce cortisol, which would act in an autocrine or paracrine manner in the splachnic territory, thus promoting abdominal obesity and associated metabolic disorders [8].
Recent studies have demonstrated that intracellular glucocorticoid metabolism is regulated at a prereceptor level by the activity of two isoforms of the 11β-hydroxysteroid dehydrogenase enzyme: 11β-Hydroxysteroid dehydrogenase type 1 and 2 (11β-HSD1 and 11β-HSD2) [10–13]. 11β-HSD1 is a microsomal enzyme, which acts mainly as a NADP (H)-dependent reductase converting inactive cortisone to active cortisol, thus regulating intracellular activation of the glucocorticoid receptor [14]. This enzyme is widely expressed not only in liver and adipose tissue, but also in adrenal, ovary, decidua, and dendritic cells [11, 15].
The pathogenic role of 11β-HSD1 in the development of the key features of metabolic disorders of central obesity has been demonstrated in transgenic mice models. Selective overexpression of 11β-HSD1 in adipose tissue is associated to visceral obesity, pronounced insulin-resistant diabetes, and dyslipidemia [16]. Hepatic overexpression of 11β-HSD1 in mice also produced MS without obesity, associated with fatty liver, dyslipidemia, mild insulin resistance, and increased angiotensinogen expression [17]. The opposite effects are seen in 11β-HSD1 knock-out mice that, under hypercaloric diets, did not develop insulin resistance or glucose intolerance [18]. Furthermore, human studies had confirmed these results, and available clinical data have shown a potential pathogenic role of 11β-HSD1 in type 2 diabetes and MS [19–22], dyslipidemia [23], hypertension [24], and osteoporosis [25].
Obesity in humans appears to be associated with increased 11β-HSD1 activity in adipose tissue compared with lean subjects or patients with Cushing syndrome [26, 27], with portal and splanchnic hypercortisolism but normal systemic cortisol [21, 28]. Recently, our group demonstrated that 11β-HSD1 expression levels were higher in subcutaneous adipose tissue (SAT) compared with visceral adipose tissue (VAT) of morbidly obese patients [29].
The aim of the present study was to evaluate the hepatic expression of 11β-HSD1 in morbidly obese patients, to compare this with the expression of 11β-HSD1 in VAT and SAT, and to establish the presence of correlations with clinical, anthropometric, and biochemical values in these patients.
Materials and Methods
Patients
All patients were prospectively recruited from September 2004 to December 2006 from the Department of Digestive Surgery Obesity Program of the Pontificia Universidad Católica de Chile. Patients that met criteria for surgical treatment of morbid obesity were evaluated by a multidisciplinary team and underwent a complete clinical and biochemical work-up, including a liver ultrasound (US) as part of routine clinical examination [30]. Only morbidly obese patients with no endocrine or genetic disease causing their obesity were enrolled in this study.
This study was approved by the Institutional Review Board for Human Studies of the Pontificia Universidad Católica de Chile and all patients provided informed consent prior to laparoscopic Roux-en-Y gastric bypass (LRYGBP) or laparoscopic sleeve gastrectomy (LSG).
The database included age, gender, preoperative body mass index (BMI), and associated diseases (hypertension, dyslipidemia, diabetes mellitus, glucose intolerance, obstructive sleep apnea, and cholelithiasis or fatty liver).
Blood samples for each individual were obtained. Serum fasting glucose, serum levels of alanine aminotransferase (ALT), and serum lipid profile measurements were performed in an automatized Roche Hitachi Modular chemistry analyzer (Hitachi, Tokyo, Japan). Fasting insulin serum level was measured with the Immulite 2000 equipment with DPC reactive (Diagnostics Product, Los Angeles, CA, USA). Insulin resistance was estimated using the homoeostasis model assessment-insulin resistance (HOMA-IR) method, according to the formula: \( {\text{insulin}}\,{\left( {{\mu {\text{IU}}} \mathord{\left/ {\vphantom {{\mu {\text{IU}}} {{\text{mL}}}}} \right. \kern-\nulldelimiterspace} {{\text{mL}}}} \right)} \times {\text{fasting}}\,{\text{plasma}}\,{\text{glucose}}\,{{\left( {{{\text{mmol}}} \mathord{\left/ {\vphantom {{{\text{mmol}}} {\text{L}}}} \right. \kern-\nulldelimiterspace} {\text{L}}} \right)}} \mathord{\left/ {\vphantom {{{\left( {{{\text{mmol}}} \mathord{\left/ {\vphantom {{{\text{mmol}}} {\text{L}}}} \right. \kern-\nulldelimiterspace} {\text{L}}} \right)}} {22.5}}} \right. \kern-\nulldelimiterspace} {22.5}\). Insulin resistance was defined in Chile by Acosta et al. according to the WHO criteria. HOMA-IR >2.6 is considered insulin resistance in non-diabetic subjects in Chile [31]. Determination of serum high-sensitivity C-reactive protein (hs-CRP) was performed with a latex particle enhanced nephelometric immune assay, in BN ProSpec equipment, Dade Behrings (Deerfield, IL, USA) and adiponectin by ELISA Quantikine™ [Human Adiponectin] (R&D Systems, Minneapolis, MN, USA).
Patients were classified as having MS if they had at least three of the following variables, according to modified NCEP-ATP III criteria: (a) waist circumference ≥ 40 in. (102 cm, males) or ≥35 in. (88 cm, females), (b) HDL cholesterol ≤ 1.03 mM (40 mg/dL, males) or ≤1.3 mM (50 mg/dL, females) or taking medication for reduced HDL cholesterol, (c) triglycerides ≥ 1.7 mM (150 mg/dL) or taking medication for elevated triglycerides, (d) systolic blood pressure (BP) ≥ 130 mmHg or diastolic BP ≥ 85 mmHg or taking antihypertensive medication, and (e) fasting glucose ≥ 5.6 mM (100 mg/dL) or taking medication for hyperglycemia [32]. All patients were considered positive for the waist circumference criteria if they had preoperative BMI > 35 kg/m2.
Tissue Biopsies
Paired sample biopsies of liver, SAT, and VAT were obtained at the moment of surgery. Liver samples were obtained by intraoperative biopsy and VAT samples were obtained from greater omental fat. Liver biopsies were examined by a single pathologist using the classification for nonalcoholic fatty liver disease (NAFLD) developed by Kleiner and co-workers [33]. SAT samples were obtained by biopsy from the abdominal port-site insertion. Approximately, 400–500 mg of each fat tissue depot and 50 mg of liver tissue were retrieved and immediately stored with RNAlater® (Ambion, Austin, TX, USA). All samples were collected and stored at −80°C until further analysis.
Total RNA Isolation and Quantitation
Total RNA was extracted from 50–200 mg of liver, VAT, and SAT using RNA kit (SV Total RNA Isolation System, Promega, Madison, WI, USA). RNA integrity was assessed by electrophoresis on 1% (w/v) agarose gels, and quantity was determined spectrophotometrically in a NanoDrop ND-1000 (NanoDrop Technologies, Wilmington, DE, USA). One microgram of total RNA was reverse-transcribed in 25 μl total volume (Improm II system, Promega) and 150 pmol random hexamers according to the manufacturer's guidelines. The reaction was terminated by heating the cDNA to 70°C for 5 min and stored at −80°C until required.
Gene Expression Analysis of 11β-HSD1
For Taqman™-based real-time PCR, gene-specific reverse transcribed products were amplified with Taq DNA polymerase kit (Fermentas, Hanover, MD, USA) using the 11β-HSD1 gene-specific primers and probe (11β-HSD1 sense primer 5′-AGGAAAGCTCATGGGAGGACTAG-3′, 11β-HSD1 antisense primer 5′-ATGGTGAATATCATCATGAAAAAGATTC-3′, and 11β-HSD1 probe 5′-6FAM-CATGCTCATTCTCAACCACATCACCAACA-TAMRA-3′) in a 72-well disk Rotor-Gene 6000 real-time Termocycler (Corbett, Concorde, Australia). Reaction conditions were 3 min at 95°C followed by 40 cycles of 15 s at 95°C and 30 s at 60°C. Real-time data were obtained during the extension phase and threshold cycle values were obtained at the log phase of each gene amplification. PCR product quantification was performed by the relative quantification method [34] and standardized against 18S RNA (18S sense primer 5′-AGGGAATTCCCGAGTAAGTGC-3′, 18S antisense primer 5′-GCCTCACTAAACCATCCAATC-3′, and 18S probe 5′-JOE-CATAAGCTTGCGTTGATTAAGTCCCTGC-TAMRA-3′). Efficiency for each primer pair was assessed by using serial dilutions of reverse transcriptase product. Results are expressed as arbitrary units (AU) and normalized against 18S RNA expression. The specificity of the PCR products was confirmed by melting temperature determination of the PCR product and high-resolution electrophoretic analysis in 4% agarose gels (Invitrogen, Carlsbad, CA, USA). Gene-specific primers were obtained from IDT (Coralville, ID, USA).
Statistical Analysis
Continuous variables are presented as mean ± standard deviation and were compared with categorical variables using Mann–Whitney nonparametric test. Gene expression is expressed as median value and was compared with continuous variables using Spearman correlation test. To compare tissue gene expression in liver, VAT, and SAT, we used a generalized linear model. A two-tailed p value <0.05 was considered statistically significant. Statistical analysis was performed using a commercially available software package (SPSS version 15.0 for Windows).
Results
A total of 49 patients met the inclusion criteria (mean age of 42.1 ± 10.1 years, mean BMI of 42.1 ± 6.1 kg/m2); 35/49 (71%) of female gender and 31 out of 49 (63%) patients had MS. The variables associated with MS were male gender, low serum HDL cholesterol and adiponectin levels, and elevated triglycerides. Baseline characteristics of morbidly obese patients according to MS are shown in Table 1.
11β-HSD1 Expression in Liver, VAT, and SAT Depots in Patients with Morbid Obesity
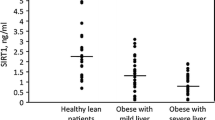
Hepatic mRNA levels of 11β-HSD1 gene in morbidly obese patients were significantly higher [31.03 ± 16.4 AU, range (2.6–90.4)] than those seen in VAT {1.36 ± 1.09 AU, range [0.2–4.6, (p < 0.001)]} and SAT [5.24 ± 4.89 AU, range (0.8–23.9) (p < 0.001)], being higher in SAT than in VAT (p < 0.001), with no gender-related differences (Fig. 1). We also found that liver 11β-HSD1 mRNA correlated positively with SAT mRNA levels (r = 0.61, p < 0.001) and VAT mRNA levels (r = 0.44, p = 0.003).
11β-HSD1 expression in analyzed tissues. To compare tissue gene expression in liver, VAT, and SAT, we used a generalized linear model observing that hepatic mRNA levels of 11β-HSD1 in morbidly obese patients were significantly higher [31.03 ± 16.4 AU, range (2.6–90.4)] than those seen in VAT [1.36 ± 1.09 AU, range (0.2–4.6)] and SAT [5.24 ± 4.89 AU, range (0.8–23.9)] with no gender-related differences
11Β-HSD1 Expression in Liver, VAT, and SAT According to Clinical and Anthropometric Variables
Hepatic 11β-HSD1 decreased consistently with the obesity status according to BMI (r = −0.32, p = 0.05) (Fig. 2), and a trend toward higher expression of the enzyme was seen in hypertensive patients (p = 0.05). On the other hand, no correlation was observed between hepatic 11β-HSD1 expression and co-morbidities such as obstructive sleep apnea or presence of gallstone disease or fatty liver. In addition, VAT 11β-HSD1 and SAT 11β-HSD1 expression did not correlate with any clinical features or anthropometric variables. There was no statistical correlation between gender and age regarding 11β-HSD1 mRNA expression in liver, SAT, or VAT.
Correlation between hepatic 11β-HSD1 and BMI. Hepatic expression of 11β-HSD1 was inversely associated with the obesity status according to BMI (r = − 0.30, p = 0.05, Spearman correlation test). We hypothesize that a down regulation of liver enzyme occurs as a result of long-term overstimulation secondary to increased visceral adiposity and probably portal hypercortisolism
11Β-HSD1 Expression in Liver, VAT, and SAT According to Biochemical Variables
We found significant correlations between hepatic 11β-HSD1 expression and biochemical variables such as fasting glucose (r = 0.29, p = 0.05) and ALT (r = 0.36, p = 0.01). An inverse trend between liver 11β-HSD1 expression and adiponectin was observed (r = −0.265, p = 0.07). Significant correlations were found between VAT 11β-HSD1 expression and biochemical variables such as fasting plasma insulin (r = 0.48, p = 0.005), ALT (r = 0.36, p = 0.02), total cholesterol (r = 0.37, p = 0.02), and LDL cholesterol (r = 0.36, p = 0.03). SAT 11β-HSD1 expression did not correlate with any of the biochemical variables analyzed.
11Β-HSD1 Expression in Liver, VAT, and SAT According to the Presence of MS
The 11β-HSD1 expression in liver, VAT, or SAT had no statistical differences when comparing patients with or without MS. However, MS had significant correlation with serum levels of HDL-cholesterol (p = 0.04), triglycerides (p = 0.03), hs-CRP (p = 0.048), and adiponectin (p < 0.001) (Table 1). Because the MS (−) group consists of one man and 17 women, while the MS (+) group consists of 13 and 18, respectively, we performed correlations by gender. When analyzing female patients with and without MS, the associations that remained with a positive correlation were hs-CRP (p = 0.01) and HDL-cholesterol (p = 0.05) and a trend with adiponectin (p = 0.081).
Discussion
In the present study, we found that, in obese subjects, hepatic mRNA 11β-HSD1 expression levels were more than 20 times greater than the expression levels seen in adipose tissue, and consistent with our previous report, SAT was statistically higher than VAT [29]. The greater expression of 11β-HSD1 is related to the important physiologic role of glucocorticoids in regulating gluconeogenic enzymes, hepatic glucose output, and glycogen stores [35, 36]. Expression of 11β-HSD1 in liver correlated positively with SAT and VAT 11β-HSD1 mRNA levels, and VAT 11β-HSD1 expression was positively correlated with SAT, suggesting important genetic and/or systemic regulation of enzyme expression.
We found that higher expression of 11β-HSD1 mRNA in the liver positively correlated with fasting glucose levels and serum ALT. We also observed a trend to a higher hepatic 11β-HSD1 expression in those patients who had diagnosis of hypertension, which could be explained by higher angiotensinogen production secondary to hepatic hypercortisolism, as was observed in the transgenic mice model with selective overexpression of 11β-HSD1 in liver [17, 37, 38]. We also found that hepatic 11β-HSD1 expression positively correlated with higher levels of fasting glucose, which could be related to an increase in hepatic gluconeogenesis induced by portal and paracrine hypercortisolism secondary to local reactivation of cortisone. The finding of an association between increased levels of serum ALT with liver and VAT 11β-HSD1 may be due to the high prevalence of NAFLD in morbidly obese patients [39]. Preliminary data from our laboratory suggest that patients with NAFLD exhibit significantly higher levels of 11β-HSD1 mRNA in VAT when compared with obese patients without NAFLD (Riquelme et al., unpublished results). However, this finding needs to be confirmed in larger series of patients. Of note, this observation could be also related with enhanced cortisol reactivation as seen in the rodent models and human studies analyzing hepatic histopathology of morbidly obese patients [40–42]. Moreover, the fact that hepatic 11β-HSD1 mRNA decreased consistently with BMI suggests the occurrence of a down regulation of the liver enzyme as a result of long-term overstimulation secondary to increased visceral adiposity and probable portal hypercortisolism. This hypothesis is supported by data published on Cushing syndrome where the 11β-HSD1 mRNA expression is lower when compared with idiopathic obesity [26].
The 11β-HSD1 expression in VAT correlated with insulin resistance and dyslipidemia. Similar findings have been described in transgenic rodent models overexpressing 11β-HSD1 in adipose tissue that develop obesity and insulin resistance [17]. Conversely, 11betaHSD1-knockout mice are protected from both [18]. In humans, the nonselective 11betaHSD1 inhibitor carbenoxolone improves insulin sensitivity [43], and studies analyzing glucocorticoid metabolism in male subjects with type 2 diabetes have detected a decrease in 11beta-HSD1 down-regulation that may potentiate dyslipidemia, insulin resistance, and obesity [22, 44, 45]. These studies may also explain our observation that increased total cholesterol and LDL cholesterol are associated with higher expression of 11β-HSD1 in VAT. However, there is some controversy about lipid metabolism in endogenous hypercortisolism, with some authors describing features similar to MS with elevated total cholesterol and triglycerides [46], while others suggest increased LDL cholesterol [47, 48]. New findings demonstrate that 11β-HSD1 inhibition reduced liver VLDL cholesterol secretion and partitions lipids toward oxidative tissues [23, 49]. In a previous study, we did not find association with VAT expression with metabolic parameters probably because we included patients with lower BMI, and it is known that the prevalence of co-morbidities increase in parallel with the obesity status.
The associations of VAT to metabolic dysregulation occur despite lower expression in this tissue compared to SAT, which was not associated to any clinical or biochemical variables. This finding could be explained by two factors: fat mass and localization. In obese patients, the splachnic and portal hypercortisolism is mainly the result of 11β-HSD1 activity in VAT. In fact, it has been estimated that approximately two-thirds of the splanchnic cortisol appears to originate from VAT secretion, sufficient to allow VAT, and not SAT, to increase cortisol input to the liver and pancreas via the portal vein [21, 28]. In humans, the rate of cortisol regeneration in peripheral tissues is of similar magnitude to adrenal secretion of cortisol and occurs principally in the splanchnic circulation [21]. With these data, we can hypothesize that increasing VAT during lifetime in patients with higher expression of 11β-HSD1 in VAT starts a vicious circle because this enzyme was shown to promote increased visceral adiposity [50].
One limitation in the interpretation of our results is the fact that, in these patients, we could only evaluate 11β-HSD1 mRNA expression. Previous studies have addressed this issue and have found a good correlation between 11β-HSD1 mRNA and protein levels in adipose tissue [26]. A complementary assessment of 11β-HSD1 activity in vivo has been performed by a determination of urinary corticosteroid metabolites [26, 51, 52] using the ratio of tetrahydrometabolites of cortisol (THF + 5αTHF) to those of the cortisone (THE). Moreover, unpublished data of our group have shown reliable assessment of 11β-HSD1 activity in ambulatory patients.
In conclusion, our results suggest that liver and VAT expression of 11β-HSD1, through a higher reactivation of cortisone to cortisol in these tissues, might have a pathogenic role in obesity and related metabolic disorders, such as hypertension, dyslipidemia, and insulin resistance.
Abbreviations
- GC:
-
glucocorticoid
- 11β-HSD1:
-
11β-hydroxysteroid dehydrogenase type 1
- HOMA-IR:
-
homeostasis model of assessment insulin resistance index
- VAT:
-
visceral adipose tissue
- SAT:
-
subcutaneous adipose tissue
- hs-CRP:
-
high-sensitivity C-reactive protein
- MS:
-
metabolic syndrome
References
Reaven GM. Banting lecture. Role of insulin resistance in human disease. Diabetes. 1988;37:1595–607.
Dandona P, Aljada A, Chaudhuri A, et al. Metabolic syndrome: a comprehensive perspective based on interactions between obesity, diabetes, and inflammation. Circulation. 2005;111:1448–54.
Tataranni PA. Metabolic syndrome: is there a pathophysiological common denominator? World Rev Nutr Diet. 2005;94:75–83.
National Cholesterol Education Program. Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, And Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III). JAMA. 2001;285:2486–97.
Alberti KG, Zimmet P, Shaw J. Metabolic syndrome—a new world-wide definition. A consensus statement from the international diabetes federation. Diabet Med. 2006;23:469–80.
Gholam PM, Flancbaum L, Machan JT, et al. Nonalcoholic fatty liver disease in severely obese subjects. Am J Gastroenterol. 2007;102:399–408.
Salgado Junior W, Santos JS, Sankarankutty AK, et al. Nonalcoholic fatty liver disease and obesity. Acta Cir Bras. 2006;21(Suppl 1):72–8.
Bujalska IJ, Kumar S, Stewart PM. Does central obesity reflect “Cushing's disease of the omentum”? Lancet. 1997;349:1210–3.
Siminialayi IM, Emem-Chioma PC. Glucocorticoids and the insulin resistance syndrome. Niger J Med. 2004;13:330–5.
Krozowski Z, Li KX, Koyama K, et al. The type I and type II 11beta-hydroxysteroid dehydrogenase enzymes. J Steroid Biochem Mol Biol. 1999;69:391–401.
Ricketts ML, Verhaeg JM, Bujalska I, et al. Immunohistochemical localization of type 1 11beta-hydroxysteroid dehydrogenase in human tissues. J Clin Endocrinol Metab. 1998;83:1325–35.
Tomlinson JW, Walker EA, Bujalska IJ, et al. 11beta-hydroxysteroid dehydrogenase type 1: a tissue-specific regulator of glucocorticoid response. Endocr Rev. 2004;25:831–66.
Bujalska IJ, Walker EA, Tomlinson JW, et al. 11Beta-hydroxysteroid dehydrogenase type 1 in differentiating omental human preadipocytes: from de-activation to generation of cortisol. Endocr Res. 2002;28:449–61.
Atanasov AG, Nashev LG, Schweizer RA. et al Hexose-6-phosphate dehydrogenase determines the reaction direction of 11beta-hydroxysteroid dehydrogenase type 1 as an oxoreductase. FEBS Lett. 2004;571:129–33.
Freeman L, Hewison M, Hughes SV, et al. Expression of 11beta-hydroxysteroid dehydrogenase type 1 permits regulation of glucocorticoid bioavailability by human dendritic cells. Blood. 2005;106:2042–9.
Masuzaki H, Paterson J, Shinyama H, et al. A transgenic model of visceral obesity and the metabolic syndrome. Science. 2001;294:2166–70.
Paterson JM, Morton NM, Fievet C, et al. Metabolic syndrome without obesity: Hepatic overexpression of 11beta-hydroxysteroid dehydrogenase type 1 in transgenic mice. Proc Natl Acad Sci U S A. 2004;101:7088–93.
Morton NM, Paterson JM, Masuzaki H, et al. Novel adipose tissue-mediated resistance to diet-induced visceral obesity in 11 beta-hydroxysteroid dehydrogenase type 1-deficient mice. Diabetes. 2004;53:931–8.
Alberti L, Girola A, Gilardini L, et al. Type 2 diabetes and metabolic syndrome are associated with increased expression of 11beta-hydroxysteroid dehydrogenase 1 in obese subjects. Int J Obes (Lond). 2007;31:1826–31.
Paulsen SK, Pedersen SB, Fisker S, et al. 11Beta-HSD type 1 expression in human adipose tissue: impact of gender, obesity, and fat localization. Obesity (Silver Spring). 2007;15:1954–60.
Walker BR, Andrew R. Tissue production of cortisol by 11beta-hydroxysteroid dehydrogenase type 1 and metabolic disease. Ann N Y Acad Sci. 2006;1083:165–84.
Valsamakis G, Anwar A, Tomlinson JW, et al. 11beta-hydroxysteroid dehydrogenase type 1 activity in lean and obese males with type 2 diabetes mellitus. J Clin Endocrinol Metab. 2004;89:4755–61.
Berthiaume M, Laplante M, Festuccia WT, et al. 11beta-HSD1 inhibition improves triglyceridemia through reduced liver VLDL secretion and partitions lipids toward oxidative tissues. Am J Physiol Endocrinol Metab. 2007;293:E1045–52.
Atanasov AG, Odermatt A. Readjusting the glucocorticoid balance: an opportunity for modulators of 11beta-hydroxysteroid dehydrogenase type 1 activity? Endocr Metab Immune Disord Drug Targets. 2007;7:125–40.
Tomlinson JW, Bujalska I, Stewart PM, et al. The role of 11 beta-hydroxysteroid dehydrogenase in central obesity and osteoporosis. Endocr Res. 2000;26:711–22.
Mariniello B, Ronconi V, Rilli S, et al. Adipose tissue 11beta-hydroxysteroid dehydrogenase type 1 expression in obesity and Cushing's syndrome. Eur J Endocrinol. 2006;155:435–41.
Desbriere R, Vuaroqueaux V, Achard V, et al. 11beta-hydroxysteroid dehydrogenase type 1 mRNA is increased in both visceral and subcutaneous adipose tissue of obese patients. Obesity (Silver Spring). 2006;14:794–8.
Basu R, Singh RJ, Basu A, et al. Splanchnic cortisol production occurs in humans: evidence for conversion of cortisone to cortisol via the 11-beta hydroxysteroid dehydrogenase (11beta-hsd) type 1 pathway. Diabetes. 2004;53:2051–9.
Munoz R, Carvajal C, Escalona A, et al. 11beta-Hydroxysteroid Dehydrogenase Type 1 is Overexpressed in Subcutaneous Adipose Tissue of Morbidly Obese Patients. Obes Surg. 2009;19:764–70.
Carrasco F, Klaassen J, Papapietro K, et al. A proposal of guidelines for surgical management of obesity. Rev Med Chil. 2005;133:699–706.
Acosta AM, Escalona M, Miz A, et al. Determination of the insulin resistance index by the Homeostasis Model Assessment in a population of Metropolitan Region in Chile. Rev Med Chil. 2002;130(11):1227–31.
Grundy SM, Cleeman JI, Daniels SR, et al. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation. 2005;112:2735–52.
Kleiner DE, Brunt EM, Van Natta M, et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41:1313–1321.
Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29:e45.
Altuna ME, Lelli SM, de Viale San Martin LC, et al. Effect of stress on hepatic 11beta-hydroxysteroid dehydrogenase activity and its influence on carbohydrate metabolism. Can J Physiol Pharmacol. 2006;84:977–84.
Holmes MC, Kotelevtsev Y, Mullins JJ, et al. Phenotypic analysis of mice bearing targeted deletions of 11beta-hydroxysteroid dehydrogenases 1 and 2 genes. Mol Cell Endocrinol. 2001;171:15–20.
Seckl JR, Morton NM, Chapman KE, et al. Glucocorticoids and 11beta-hydroxysteroid dehydrogenase in adipose tissue. Recent Prog Horm Res. 2004;59:359–93.
Walker EA, Stewart PM. 11beta-hydroxysteroid dehydrogenase: unexpected connections. Trends Endocrinol Metab. 2003;14:334–9.
Boza C, Riquelme A, Ibanez L, et al. Predictors of nonalcoholic steatohepatitis (NASH) in obese patients undergoing gastric bypass. Obes Surg. 2005;15:1148–53.
Paterson JM, Seckl JR, Mullins JJ. Genetic manipulation of 11beta-hydroxysteroid dehydrogenases in mice. Am J Physiol Regul Integr Comp Physiol. 2005;289:R642–52.
Liew PL, Lee WJ, Lee YC, et al. Hepatic histopathology of morbid obesity: concurrence of other forms of chronic liver disease. Obes Surg. 2006;16:1584–93.
Arun J, Clements RH, Lazenby AJ, et al. The prevalence of nonalcoholic steatohepatitis is greater in morbidly obese men compared to women. Obes Surg. 2006;16:1351–8.
Andrews RC, Rooyackers O, Walker BR. Effects of the 11 beta-hydroxysteroid dehydrogenase inhibitor carbenoxolone on insulin sensitivity in men with type 2 diabetes. J Clin Endocrinol Metab. 2003;88:285–91.
Kannisto K, Pietilainen KH, Ehrenborg E, et al. Overexpression of 11beta-hydroxysteroid dehydrogenase-1 in adipose tissue is associated with acquired obesity and features of insulin resistance: studies in young adult monozygotic twins. J Clin Endocrinol Metab. 2004;89:4414–21.
Hughes KA, Webster SP, Walker BR. 11-Beta-hydroxysteroid dehydrogenase type 1 (11beta-HSD1) inhibitors in type 2 diabetes mellitus and obesity. Expert Opin Investig Drugs. 2008;17:481–96.
Friedman TC, Mastorakos G, Newman TD, et al. Carbohydrate and lipid metabolism in endogenous hypercortisolism: shared features with metabolic syndrome X and NIDDM. Endocr J. 1996;43:645–55.
Jezkova J, Marek J, Prazny M, et al. Effect of hypercortisolism on development of atherosclerotic changes in blood vessels. Vnitr Lek. 2003;49:656–67.
Yoshida C, Miyachi Y. Lipid metabolism in Cushing's syndrome. Nippon Rinsho. 2001;59(Suppl 3):177–80.
Nuotio-Antar AM, Hachey DL, Hasty AH. Carbenoxolone treatment attenuates symptoms of metabolic syndrome and atherogenesis in obese, hyperlipidemic mice. Am J Physiol Endocrinol Metab. 2007;293:E1517–28.
Wolf G. Glucocorticoids in adipocytes stimulate visceral obesity. Nutr Rev. 2002;60:148–51.
Wake DJ, Rask E, Livingstone DE, et al. Local and systemic impact of transcriptional up-regulation of 11beta-hydroxysteroid dehydrogenase type 1 in adipose tissue in human obesity. J Clin Endocrinol Metab. 2003;88:3983–8.
Turpeinen U, Markkanen H, Sane T, et al. Determination of free tetrahydrocortisol and tetrahydrocortisone ratio in urine by liquid chromatography-tandem mass spectrometry. Scand J Clin Lab Invest. 2006;66:147–59.
Acknowledgements
This work was supported by grant from Sociedad Chilena de Endocrinología (SOCHED) Nº 2008-03 to R.B. and partially supported by grants from the National Commission for Scientific and Technological Research of the Chilean Government (CONICYT); FONDECYT Nº 1070876 to C.F. and Nº 1080170 to M.A.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Baudrand, R., Carvajal, C.A., Riquelme, A. et al. Overexpression of 11β-Hydroxysteroid Dehydrogenase Type 1 in Hepatic and Visceral Adipose Tissue is Associated with Metabolic Disorders in Morbidly Obese Patients. OBES SURG 20, 77–83 (2010). https://doi.org/10.1007/s11695-009-9937-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11695-009-9937-0