Abstract
Fruit seeds are by-products of fruit processing industries which are rich in bioactive compounds including phenolic compounds. In this study, the efficiency of the microwave-assisted aqueous extraction (MAAE) technique on the recovery of total phenolic content (TPC) from jackfruit, jamun and papaya seeds was explored. The process with three independent parameters (microwave power, treatment time and seed powder to solvent ratio) was developed and optimized individually for all three seeds using both response surface methodology (RSM) and artificial neural network- genetic algorithm (ANN- GA) to maximize the response (TPC). The value of TPC at the RSM optimized condition was 8.79 mg GAE/100 g, 211.87 mg GAE/100 g and 31.38 mg GAE/100 g and at the ANN optimized condition were 30.09 mg GAE/100 g, 252.01 mg GAE/100 g and 37.55 mg GAE/100 g for jackfruit, jamun and papaya seed, respectively, which were significantly higher than that of the control extract. Moreover, the ANN model for all three seeds displayed better performance with higher extraction yield and fewer statistical errors as compared to RSM. Hence, the study concludes that MAAE could be an excellent alternative to extract phenolic compounds from fruit seeds with maximum yield.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Embodying a crucial role in the diet and human life, fruits and vegetables comprehend as the major contributors to the constantly growing rate of food lost and wasted every year [1]. Around one-third of all fruits and vegetables produced across the globe is wasted due to poor postharvest handling practices [2]. Major waste volumes in the form of solid and liquid from the fruits and vegetable processing industry majorly include leftovers like pomace or skin, pulp, seed, kernel etc. enumerating 10–35% of the raw mass [3]. Management of the waste generated is of utmost importance as this entails a connection of sustainability to the whole process.
Although horticultural waste constitutes higher fractions of potentially valuable bioactive components, they were not pondered as beneficial resources in the past [4]. Therefore, habitual utilization of these by-products involves the production of biomaterials, biofuel, bio-fertilizers, biogas etc. and is used also in animal feeds and landfills [5]. With a shifting scenario, the management of these by-products intends at apprehending the potential and resolving the purpose. Horticultural by-products are considered to be natural and excellent sources of organic acids, pigments, phenolic compounds, dietary fibres, sugar derivatives and other compounds [6]. Acknowledging the antioxidant, antiviral, antitumor, antimutagenic, antibacterial and cardioprotective activities of these compounds, their healthy contribution to human life is irrefutable [7].
Extraction of these bioactive components can be performed by means of different technologies and can be employed to develop different valorized products [4]. The selection of these techniques is purely based on different factors like cost effectivity, the complexity of the matrix involved, process efficiency and sustainability. Conferring to the type of matrix involved and the type of compound extracted there will be variations in the process enmeshed and procedures followed. The current study attempts to impregnate the idea of effective utilization and valorization of bioactive compounds extracted from different fruit seeds with the purpose of refining healthy course by plummeting the waste disposal burdens and extenuating the environmental problems.
Among the different bioactive components in fruit by-products, the most significant and demonstrative clusters are coined as polyphenolic compounds [8]. With molecular structure pertaining to different phenolic hydroxyl groups, they are found to be beneficial to human health accomplishing different formats such as flavonoids, tannin, phenolic acids etc. in fruit waste [9]. The most conceivable health benefits of these polyphenols include their antioxidative and anti-inflammatory properties with evidence and experiments circling around their ability to the prevention of cancer and cardiovascular diseases [7]. Lately owing to its significance, there is a hype gyrating around the research involving the extraction and characterization of these compounds.
Conventionally polyphenol extraction was performed using techniques like solid–liquid extraction, liquid–liquid extraction and other solvent-based extraction. Time period, extraction efficiency and economic feasibility of processes involved in traditional practices acts as an obstacle to their workable relevance [10]. This endorsed the practice of novel techniques like ultrasound/enzyme/microwave-assisted extraction in the separation of polyphenols from horticultural wastes [11]. Microwave-assisted aqueous extraction (MAAE) of polyphenols is advantageous over other practices due to the shorter process time, non-toxic solvent, higher extraction rates, superior extraction selectivity, and low cost. Owing to this, the current study was aimed at the effectual extraction of polyphenols from different fruit seeds using a microwave-assisted extraction technique. The specific objectives of the study are as follows: (a) to investigate the impact of MAAE technique on the extraction of total phenolic content (TPC) from jackfruit, jamun and papaya seeds; (b) to analyze the linear and collective effects of process paramerts (microwave power, treatment time and seed powder to solvent ratio) on the extracted TPC; and (c) to employ and compare response surface methodology (RSM) and artificial neural network—genetic algorithm (ANN-GA) techniques to optimize the process paraneters to extract the TPC from jackfruit, jamun and papaya seeds with maximum yield.
Materials and methods
Collection of raw materials
As per the premeditated design, fruits like jackfruit, jamun and papaya garnered from the neighbouring localities of Kottayam, Kerala, India was sourced for seed assortment. Seeds were separated from the fruit sample and were stored in plastic bags. Thorough cleaning of the seed samples was done to evade the unwanted particles followed by size reduction of larger seeds. Size reduction of larger fractions was performed to ensure the uniformity of operation. Drying of these seed fractions was operated in a tray drier at 50 °C until the constant moisture content is achieved. Dried fruit seeds were powdered in a mixer grinder and stored in a refrigerator for further experiments (4 ± 1 °C).
Microwave-assisted extraction
A microwave oven (Morphy Richards, 25 CG, India) having a constant microwave frequency of 2450 MHz, power of 1400 W and a maximum time of 30 min was used for this experiment. One gram of dried and powdered seed fractions of each fruit seed was taken as a base quantity for the extraction process. Distilled water was used as the solvent of extraction for all samples. As per the combinations integrated in the experimental design (Table 1), treatments were performed at different power, varied extraction time and different powder/solvent ratios. After the treatment, microwaved samples were filtered and collected in bottles for the analysis of phenol fractions. The initial temperature for all the treatments was kept constant at room temperature (25 ± 1 °C). The flowchart of MAAE of phenolic fractions from different fruit seeds is shown in Fig. 1 [4].
Experimental design
The present work exercises the use of Box–Behnken design (BBD) using the Design-Expert (Stat- Ease Inc., version 10.0.3.0.) software to acquire the combinations of independent variables and the number of experiments. Microwave power (X1), treatment time (X2), and seed powder: solvent ratio (X3) were the independent variables, and the TPC (Y1) was taken as the responses. The entire experiment consists of 17 runs with five centre points, as given in Table 2. The influence of process variables on the TPC for all three seed fractions was mathematically modelled using RSM and ANN.
Similarly, the control extract was prepared according to Patra et al. [8] by dipping one gram of jackfruit, jamun and papaya seed powders for 60 min in 50 mL solvent (distilled water). Later, the solvent was filtered to collect the supernatant for TPC analysis.
Determination of total phenolic content (TPC)
The TPC was analysed by Folin-Ciocalteu (FC) method given by Abdullah et al. [12] and Simsek et al. [13]. 0.1 mL of filtrate sample of each seed fraction was taken and made up to into 2 mL by using distilled water. 0.5 mL of FC reagent was added to the sample and it is kept for incubation for 3 min. This is followed by the addition of 2 mL of 7.5% Na2CO3. Then the sample was thoroughly mixed and retained in a dark room for 30 min. The absorbance values of these samples were analysed against a blank at 760 nm using a UV–Vis Spectrophotometer. Gallic acid was used as the reference standard and the TPC was expressed as milligrams of gallic acid equivalent (mg GAE) in one gram of seed powder.
Modeling
RSM modelling and numerical optimization
The RSM modelling and numerical optimization of the developed data were carried out using the design expert (Stat- Ease Inc., version 10.0.3.0.) software. The empirical regression model equation (Eq. 1) recommended by RSM was exercised to evaluate the influence of independent variables on the TPC (response) and to predict its optimal values.
where Y = Predicted value of the response, b(0,1,2,3) = Regression coefficients, X(1,2,3) = Independent variables
The developed mathematical models were evaluated for their validation and significance based on the coefficient of determination (R2), adjusted R2 (adj. R2), predicted R2 (Pred. R2) and lack of fit (LoF) and p-value with the help of ANOVA (Analysis of variance).
Similarly, numerical optimization was carried out to maximize the yield. Hence, the numerical optimization for all three seed fractions was carried out by maximizing the response (TPC) were maximized and keeping the independent variables within range.
ANN modelling and GA optimization
The ANN modelling of the experimental data was carried out using the neural network fitting tool present in MATLAB software (The Math Works, Inc., Natick, MA, version R2015a). The proposed ANN models for all three seed fractions comprise input (representing independent parameters), hidden and output (representing the response) layers with numerous neurons in them. The number of neurons in the input layer was three, representing Microwave power (X1), treatment time (X2), and seed powder: solvent ratio (X3). Similarly, the input layer is comprised of a single neuron representing TPC (Y).
The modelling and prediction of the TPC yield for jackfruit, jamun and papaya seed were executed using the Levenberg–Marquardt backpropagation algorithm and the number of neurons in the hidden layer was selected based on trial and error, as reported by [8, 14,15,16]. To begin with, the 17 experimental runs developed with the help of Box–Behnken design were randomly grouped into three, training (70% of data), testing (15% of data) and validation (15% of data). The training was performed to determine the network parameters, and testing and validation were performed to control its errors and to confirm its robustness, respectively [14, 15, 17, 18]. The best ANN network was designated based on the correlation coefficient, performance plot, coefficient of determination and mean square error.
Similarly, the independent parameters of the ANN-modelled data were optimized using GA to maximize the yield of TPC. A GA is a valuable technique for the optimization of near-linear and non-linear diverse processes in science and engineering sectors [19, 20]. To obtain the maximum TPC yield, the dependent parameter was maximized at the optimum microwave power, treatment time and powder-to-solvent ratio. In addition, the various parameters of GA selected for the current study were mutation function (adaptive feasibility), crossover function (scattered), selection function (roulette wheel), fitness scaling function (rank), creation function (feasible population), migration (forward migration), crossover fraction (0.8) and population type (double vector) [14, 15, 19, 21].
Statistical analysis
Succeeding equations were employed in calculating average absolute deviation (AAD), mean square error (MSE), root mean square error (RMSE), normalized mean square error (NMSE), normalized root mean square error (NRMSE), mean percentage error (MPE), and coefficient of determination (R2).
where xact is the experimental data, xmean is the mean experimental data, xpre is the predicted data, and n is the no of experiments. The model with minimum values of AAD, MSE, RMSE, NMSE, NRMSE, MPE and maximum R2 value was taken as the best model for response expression.
The statistically significant difference between experimental and predicted results from the optimized condition was statically scrutinized via the Duncan homogeneity test by SPSS software 23.0. All the experiments were conducted in triplicate, and data were reported as mean ± standard deviation (SD).
Results and discussion
Response surface methodology
Model fitting
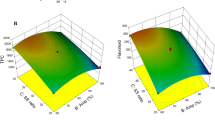
The impact of three process parameters such as microwave power, treatment time and seed powder to solvent ratio on the TPC extraction of jackfruit, jamun and papaya seeds are tabulated in Table 2. The maximum TPC was obtained at 700 W microbial power, 75 s and 30 seed powder to the solvent ratio for jackfruit, 700 W microbial power, 120 s and 25 seed powder to the solvent ratio for Jamun and 1050 W microbial power, 30 s and 20 seed powder to the solvent ratio for papaya seed. Also, the experimental results were employed to create second-degree polynomial models (Eqs. 9–11) with the help of nonlinear multiple regression analysis.
where TPCJackfruit, TPCJamun and TPCPapaya denote the total phenolic content of jackfruit, jamun and papaya seeds, respectively. Similarly, X1, X2 and X3 represent microwave power, treatment time and seed powder-to-solvent ratio, respectively.
Correspondingly, the regression coefficient and the corresponding R2, adjusted R2, predicted R2 and lack of fit of the developed quadratic models are given in Table 3. These values signify the adequacy of the developed models. The regression models could be acceptable if its R2 is > 0.8 [14, 15] and the deviation between adjusted and predicted R2 is < 0.2. Here, the models were statistically significant (p < 0.05) with a non-significant lack of fit (p > 0.05). Also, for all three models, the R2 value was more than 0.9 and the difference between adjusted R2 and predicted R2 was less than 0.2. These results suggest that the selected models are acceptable with a reasonable agreement with the experimental outcomes.
Effect of process parameters on TPC
The impact of process parameters (microwave power, treatment time and seed powder to solvent ratio) on the TPC extraction of the jackfruit, jamun and papaya seeds were represented by the coefficient of significance (p < 0.05) of the developed quadratic model. From Table 3, the first-order linear effect of all the process parameters, such as microwave power (X1), treatment time (X2) and seed powder to the solvent ratio (X3), the second-order quadratic effect of treatment time (X22) and the interactive effect of treatment time and seed powder to the solvent ratio (X2X3) has influenced the TPC extraction of the jackfruit seed significantly (p < 0.05). However, the linear effect of microwave power (X1) and the quadratic effect of treatment time (X22) had a negative correlation and all the other parameters have a positive correlation with the TPC. Similarly for jamun seeds, the first-order linear effect and the second-order quadratic effect of microwave power (X1 and X12), and the interactive effect of all the process parameters (X1X2, X1X3 and X2X3 had a significant effect on the TPC. Here, the interactive effect of microwave power and seed powder to the solvent ratio (X1X3) and treatment time and seed powder to the solvent ratio (X2X3) impacted positively and the remaining three parameters impacted negatively on the TPC of the jamun seed.
By the same token, the first-order linear effect of microwave power (X1) and treatment time (X2), the second-order quadratic effect of seed powder to the solvent ratio (X32) and the interactive effect of treatment time and seed powder to the solvent ratio (X2X3) had a significant effect on the TPC extraction of papaya seeds (p < 0.05). Though the second-order quadratic effect of seed powder to the solvent ratio (X22) and the interactive effect of treatment time and seed powder to the solvent ratio (X2X3) impacted positively, the linear effect of microwave power (X1) and treatment time (X2) had a significant negative impact on the TPC. In general, for all three seeds, the linear effect of the microwave power showed a significant negative effect and the interaction effect of treatment time and seed powder to the solvent ratio (X2X3) showed a significant positive effect on the yield of TPC. The positive influence of the determined factors represents that their positive changes can result in an increase in the yield of TPC and vice versa. The negative effect of the microwave power might be due to the longer treatment time which might result in the thermal degradation of certain phenolic compounds, as reported by Sahin et al. [22]. Simsek et al. [13] also found that the TPC might decrease due to thermal degradation after certain exposure time during microwave treatment. Correspondingly, the negative effect of the interaction between microwave power and treatment time on the yield of TPC is also due to the thermal degradation as a result of the synergic effect of high microwave power and treatment time. Similarly, Patra et al. [23] and Bai et al. [24] also observed a similar interaction impact (positive) of treatment time and sample-to-solvent ratio on the TPC during microwave extraction. This increase in the TPC extraction yield is because the longer treatment time breaks maximum seed cell walls [25] and the rise in the solvent amount increases the concentration gradient in and out of the seed cell wall [26].
On the other hand, the interactive terms X1X2 and X1X3 and the second-order quadratic terms X12 and X32 for the jackfruit seed, the linear terms X2 and X3 and the quadratic terms X22 and X32 for the jamun seed and the linear term X3, the interactive terms X1X2 and X1X3 and the quadratic terms X12 and X32 for the papaya seeds do not have a significant influence on the yield of TPC. Hence the developed models were reformed and simplified by eliminating the insignificant factors that are given in the Eqs. (12–14).
where TPCJackfruit, TPCJamun and TPCPapaya denote the TPC of jackfruit, jamun and papaya seeds, respectively. Similarly, X1, X2 and X3 represent microwave power, treatment time and seed powder-to-solvent ratio, respectively.
Numerical optimization
Numerical optimization from the design expert software was used to optimize the process parameters for the extraction of TPC with maximum yield. For this purpose, the process parameters of all the seeds were provided with the importance of ‘5’ and kept ‘in range’, i.e., seed powder to solvent ratio from 20 to 30 g/mL, treatment time from 30 to 120 s and microwave power from 700 to 1400 W. Whereas the TPC (response) was maximized and the combination with maximum desirability was selected as the optimum condition. The attained optimum conditions were 720 W microwave power, 97 s treatment time and 1:29 seed powder to the solvent ratio for jackfruit seed, 747 W microwave power, 109 s treatment time and 1:22 seed powder to the solvent ratio for jamun seed and 948 W microwave power, 34 s treatment time and 1:20 seed powder to the solvent ratio for papaya seed, respectively. The predicted TPC at these optimized conditions were 28.79 mg GAE/100 g, 211.87 mg GAE/100 g and 31.38 mg GAE/100 g for jackfruit, jamun and papaya seed, respectively. Further, these predicted results were validated by conducting experiments at these optimum conditions and confirming that there is no significant difference between the predicted and experimental yield of TPC.
ANN modelling
Model fitting
The optimum ANN model architecture for the TPC of all the seed extracts involves one input layer, one hidden layer and one output layer with three, ten and one neurons respectively as presented in Fig. 2. To begin with, the 17 experimental runs developed with the help of Box–Behnken design were randomly grouped into three, training (70% of data), testing (15% of data) and validation (15% of data). The training was performed to determine the network parameters, and testing and validation were performed to control its errors and to confirm its robustness, respectively [14, 15, 17, 18]. For jackfruit seeds, the experimental runs 2, 5, 6, 7, 8, 11, 12, 13, 14, 15 and 17 of Table 2 were used for training, 1, 3 and 9 for validation and 4, 10 and 16 for testing, respectively. The MSE values of the training, validation and testing were 0.2341, 0.2692 and 1.9203 and R values for these runs were 0.9914, 0.9999 and 0.9035, respectively. Similarly, for jamun seeds, the experimental runs 1, 3, 5, 6, 8, 9, 10, 11, 14, 15 and 17 of Table 2 were used for training, 4, 12 and 13 for validation and 2, 7 and 16 for testing, respectively. The corresponding MSE and R values for training, validation and testing were 34.9623 and 0.9767, 77.7139 and 0.9955, and 3.2725 and 0.9984, respectively. By the same token, for papaya seeds, the experimental runs 1, 2, 3, 5, 6, 7, 8, 9, 12, 15 and 16 of Table 2 were used for training, 4, 11 and 14 for validation and 10, 13 and 17 for testing, respectively. The corresponding MSE and R values for training, validation and testing were 0.1270 and 0.9944, 5.2781 and 0.9729, and 2.6957 and 0.8923, respectively.
The accuracy of the developed model to predict the unknown data is confirmed by its maximum R2 and minimum MSE value [19, 21]. The regression analysis and the error histogram of the developed ANN models are given in Fig. 3. The error histogram illustrates that the majority of data has an error of − 0.0034 for jackfruit seed (Fig. 3b), − 0.5509 for jamun seed (Fig. 3d) and 0.0717 for papaya seed (Fig. 3f), respectively, which is very close to zero. Similarly, the regression analysis given in Fig. 3a (for jackfruit seed), Fig. 3c (for jamun seed) and Fig. 3e (for papaya seed) explains how well the experimental and predicted values fit together. The TPC of all three seed extracts predicted by the ANN model is tabulated in Table 2. This study achieved better overall R2 and MSE values of 0.9586 and 0.5379, 0.9537 and 36.9047, and 0.9078 and 1.4893 for the TPC yield of jackfruit, jamun and papaya seeds, respectively. A good agreement exists between the experimental and predicted data confirming the reliability of the model fit. In addition, the weights and bias values of the developed ANN models for all three seeds are given in the following matrices (Eqs. 15 to 25).
where U is the connection weight between the input and hidden layer, W is the connection weight between the hidden and output layer, TH is the threshold value between the input to the hidden layer and TO is the threshold value between the hidden to the outer layer, respectively and suffix a, b and c signifies the jackfruit, jamun and papaya seeds, respectively.
GA optimization
The GA optimization comprises a repeated cycle of processes involving reproduction, crossover, and mutations till the lowest RSME and MSE values between the individual fitness and mean values are attained. This condition was accomplished after the 150th generation with a mean fitness value of − 30.0937 for jackfruit seed (Fig. 4a), after the 80th generation with a mean fitness value of − 251.998 for jamun seed (Fig. 4b) and after the 180th generation with a mean fitness value of − 37.32 for papaya seed, respectively. The optimum process conditions obtained at this best fitness points were 731 W microwave power, 94 s treatment time and 1:30 seed powder to the solvent ratio for jackfruit seed, 745 W microwave power, 120 s treatment time and 1:20 seed powder to the solvent ratio for jamun seed and 901 W microwave power, 47 s treatment time and 1:25 seed powder to the solvent ratio for papaya seed, respectively. The predicted TPC at these optimized conditions were 30.09 mg GAE/100 g, 252.01 mg GAE/100 g and 37.55 mg GAE/100 g for jackfruit, jamun and papaya seed, respectively. Similar to numerical optimization, these predicted results were further validated by conducting experiments at these optimum conditions and confirming that there is no significant difference between the predicted and experimental yield of TPC.
Model comparison
The performance of the generated models (ANN and RSM) was compared employing statistical indicators such as AAD, MPE, MSE, RMSE, NMSE, NRMSE and R2 which is tabulated in Table 4. An R2 > 0.8 and a minimum AAD, MPE, MSE, RMSE, NMSE, and NRMSE value are desirable for the best model [27, 28]. It is clear from Table 4 that the R2 values of the generated RSM and ANN models for jackfruit, jamun and papaya seeds were more than 0.9 and AAD, MPE, MSE, RMSE, NMSE and NRMSE were minimum, which endorses the validity of the generated models.
Furthermore, while comparing the models, the R2 values of the developed ANN models for jackfruit, jamun and papaya seeds were higher than that of the RSM models. Correspondingly, for all three seeds, the least AAD, MPE, MSE, RMSE, NMSE and NRMSE values were achieved for ANN compared to RSM models. This indicates the ANN data has fewer errors as compared to RSM and hence the generated ANN models can explain the data sets better than the RSM models. In addition, for all three seeds, the yield of TPC obtained at the ANN-GA optimized conditions is comparatively higher than at the RSM optimized conditions (depicted in Table 5). Therefore, the models developed using the ANN technique exhibited dominance and were found better than the RSM models for the MAAE of TPC from jackfruit, jamun and papaya seeds. Pradhan et al. (2022a) compared both the models and reported that the ANN is dominant over RSM technique in predicting and optimizing the process parameters. A similar observation was reported by various other researchers while comparing RSM and ANN techniques [17,18,19, 29, 30]
In the same way, the yield of TPC achieved at the optimum conditions of ANN-GA and RSM techniques was compared with the yield obtained from the control extract and displayed in Table 5. The yield of TPC obtained from the control extract for jackfruit, jamun and papaya seeds were 25.12, 196.09 and 25.60 mg GAE/100 g, respectively, which is a minimum of 14.59%, 8.05% and 22.54% less than the yield obtained for the microwave-assisted extract of jackfruit, jamun and papaya seeds, respectively. These results indicate that MAAE can be explored as an alternate technique to extract phenols from jackfruit, jamun and papaya seeds.
Conclusion
The influence of microwave and its process parameters on the TPC extraction from jackfruit, jamun and papaya seeds were determined, modelled and optimized using ANN-GA and RSM. The optimum process condition obtained by RSM to maximize the TPC yield for jackfruit seed was 720 W microwave power, 97 s treatment time and 1:29 seed powder to solvent ratio, for jamun seed, the optimized condition was 747 W microwave power, 109 s treatment time and 1:22 seed powder to solvent ratio and for papaya seed, it was 948 W microwave power, 34 s treatment time and 1:20 seed powder to the solvent ratio, respectively. Also, the TPC at these optimized conditions were 28.79 mg GAE/100 g, 211.87 mg GAE/100 g and 31.38 mg GAE/100 g for jackfruit, jamun and papaya seed, respectively. Similarly, the optimum process condition obtained by ANN-GA for jackfruit seed was 731 W microwave power, 94 s treatment time and 1:30 seed powder to solvent ratio, for jamun seed 745 W microwave power, 120 s treatment time and 1:20 seed powder to solvent ratio, and papaya seed, it was 901 W microwave power, 47 s treatment time and 1:25 seed powder to solvent ratio, respectively. Correspondingly the TPC at these optimized conditions were 30.09 mg GAE/100 g, 252.01 mg GAE/100 g and 37.55 mg GAE/100 g for jackfruit, jamun and papaya seed, respectively. The predictability of both the generated models was good, but, ANN was found to be a better model with minimum MPE, MSE, NMSE, RMSE, NRMSE, AAD and maximum R2 values. Besides, the TPC yield of microwave treated extract was found significantly higher than the control extract. Therefore, the study concludes that microwave treatment can be utilized as a preferred technique to extract phenolic compounds from fruit seeds and also ANN-GA is a superior technique to perform modelling and optimizing process parameters than RSM.
Abbreviations
- MAAE:
-
Microwave-assisted aqueous extraction
- RSM:
-
Response surface methodology
- ANN-GA:
-
Artificial neural network- genetic algorithm
- TPC:
-
Total phenolic content
- GAE:
-
Gallic acid equivalent
- BBD:
-
Box-Behnken design
- FC:
-
Folin-Ciocalteu
- ANOVA:
-
Analysis of variance
- Y:
-
Predicted value of the response
- b:
-
Regression coefficients
- X:
-
Independent variables
- AAD:
-
Average absolute deviation
- MSE:
-
Mean square error
- RMSE:
-
Root mean square error
- NMSE:
-
Normalized mean square error
- NRMSE:
-
Normalized root mean square error
- MPE:
-
Mean percentage error
- R2 :
-
Coefficient of determination
- SD:
-
Standard deviation
References
P. Rao, V. Rathod, Valorization of food and agricultural waste: a step towards greener future. Chem. Rec. 19(9), 1858–1871 (2019)
N.A. Sagar, S. Pareek, S. Sharma, E.M. Yahia, M.G. Lobo, Fruit and vegetable waste: bioactive compounds, their extraction, and possible utilization. Compr. Rev. Food Sci. Food Saf. 17(3), 512–531 (2018)
J. Majerska, A. Michalska, A. Figiel, A review of new directions in managing fruit and vegetable processing by-products. Trends Food Sci. Technol. 88, 207–219 (2019)
A. Patra, S. Abdullah, R.C. Pradhan, Review on the extraction of bioactive compounds and characterization of fruit industry by-products. Bioresour. Bioprocess. (2022). https://doi.org/10.1186/s40643-022-00498-3
J. Banerjee, R. Singh, R. Vijayaraghavan, D. MacFarlane, A.F. Patti, A. Arora, Bioactives from fruit processing wastes: green approaches to valuable chemicals. Food Chem. 225, 10–22 (2017)
S. Pathania, N. Kaur, Utilization of fruits and vegetable by-products for isolation of dietary fibres and its potential application as functional ingredients. Bioactive Carbohydr. Diet. Fibre 27, 100295 (2022)
H.H. Wijngaard, C. Rößle, N. Brunton, A survey of Irish fruit and vegetable waste and by-products as a source of polyphenolic antioxidants. Food Chem. 116(1), 202–207 (2009)
A. Patra, S. Abdullah, R.C. Pradhan, Optimization of ultrasound-assisted extraction of ascorbic acid, protein and total antioxidants from cashew apple bagasse using artificial neural network-genetic algorithm and response surface methodology. J. Food Process. Preserv. 46(3), 1–17 (2022). https://doi.org/10.1111/jfpp.16317
J. Ou, M. Wang, J. Zheng, S. Ou, Positive and negative effects of polyphenol incorporation in baked foods. Food Chem. 284, 90–99 (2019)
R. Romeo, A. De Bruno, V. Imeneo, A. Piscopo, M. Poiana, Evaluation of enrichment with antioxidants from olive oil mill wastes in hydrophilic model system. J. Food Process. Preserv. 43(11), e14211 (2019)
H. Cai, S. You, Z. Xu, Z. Li, J. Guo, Z. Ren, C. Fu, Novel extraction methods and potential applications of polyphenols in fruit waste: a review. J. Food Meas. Charact. 15(4), 3250–3261 (2021)
S. Abdullah, S. Karmakar, S. Mishra, R.C. Pradhan, Ultrafiltration of cashew apple juice using hollow fibers for shelf life extension: process optimization, flux modelling and storage study. J. Food Meas. Charact. (2022). https://doi.org/10.1007/s11694-022-01790-8
M. Simsek, G. Sumnu, S. Sahin, Microwave assisted extraction of phenolic compounds from sour cherry pomace. Sep. Sci. Technol. 47(8), 1248–1254 (2012)
S. Abdullah, R.C. Pradhan, S. Mishra, Effect of cellulase and tannase on yield, ascorbic acid and other physicochemical properties of cashew apple juice. Fruits 76(2), 51–60 (2021). https://doi.org/10.17660/TH2021/76.2.1
S. Abdullah, R.C. Pradhan, D. Pradhan, S. Mishra, Modeling and optimization of pectinase-assisted low-temperature extraction of cashew apple juice using artificial neural network coupled with genetic algorithm. Food Chem. 339, 127862 (2021). https://doi.org/10.1016/j.foodchem.2020.127862
J.L. Pilkington, C. Preston, R.L. Gomes, Comparison of response surface methodology (RSM) and artificial neural networks (ANN) towards efficient extraction of artemisinin from Artemisia annua. Ind. Crops Prod. 58, 15–24 (2014). https://doi.org/10.1016/j.indcrop.2014.03.016
S. Jaddu, S. Abdullah, M. Dwivedi, R.C. Pradhan, Optimization of functional properties of plasma treated kodo millet (open air multipin) using response surface methodology (RSM) and artificial neural network with genetic algorithm (ANN-GA). J. Food Process. Eng. (2022). https://doi.org/10.1111/jfpe.14207
S. Jaddu, S. Abdullah, M. Dwivedi, R.C. Pradhan, Multipin cold plasma electric discharge on hydration properties of kodo millet flour: modelling and optimization using response surface methodology and artificial neural network—genetic algorithm. Food Chem. 5, 100132 (2022). https://doi.org/10.1016/j.fochms.2022.100132
G. Patel, A. Patra, S. Abdullah, M. Dwivedi, Indian jujube (Ziziphus mauritiana L.) fruit juice extraction using cellulase enzyme: Modelling and optimization of approach by ANN-GA. Appl. Food Res. 2(1), 100080 (2022). https://doi.org/10.1016/j.afres.2022.100080
D. Pradhan, S. Abdullah, R.C. Pradhan, Optimization of pectinase assisted extraction of chironji (Buchanania lanzan) fruit juice using response surface methodology and artificial neural network. Int. J. Fruit Sci. 20(S2), S318–S336 (2020). https://doi.org/10.1080/15538362.2020.1734895
D. Pradhan, S. Abdullah, C. Pradhan, Chironji (Buchanania lanzan) fruit juice extraction using cellulase enzyme: modelling and optimization of process by artificial neural network and response surface methodology. J. Food Sci. Technol. (2020). https://doi.org/10.1007/s13197-020-04619-8
S. Sahin, R. Samli, Z. Birteks, A.S. Tan, F.J. Barba, F. Chemat, G. Cravotto, J.M. Lorenzo, Solvent-free microwave-assisted extraction of polyphenols from olive tree leaves: antioxidant and antimicrobial properties. Molecules (2017). https://doi.org/10.3390/molecules22071056
A. Patra, S. Abdullah, R.C. Pradhan, Microwave-assisted extraction of bioactive compounds from cashew apple (Anacardium occidenatale L.) bagasse: modeling and optimization of the process using response surface methodology. J. Food Meas. Charact. 15(5), 4781–4793 (2021)
X.L. Bai, T.L. Yue, Y.H. Yuan, H.W. Zhang, Optimization of microwave-assisted extraction of polyphenols from apple pomace using response surface methodology and HPLC analysis. J. Sep. Sci. 33(23–24), 3751–3758 (2010). https://doi.org/10.1002/jssc.201000430
O.R. Alara, N.H. Abdurahman, Microwave-assisted extraction of phenolics from Hibiscus sabdariffa calyces: kinetic modelling and process intensification. Ind. Crops Prod. 137, 528–535 (2019)
T.V. Fonteles, A.K.F. Leite, A.R.A. da Silva, F.A.N. Fernandes, S. Rodrigues, Sonication effect on bioactive compounds of cashew apple bagasse. Food Bioprocess. Technol. 10(10), 1854–1864 (2017)
H.N. Sin, S. Yusof, N.S.A. Hamid, R.A. Rahman, Optimization of enzymatic clarification of sapodilla juice using response surface methodology. J. Food Eng. 73(4), 313–319 (2006). https://doi.org/10.1016/j.jfoodeng.2005.01.031
K. Vivek, S. Mishra, R.C. Pradhan, Optimization of ultrasound-assisted enzymatic extraction of Sohiong (Prunus nepalensis) juice. J. Food Process. Eng. 42(1), 1–9 (2018). https://doi.org/10.1111/jfpe.12948
H. Nourbakhsh, Z. Emam-Djomeh, M. Omid, H. Mirsaeedghazi, S. Moini, Prediction of red plum juice permeate flux during membrane processing with ANN optimized using RSM. Comput. Electron. Agric. 102, 1–9 (2014). https://doi.org/10.1016/j.compag.2013.12.017
J. Prakash Maran, V. Sivakumar, K. Thirugnanasambandham, R. Sridhar, Artificial neural network and response surface methodology modeling in mass transfer parameters predictions during osmotic dehydration of Carica papaya L. Alex. Eng. J. 52(3), 507–516 (2013). https://doi.org/10.1016/j.aej.2013.06.007
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Manoj, A.A., Fathima, A., Naushad, B. et al. Valorization of fruit seeds by polyphenol recovery using microwave-assisted aqueous extraction: modelling and optimization of process parameters. Food Measure 17, 4280–4293 (2023). https://doi.org/10.1007/s11694-023-01955-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11694-023-01955-z