Abstract
Background and objective:
CD36 is implicated in fatty-acid uptake in multiple tissues, including hepatocytes and adipocytes. Circulating CD36 (sCD36) is increased in non-alcoholic fatty liver disease (NAFLD). We explored this association further by investigating correlations between sCD36 levels, intrahepatic lipid content and markers of obesity in NAFLD patients and controls.
Methods:
In total, 111 NAFLD patients and 33 normal/overweight controls were included. Intrahepatic lipid content was measured by magnetic resonance spectroscopy; and subgroups of participants had a dual-energy X-ray absorptiometry (n=99), magnetic resonance imaging (n=94, subcutaneous and visceral adipose tissue) and liver biopsy (n=28 NAFLD patients) performed. Plasma sCD36 was assessed by enzyme-linked immunosorbent assay.
Results:
NAFLD patients had elevated sCD36 levels compared with controls (0.68 (0.12–2.27) versus 0.43 (0.10–1.18), P<0.01). sCD36 correlated with intrahepatic lipid (rs=0.30), alanine transaminase (ALT) (r=0.31), homeostasis model assessment index-insulin resistance (r=0.24), high-density lipoprotein (r=−0.32) and triglyceride (r=0.44, all P<0.01). Intrahepatic lipid and plasma triglyceride were independent predictors of sCD36 levels in a multiple regression analysis. Further, sCD36 and body mass index were weakly correlated (r=0.17, P=0.04); yet, we found no correlations between sCD36 and other measures of fat distribution except an inverse relation to visceral adipose tissue (rs=−0.21, P<0.05). We observed a trend for correlation between sCD36 and hepatic CD36 mRNA expression (r=0.37, P=0.07).
Conclusions:
sCD36 levels increased with the level of intrahepatic lipid, insulin resistance and dyslipidemia. The weak association with markers of obesity and the association with hepatic CD36 mRNA expression suggest that excess sCD36 in NAFLD patients is derived from the hepatocytes, which may support that CD36 is involved in NAFLD development. An unhealthy and unbalanced CD36 expression in adipose and hepatic tissue may shift the fatty-acid load to the liver.
Similar content being viewed by others
Introduction
Lifestyle has become increasingly sedentary and dietary patterns have changed over the past decades. This has led to an increased prevalence of obesity, insulin resistance and metabolic syndrome.1 Non-alcoholic fatty liver disease (NAFLD) is present in up to 90% of the obese patients,2 and increasingly common in the adolescent population.3 Indeed, NAFLD is recognized as part of the metabolic syndrome,4 and NAFLD is a risk factor for other conditions such as type 2 diabetes mellitus, cardiovascular disease and cancer.4 NAFLD is a spectrum of diseases ranging from simple steatosis over steatohepatitis (NASH) to NASH fibrosis and cirrhosis. Currently, more information regarding pathogenic pathways, accurate biomarkers and effective therapeutic agents of NAFLD are needed.
The increased release and transport of free fatty acids (FFAs) from insulin-resistant adipose tissue to the liver is an essential step for excessive fat accumulation within hepatocytes. Indeed, circulating fatty acids are a major source of hepatic lipids in patients with NAFLD,5 suggesting that especially the rate of influx of FFAs to the hepatocytes is important for the development of steatosis. Plasma membrane-bound fatty-acid translocase CD36 has an important role in facilitating uptake and intracellular trafficking of long-chain FFAs in hepatocytes and other cell types.6 Miquilena-Colina et al.7reported increased hepatic CD36 expression levels in a NAFLD cohort of NAFLD and NASH patients with generally mild steatosis. Along these lines, García-Monzón et al.8 recently demonstrated that the serum level of circulating CD36 (sCD36) correlates with the histological steatosis grade and hepatic CD36 protein expression. Similarly, we have previously shown that sCD36 is progressively associated with biomarkers for NAFLD as well as insulin sensitivity and atherosclerosis.9 Aiming to further investigate sCD36 levels in NAFLD patients, we hypothesized that sCD36 would be positively associated with intrahepatic lipid content (quantified by magnetic resonance (MR) spectroscopy) in a local cohort of NAFLD patients and controls. We also aimed to confirm that sCD36 is positively associated with hepatic CD36 mRNA expression. Finally, we aimed to describe the relationship between sCD36 and obesity and body composition, speculating if plasma sCD36 is perhaps less dependent on obesity than liver lipid content.
Methods
Patients
The study cohort consists of 144 participants that were included in three randomized, clinical trials on obesity and NAFLD, conducted at Aarhus University Hospital, Denmark, from August 2011 to August 2014, all registered in Clinical Trials.gov (NCT01464801, NCT01412645, NCT01446276). The trials did not include overt diabetic patients and further details on inclusion/exclusion criteria are found in.10, 11, 12 All trial participants with MR spectroscopy data were included in the present study, also including a control group of healthy, normal-weight participants, matched in age and gender to the NAFLD patients of.10
The NAFLD diagnosis was established on the basis of an intrahepatic lipid content of >5%, and in the absence of any other explanation for hepatic steatosis, including excess alcohol consumption. A body mass index (BMI) of ⩾25.0 was considered overweight. Participants were divided into the main categories, based on the presence/absence of NAFLD and overweight; (1) normal-weight controls (n=13); (2) overweight controls (n=20); and (3) NAFLD patients (n=111).
The studies were performed in accordance with the ethical guidelines of the Helsinki Declaration, and approved by the Danish National Committee on Health Research Ethics and the Danish Data Protection Agency. All participants gave written informed consent. All data included were retrieved at the baseline trial visit.
MR spectroscopy
The intrahepatic lipid content was measured by MR spectroscopy using the same Signa Excite 1.5 tesla twin-speed scanner (GE Medical Systems, WI, USA), and the spectra were quantified using the LC model software package (version 6.2, details specified in Heebøll et al.10). The intrahepatic lipid content is accurately assessed at both low and high lipid concentrations,13 and therefore in both normal-weight and overweight study participants.12
Body composition
A further investigation of body composition was performed in a total of 99 overweight male participants, who were included in two sub-studies.12, 14 Body composition was assessed by dual-energy X-ray absorptiometry (QDR-2000; Hologic, Marlborough, MA, USA), and results were expressed as total body fat percentage. SAT (subcutaneous adipose tissue) and visceral adipose tissue (VAT) depots were quantified by magnetic resonance imaging from caput femoris to the upper rim of the kidney and analyzed using the software tool Hippo fat, version 6.3. Technical difficulties caused missing magnetic resonance imaging data in five of the 99 tested patients.
Biochemistry
Blood samples were taken after an overnight fast. Routine biochemistry was analyzed continuously throughout the study at the Department of Clinical Biochemistry, Aarhus University Hospital. The homeostasis model assessment index (HOMA) was calculated on the basis of fasting glucose and insulin levels, using the HOMA2 calculator (The University of Oxford 2013(ref. 15)) and used as an estimate of insulin sensitivity.
The sCD36 measurements were performed in batch on a well-established, in-house kit, as specified elsewhere16 and expressed in arbitrary units l−1 (AU).
Liver biopsy and histological assessment
Liver biopsy material was available for assessment in 28 NAFLD patients, included in one of the three sub-studies. This study aimed to investigate effects of resveratrol in NAFLD/NASH patients.10 Besides steatosis on imaging, criteria for liver biopsy included BMI⩾25 kg m−2, transaminasemia (ALT>70/45 U l−1 for men/women) and at least one additional element of the metabolic syndrome (as defined in the Adult Treatment Panel III). Sections were evaluated in a blinded manner by two experienced pathologists and scored according to the criteria proposed by the NASH-Clinical Research Network.17 Differentiation between NAFLD and NASH was performed according to the FLIP algorithm.18
RNA isolation, cDNA synthesis and RT-PCR
Liver tissue was available for real-time PCR (RT-PCR) in 27 NAFLD patients. Total RNA was extracted from liver biopsy samples using TriZol reagent (Cat. 15596018, Life Technologies Inc., Nærum, Denmark), according to the manufacturer’s protocol. RNA was quantified by measuring absorbance at 260 nm and 280 nm using a NanoDrop 8000 (NanoDrop Products, DE, USA). Quality was checked on a BioRad Experion RNA analyzer (Bio-Rad Laboratories, Inc., Hercules, CA, USA), and the RNA quality index-factor was 6.3 (±1.4). cDNA was synthesized using random hexamer primers (Verso cDNA kit, Thermo Fisher Scientific Inc., Waltham, MA, USA).
The quantitative RT-PCRs were performed in duplicate using the KAPA SYBR FAST qPCR kit (Kapa Biosystems, Inc., Wilmington, MA, USA) in a LightCycler 480 (Roche Applied Science, Mannheim, Germany) using the following protocol: One step at 95 °C for 3 min, then 95 °C for 10 s, 60 °C for 20 s, and 72 °C for 10 s. CD36 primer sequence sense; TCTGTGCCTGTTTTAACCCAA, and anti-sense; GCCAGTTGGAGACCTGCTTA, were used. The specificity of the primers was tested by melting curve analysis and agarose gel electrophoresis. The relative gene expression was estimated using the default 'Advanced Relative Quantification' mode of the software version LCS 480 1.5.0.39 (Roche Applied Science). Housekeeping gene β2-microglobulin served as the internal control.
Statistical analysis
Statistical analysis was performed using the STATA software (11.0). Categorical data are summarized as frequencies (percentages) and continuous variables as mean (±s.d.) for parametric data or median (range) for non-parametric data. Normality of data was checked by QQ-plots. When appropriate, data were ln-transformed to normality. Patient characteristics were compared using the ANOVA (parametric data) or the Kruskal–Wallis test by ranks (non-parametric data). Correlations were performed using Pearson’s test (correlation coefficient; r, parametric data) or Spearman’s test (correlation coefficient; rs, non-parametric data). After due test of assumptions, robust multiple regression analyses were performed, including all predictive variables that were tested on the full cohort and that were significant on linear regression. In the situation of missing data, only available cases were included in the specific analysis. The level of statistical significance was set at P<0.05 (two-sided).
Results
Patient characteristics
Table 1 includes the demographic, metabolic and biochemical characteristics of the study participants, and especially demonstrates a NAFLD patient group with significant steatosis of median 22% intrahepatic lipid content with a large variance (5−45%). Also, the NAFLD group had a higher alanine transaminase (ALT) level, insulinemia and dyslipidemia (Table 1). We found a slightly higher BMI and waist circumference in the NAFLD group than the overweight control group, however other markers of obesity and measures of body composition such as, SAT, VAT and total body fat percentage were non-significantly elevated in the NAFLD patients.
A liver biopsy performed in 28 NAFLD patients showed that 12 of these patients suffered from histological NASH.10 We found a higher ALT (89 U l−1 (43−150 U l−1) versus 43 U l−1 (18–235 U l−1), P<0.01), intrahepatic lipid content (30% (18–35%) versus 19% (5–45%), P=0.02) and a trend higher HOMA level (2.0 (0.6–4.0) versus 1.6 (0.5−4.8), P=0.08) in the subgroup of NAFLD patients with histologically confirmed NASH, in comparison with the NAFLD patients in general.
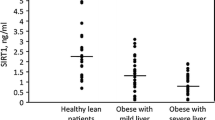
Soluble CD36 is elevated in NAFLD and NASH and increases with increasing levels of intrahepatic lipid content
Compared with both normal-weight and overweight controls, NAFLD patients had an elevated sCD36 level (P<0.01, Table 1). Also, we found a significantly higher sCD36 level in patients with histologically confirmed NASH (0.92 AU (0.41−1.95 AU)), compared with the remaining NAFLD patients (0.63 AU (0.10−1.97 AU), P=0.01). Yet, sCD36 was not associated with histological NAFLD activity score or fibrosis score in the available biopsies (data not shown).
The level of sCD36 correlated with the level of intrahepatic lipid as assessed by MR spectroscopy (n=144, rs=0.30, P<0.01, Figure 1a). Corroborating this finding, we found a significant association between plasma ALT and sCD36 levels (n=144, r=0.31, P<0.01, Figure 1b).
Correlation between sCD36 and hepatic CD36 expression
We found a moderate but non-significant correlation between sCD36 levels and hepatic CD36 mRNA expression (n=27, r=0.36, P=0.07, Figure 2).
sCD36 correlation with measures of obesity and fat distribution
sCD36 and BMI were weakly correlated (n=144, r=0.17, P=0.04, Figure 3a). Yet, we found no significant correlations between sCD36 and most other measures of obesity and fat distribution, including total body fat percentage (n=99, r=−0.02, P=0.85), waist circumference (n=144, r=0.15, P=0.07) and SAT (n=94, r=−0.08, P=0.42). A notable exception was an inverse relation between sCD36 and VAT (n=94, rs =−0.21, P<0.05, Figure 3b).
sCD36 association with gender, age and biochemical metabolic risk factors
We found no difference in sCD36 levels between the genders, albeit our study included only few female participants (Table 1). We found a significant increase in sCD36 with increasing age in the control subjects (r=0.43, P=0.01), irrespective of no age-related increase in BMI or intrahepatic lipid content (data not shown). In contrast, sCD36 decreased with age in NAFLD patients (r=0.32, P<0.01).
As expected, sCD36 levels were inversely correlated with plasma HDL (n=141, r=−0.31, P<0.01) and positively correlated with plasma triglycerides (n=141, r=0.44, P<0.01), whereas sCD36 and LDL levels were not correlated. Also, sCD36 was positively associated with HOMA (n=143, r=0.24, P<0.01), and insulin levels (n=143, r=0.17, P<0.05).
Multiple regression analyses
In a multiple regression analysis, we included variables that may influence sCD36 levels and that were available for the full cohort, thus testing age, intrahepatic lipid content (assessed by MR spectroscopy), BMI, waist circumference, plasma lipids (triglyceride, HDL, LDL) and HOMA. Variables that proved significant in a univariate analysis were included in a robust multiple regression analysis, thus including intrahepatic lipid, BMI, triglyceride, HDL and HOMA. In this model, only intrahepatic lipid content (n=141, P=0.01) and triglyceride (P<0.01) were independently associated with sCD36 levels.
We also tested which variables were independent contributors to intrahepatic lipid content as defined as independent variables in a robust multiple regression model. Tested variables included waist circumference, BMI, triglyceride, HDL, HOMA and sCD36 that were all significantly associated with intrahepatic lipid content in a univariate regression analysis (data not shown). Waist circumference was omitted due to the close correlation with BMI. In this model, sCD36 (n=141, P<0.01) and HOMA (P<0.01) were independently associated with intrahepatic lipid content.
Discussion
In this study, our main finding was a significant correlation of sCD36 and intrahepatic lipid content as assessed by MR spectroscopy, which also proved significant in a multiple regression model. sCD36 levels were elevated in NAFLD patients in comparison with both normal-weight and overweight controls. Furthermore, we related this sCD36 to the hepatic mRNA expression in a subgroup of NAFLD patients. As a novel finding, we demonstrated an inverse relationship between sCD36 and VAT.
CD36 is a multifunctional receptor, which inter alia mediates the uptake of long-chain fatty acids in metabolically active tissues such as liver, adipose and muscle tissue; and oxidized LDL in monocytes/macrophages, platelets and endothelial cells.6 Increased expression and recruitment of CD36 from intracellular storage sites regulates fatty-acid uptake and metabolism, inducing ectopic triglyceride accumulation and metabolic disease.19 Indeed, several lines of evidence link hepatic CD36 expression and NAFLD pathogenesis, including human data. Studies have reported elevated hepatic CD36 expression in NAFLD and NASH patients,7, 20, 21, 22 and recently, hepatic steatosis and CD36 expression were linked to sCD36 levels.8
Hence, our study corroborates the findings of previous studies with a number of definite strengths and limitations. The primary strength is the broad, non-diabetic NAFLD patient cohort, which includes patients with a wide range of intrahepatic lipid content and more patients with grade 3 steatosis, than in previous trials.7, 8 For quantification of hepatic lipid content, we used MR spectroscopy, which yields a better quantitative estimation of hepatic steatosis than histology.13, 23 Also, we included a dedicated normal- and overweight control group. Another strength is the fact that plasma sCD36 quantification was performed by a well-established method.9, 16, 24, 25 Currently, commercial sCD36 assays lack standardization and consistency.26
Limitations of our study include the incomplete data set in regards to measures of body composition, histology and hepatic CD36 mRNA expression, which restricts the analysis and conclusions we can make on the association between sCD36 and these variables. Also, inclusion of variables is limited by the retrospective design, for example, we only have data on hepatic CD36 mRNA expression with no data on protein expression or the expression in other tissue types.
In our cohort, the NASH patients had a significantly higher level of sCD36 than NAFLD patients in general. This may signify an association between CD36 and hepatic inflammation and apoptosis as suggested by both experimental and human studies,21, 27, 28 however sCD36 was not associated with histological NAFLD activity score or fibrosis score. Our data contrast the findings of García-Monzón, in which sCD36 levels were significantly more elevated in simple steatosis than in NASH patients.7 Opposite the García-Monzón study, in which the NASH patients had little steatosis, our NASH patients had a significantly higher level of steatosis than the NAFLD patients in general, and this higher level of steatosis may also explain the elevated sCD36 level in our NASH group. Obviously, we did not include enough patients with histological verification of simple steatosis/NASH to formally test this, and the effect of hepatic inflammation, ballooning and fibrosis on sCD36 levels remains unresolved.
It is well-established that insulin induces CD36 gene expression and CD36 protein translocation to the plasma membrane in macrophages, adipocytes, skeletal muscle cells29, 30 and probably hepatocytes.7, 31, 32 In contrast, experimental studies have suggested that HDL inhibits CD36 expression.33 Parallel to previous studies,7, 16, 34, 35 we confirmed a significant correlation between sCD36 and insulin resistance (insulin and HOMA) in our cohort as well as a correlation between sCD36 and HDL. An increase in hepatic CD36 activity may increase the fatty-acid load on the liver (and skeletal muscle), and may thus further impair insulin signaling and stimulate hepatic VLDL-triglyceride secretion. Accordingly, we found a strong correlation between sCD36 and triglyceride levels, and triglyceride was an independent predictor of sCD36 in a multiple regression model.
Interestingly, we found a moderate though non-significant correlation between sCD36 and hepatic CD36 mRNA expression, although our expression data are based on only a small subgroup of NAFLD patients with no controls. Indeed, our results parallel the previous findings of García-Monzón et al.8 The correlation may signify that a considerable portion of sCD36 released for circulation is derived from the liver.
Generally, sCD36 circulates in microvesicles,36 which mainly derive from platelets in healthy individuals.37 However, many other cell types may add to this under pathological conditions,38 including hepatocytes and leukocytes involved in NAFLD pathogenesis. Other mechanism for the increased sCD36 in NAFLD patients may also be at play. Miquilena-Colina et al. report a shift in subcellular CD36 protein location in the NAFLD patients, demonstrating more CD36 in the hepatocyte plasma membrane of NAFLD patients as opposed to the intracellular storage seen in normal livers.7 Therefore, the increased CD36 release to the circulation may be explained by the membranous location in NAFLD patients, whether released in microvesicles or released unbound. Finally, sCD36 may be released from degrading hepatocytes and macrophages (Kupffer cells) as a consequence of necroinflammation.
Another significant source of sCD36 may be the adipose tissue.39 Previous studies have suggested an association between sCD36 and obesity as measured most commonly by BMI,8, 16 which is in accordance with an increased adipose tissue volume as a source of sCD36. In the current study, however, sCD36 was only weakly correlated to BMI and not correlated to SAT, waist circumference or total fat mass; and we even showed an inverse correlation between sCD36 and VAT. Further, we found no difference in the sCD36 level between normal and overweight/obese controls. Methodological issues may be important for these inconsistencies, since absolute incongruity was previously demonstrated between results derived from the commercial assay used by García-Monzón et al. and our in-house method, which has been extensive validated on clinical data.26 Another issue is that previous sCD36 studies include no detailed information on concomitant intrahepatic lipid content and hence, the adipose tissue contribution to sCD36 may be overestimated in overweight individuals.
There are numerous possible interpretations of the inverse correlation between sCD36 and VAT. Especially, sCD36 may be released more readily from liver than adipose tissue owing to anatomy. Also, the hepatic CD36 expression may increase in the situation of low adipose tissue CD36 expression, resulting in a higher FFA load on the liver. In line with this theory, an imbalanced adipocyte/hepatocyte CD36 expression may be part of the NAFLD pathogenesis as suggested by Fabbrini et al.19 Matching obese study subjects on VAT, they found a significantly lower adipose tissue CD36 expression, a lower insulin sensitivity and an increased hepatic VLDL-triglyceride secretion rate in subjects with a high than with a low intrahepatic lipid content.19 In contrast, matching subjects on intrahepatic lipid content identified no such differences. Also, Glintborg et al. showed a decrease in sCD36 levels during pioglitazone treatment, a peroxisome proliferator-activated receptor-γ agonist that increases adipose tissue CD36 expression.34 Indeed, these findings agree with ours in the sense that an inverse correlation between sCD36 and VAT may be mediated by a higher hepatic contribution and a lower adipocyte contribution to sCD36 in the overweight/obese subjects.
In conclusion, sCD36 levels increase with the level of intrahepatic lipid, insulin resistance and dyslipidemia. The weak association with markers of obesity and the association with hepatic CD36 mRNA expression suggest that the excess sCD36 in NAFLD patients is derived from the hepatocytes, which may indicate a role for CD36 in NAFLD development. In theory, the inverse correlation between sCD36 and VAT may reflect an unhealthy and unbalanced CD36 expression in adipose and hepatic tissue, which may shift the FFA load to the liver.
References
Ng M, Fleming T, Robinson M, Thomson B, Graetz N, Margono C et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet 2014; 384: 766–781.
Mottin CC, Moretto M, Padoin AV, Swarowsky AM, Toneto MG, Glock L et al. The role of ultrasound in the diagnosis of hepatic steatosis in morbidly obese patients. Obes Surg 2004; 14: 635–637.
Welsh JA, Karpen S, Vos MB . Increasing prevalence of nonalcoholic fatty liver disease among United States adolescents, 1988-1994 to 2007-2010. J Pediatr 2013; 162: 496–500 e1.
Vernon G, Baranova A, Younossi ZM . Systematic review: the epidemiology and natural history of non-alcoholic fatty liver disease and non-alcoholic steatohepatitis in adults. Aliment Pharmacol Ther 2011; 34: 274–285.
Donnelly KL, Smith CI, Schwarzenberg SJ, Jessurun J, Boldt MD, Parks EJ . Sources of fatty acids stored in liver and secreted via lipoproteins in patients with nonalcoholic fatty liver disease. J Clin Invest 2005; 115: 134351.
Koonen DP, Jensen MK, Handberg A . Soluble CD36- a marker of the (pathophysiological) role of CD36 in the metabolic syndrome? Arch Physiol Biochem 2011; 117: 57–63.
Miquilena-Colina ME, Lima-Cabello E, Sanchez-Campos S, Garcia-Mediavilla MV, Fernandez-Bermejo M, Lozano-Rodriguez T et al. Hepatic fatty acid translocase CD36 upregulation is associated with insulin resistance, hyperinsulinaemia and increased steatosis in non-alcoholic steatohepatitis and chronic hepatitis C. Gut 2011; 60: 1394–1402.
Garcia-Monzon C, Lo Iacono O, Crespo J, Romero-Gomez M, Garcia-Samaniego J, Fernandez-Bermejo M et al. Increased soluble CD36 is linked to advanced steatosis in nonalcoholic fatty liver disease. Eur J Clin Invest 2014; 44: 65–73.
Handberg A, Højlund K, Gastaldelli A, Flyvbjerg A, Dekker JM, Petrie J et al. Plasma sCD36 is associated with markers of atherosclerosis, insulin resistance and fatty liver in a nondiabetic healthy population. J Intern Med 2012; 271: 294–304.
Heebøll S, Kreuzfeldt M, Hamilton-Dutoit S, Kjær Poulsen M, Stødkilde-Jørgensen H, Møller HJ et al. Placebo-controlled, randomised clinical trial: high-dose resveratrol treatment for non-alcoholic fatty liver disease. Scand J Gastroenterol 2016; 51: 456–464.
Ornstrup MJ, Harsløf T, Kjær TN, Langdahl BL, Pedersen SB . Resveratrol increases bone mineral density and bone alkaline phosphatase in obese men: a randomized placebo-controlled trial. J Clin Endocrinol Metab 2014; 99: 4720–4729.
Poulsen MK, Nellemann B, Stødkilde-Jørgensen H, Pedersen SB, Grønbæk H, Nielsen S . Impaired insulin suppression of VLDL-triglyceride kinetics in non-alcoholic fatty liver disease. J Clin Endocrinol Metab 2016; 101: 1637–1646.
Szczepaniak LS, Nurenberg P, Leonard D, Browning JD, Reingold JS, Grundy S et al. Magnetic resonance spectroscopy to measure hepatic triglyceride content: prevalence of hepatic steatosis in the general population. Am J Physiol Endocrinol Metab 2005; 288: E462–E468.
Ornstrup MJ, Kjær TN, Harsløf T, Stødkilde-Jørgensen H, Hougaard DM, Cohen A et al. Adipose tissue, estradiol levels, and bone health in obese men with metabolic syndrome. Eur J Endocrinol 2015; 172: 205–216.
Wallace TM, Levy JC, Matthews DR . Use and abuse of HOMA modeling. Diabetes Care 2004; 27: 1487–1495.
Handberg A, Levin K, Hojlund K, Beck-Nielsen H . Identification of the oxidized low-density lipoprotein scavenger receptor CD36 in plasma: a novel marker of insulin resistance. Circulation 2006; 114: 1169–1176.
Kleiner DE, Brunt EM, Van Natta M, Behling C, Contos MJ, Cummings OW et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology 2005; 41: 1313–1321.
Bedossa P, Poitou C, Veyrie N, Bouillot JL, Basdevant A, Paradis V et al. Histopathological algorithm and scoring system for evaluation of liver lesions in morbidly obese patients. Hepatology 2012; 56: 1751–1759.
Fabbrini E, Magkos F, Mohammed BS, Pietka T, Abumrad NA, Patterson BW et al. Intrahepatic fat, not visceral fat, is linked with metabolic complications of obesity. Proc Natl Acad Sci USA 2009; 106: 15430–15435.
Greco D, Kotronen A, Westerbacka J, Puig O, Arkkila P, Kiviluoto T et al. Gene expression in human NAFLD. Am J Physiol Gastrointest Liver Physiol 2008; 294: G1281–G1287.
Bechmann LP, Gieseler RK, Sowa JP, Kahraman A, Erhard J, Wedemeyer I et al. Apoptosis is associated with CD36/fatty acid translocase upregulation in non-alcoholic steatohepatitis. Liver Int 2010; 30: 850–859.
Sheedfar F, Sung MM, Aparicio-Vergara M, Kloosterhuis NJ, Miquilena-Colina ME, Vargas-Castrillon J et al. Increased hepatic CD36 expression with age is associated with enhanced susceptibility to nonalcoholic fatty liver disease. Aging 2014; 6: 281–295.
Krssak M, Hofer H, Wrba F, Meyerspeer M, Brehm A, Lohninger A et al. Non-invasive assessment of hepatic fat accumulation in chronic hepatitis C by 1H magnetic resonance spectroscopy. Eur J Radiol 2010; 74: e60–e66.
Knøsgaard L, Thomsen SB, Stockel M, Vestergaard H, Handberg A . Circulating sCD36 is associated with unhealthy fat distribution and elevated circulating triglycerides in morbidly obese individuals. Nutr Diabetes 2014; 4: e114.
Liani R, Halvorsen B, Sestili S, Handberg A, Santilli F, Vazzana N et al. Plasma levels of soluble CD36, platelet activation, inflammation, and oxidative stress are increased in type 2 diabetic patients. Free Radic Biol Med 2012; 52: 1318–1324.
Lykkeboe S, Larsen AL, Handberg A . Lack of consistency between two commercial ELISAs and against an in-house ELISA for the detection of CD36 in human plasma. Clin Chem Lab Med 2012; 50: 1071–1074.
Bieghs V, Verheyen F, van Gorp PJ, Hendrikx T, Wouters K, Lutjohann D et al. Internalization of modified lipids by CD36 and SR-A leads to hepatic inflammation and lysosomal cholesterol storage in Kupffer cells. PloS One 2012; 7: e34378.
Bieghs V, Wouters K, van Gorp PJ, Gijbels MJ, de Winther MP, Binder CJ et al. Role of scavenger receptor A and CD36 in diet-induced nonalcoholic steatohepatitis in hyperlipidemic mice. Gastroenterology 2010; 138: 2477–2486 e1−3.
Glatz JF, Luiken JJ, Bonen A . Membrane fatty acid transporters as regulators of lipid metabolism: implications for metabolic disease. Physiol Rev 2010; 90: 367–417.
Liang CP, Han S, Okamoto H, Carnemolla R, Tabas I, Accili D et al. Increased CD36 protein as a response to defective insulin signaling in macrophages. J Clin Invest 2004; 113: 764–773.
Steneberg P, Sykaras AG, Backlund F, Straseviciene J, Soderstrom I, Edlund H . Hyperinsulinemia enhances hepatic expression of the fatty acid transporter Cd36 and provokes hepatosteatosis and hepatic insulin resistance. J Biol Chem 2015; 290: 19034–19043.
Buque X, Cano A, Miquilena-Colina ME, Garcia-Monzon C, Ochoa B, Aspichueta P . High insulin levels are required for FAT/CD36 plasma membrane translocation and enhanced fatty acid uptake in obese Zucker rat hepatocytes. Am J Physiol Endocrinol Metab 2012; 303: E504–E514.
Nicholson AC . Expression of CD36 in macrophages and atherosclerosis: the role of lipid regulation of PPARgamma signaling. Trends Cardiovasc Med 2004; 14: 8–12.
Glintborg D, Højlund K, Andersen M, Henriksen JE, Beck-Nielsen H, Handberg A . Soluble CD36 and risk markers of insulin resistance and atherosclerosis are elevated in polycystic ovary syndrome and significantly reduced during pioglitazone treatment. Diabetes Care 2008; 31: 328–334.
Handberg A, Norberg M, Stenlund H, Hallmans G, Attermann J, Eriksson JW . Soluble CD36 (sCD36) clusters with markers of insulin resistance, and high sCD36 is associated with increased type 2 diabetes risk. J Clin Endocrinol Metab 2010; 95: 1939–1946.
Alkhatatbeh MJ, Mhaidat NM, Enjeti AK, Lincz LF, Thorne RF . The putative diabetic plasma marker, soluble CD36, is non-cleaved, non-soluble and entirely associated with microparticles. J Thromb Haemost 2011; 9: 844–851.
Gustafson CM, Shepherd AJ, Miller VM, Jayachandran M . Age- and sex-specific differences in blood-borne microvesicles from apparently healthy humans. Biol Sex Differ 2015; 6: 10.
Alkhatatbeh MJ, Enjeti AK, Acharya S, Thorne RF, Lincz LF . The origin of circulating CD36 in type 2 diabetes. Nutr Diabetes 2013; 3: e59.
Zhou D, Samovski D, Okunade AL, Stahl PD, Abumrad NA, Su X . CD36 level and trafficking are determinants of lipolysis in adipocytes. FASEB J 2012; 26: 4733–4742.
Acknowledgements
We are thankful for the skilled technical assistance provided by Lone Larsen, Aarhus University Hospital, Denmark. The study was supported by the NOVO Nordisk Foundation, Aarhus University, the Danish Council for Independent Research, Medical Sciences (11-107912); 'Savværksejer Jeppe Juhl og hustru Ovita Juhls mindelegat'. The study is part of the research program LIRMOI Research Center (www.LIRMOI.com), which is supported by the Danish Council for Strategic Research (0-093499).
Author contributions
Declaration of contribution: SH, HG and AH conceived the study. SH collected and researched data and wrote the manuscript. MKP, MO and TNK collected the data and reviewed/edited the manuscript. SN, SBP and HG assisted in study design, data interpretation and reviewed/edited the manuscript. AH assisted in collecting and researching data and writing the manuscript. AH and HG shares last co-author ship.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Heebøll, S., Poulsen, M., Ornstrup, M. et al. Circulating sCD36 levels in patients with non-alcoholic fatty liver disease and controls. Int J Obes 41, 262–267 (2017). https://doi.org/10.1038/ijo.2016.223
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ijo.2016.223
- Springer Nature Limited
This article is cited by
-
Understanding lipotoxicity in NAFLD pathogenesis: is CD36 a key driver?
Cell Death & Disease (2020)
-
Bariatric surgery reduces CD36-bearing microvesicles of endothelial and monocyte origin
Nutrition & Metabolism (2018)
-
Anthropometric and blood parameters for the prediction of NAFLD among overweight and obese adults
BMC Gastroenterology (2018)