Abstract
Many fungi have inner-polysaccharides (IPS) that can modulate the immune response and/or have antioxidant activity. Ganoderma australe, a species from Patagonia, is a likely candidate to produce bioactive IPS. Our aims were to assess the ability of its IPS to induce maturation and activation of dendritic cells (DCs), to determine their antioxidant activity, and to evaluate the influence of the chemical composition on the bioactivity of the IPS. Mycelia were cultivated in different liquid culture media (M1, M2, and AG), and polysaccharides were extracted with hot water. Mice bone marrow-derived DCs were exposed to the different IPS, and the percentage of cells expressing CD86, MHC II, and IL-12 was determined. The antioxidant activity was evaluated through four assays. Results showed that the chemical composition of the three extracts was different. Glucose, mannose, and galactose prevail in all the extracts, although in different proportions. IPS from mycelia growing in M1 (IPS_M1) significantly increased the expression levels of MHC II and CD86 in a concentration-dependent manner, and the same tendency was found for IL-12 production. This IPS extract was mainly composed of glucose. Only IPS obtained from mycelia growing in M2 (IPS_M2) showed significant antioxidant activity, possibly related to the presence of a greater amount of phenolic compounds in this extract. This is the first study reporting the ability of aqueous extracts of mycelial polysaccharides from G. australe to maturate and activate mouse DCs, as well as to prevent oxidation, encouraging further studies of the immunomodulatory and antioxidant effects of these compounds.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Medicinal mushrooms have been used to improve human health and treat several diseases for centuries, and certain extracts have been used by Traditional Chinese Medicine for thousands of years [1]. Fungi have different chemical compounds that can act as exogenous biological response modifiers (BRMs). These BRM, such as polysaccharides and proteins, have been successful as anti-tumor, anti-radiation, anti-inflammation, anti-fatigue, anti-aging agents, and as immunomodulators [2,3,4].
Ganoderma species are the most widely used and studied among the over 2000 medicinal mushrooms reported [5, 6]; some species of this genus have polysaccharides with proven effects related to anticancer activity, immunomodulation [7,8,9,10,11], and antioxidant activity [12, 13]. Particularly, some in vitro studies in human and other mammalian cells and in vivo studies in rats and humans have shown that extracts or purified polysaccharides of G. lucidum have an immunomodulatory effect [14,15,16].
Several studies have shown the modulation of the immune system by mushroom-derived polysaccharides [17]. Particularly important is the modulation of antigen-presenting cells, such as dendritic cells (DCs), because they link innate and adaptive immunity. When the DCs capture an antigen, they become mature and develop several phenotypic changes that will trigger an effective immune response. One of these changes is associated with the up-regulation of co-stimulatory molecules, such as CD40, CD80, CD86, and those of MHC (Major Histocompatibility Complex) class II, which are important for priming lymphocyte T (T cells). Also, depending on the antigen encountered and the different signals that they receive from the environment, DCs can be activated to induce either an immunogenic or a tolerogenic response. For example, the former occurs when, through the release of IL-12, DCs promote the polarization of the immune response to a pro-inflammatory T helper1 profile. The latter occurs, for instance, through the generation of regulatory T cells [18,19,20]. Hence, DCs have a fundamental role in the initiation of the immune response; this is why their handling is an attractive point to generate a desired immune response. The identification of substances that can shape DC phenotypes and their subsequent actions, like the ability to trigger specific T cell responses, is a growing field of study [21]. The maturation and activation of DCs may occur through the stimulation of different receptors [22]. In this sense, natural ligands, such as certain polysaccharides, may induce the activation of DCs, stimulating their chemokine and cytokine production [23, 24].
Free radicals are produced in the normal natural cellular metabolism, mostly in the form of reactive oxygen species (ROS); although they play important physiological roles, the excess of ROS can result in diseases such as cancer, immune system impairment, and others, and in the acceleration of aging [25, 26]. Hence, once produced, most of them are neutralized by cellular antioxidant systems, but when excessive oxidative stress occurs, the natural antioxidant system is not able to neutralize their deleterious effects. In this sense, the identification of new natural products with antioxidant compounds is an interesting area of research, and several studies have demonstrated that the consumption of antioxidants reduces the risks of diverse diseases [26]. Polysaccharides are reported as effective free radical scavengers and antioxidants, and have been employed as antioxidants both in vitro and in vivo [27].
Most of the studies about the effects of polysaccharide extracts on immunomodulation and about antioxidant activities were performed with polysaccharides extracted from basidiomata [8, 28]. Nevertheless, submerged cultivation is an alternative for the production of polysaccharides. This type of culture involves growing mycelia in a defined medium, in a short time and under controlled conditions. In this sense, it has been reported that mycelia from Ganoderma can produce polysaccharides with significant biological activities [29]. Considering that mycelia growing in different culture media can produce polysaccharides with different characteristics and/or properties [30] that might, consequently, modify their potential biological activity, it is important to evaluate the composition of the culture medium and of the polysaccharide. Most of the bioactive polysaccharides reported are composed of α/β-glucans, glycoproteins, and water-soluble heteropolysaccharides with glucose, mannose, galactose, fucose, xylose, and arabinose [31, 32].
Ganoderma australe, commonly found in native forests of Patagonia, is another species of this genus that has medicinal properties [33, 34]. Although this species is also present in Australia, New Zealand, and Malaysia [35], it has not been thoroughly studied. Indeed, there are no studies about the potential effects of polysaccharides from mycelia cultures on immune response, and only de Melo et al. [11] reported that a polysaccharide from its basidiomata is a BRM that increases phagocytic activity and the production of IL-6 in mouse macrophages. Regarding antioxidant activity, G. australe ethanolic extracts from basidiomata and mycelia were reported to have antioxidant activity [36,37,38], but the role of polysaccharides as antioxidants was not evaluated.
The objectives of this study were to determine whether aqueous inner-polysaccharide (IPS) extracts from submerged culture of G. australe produced an immunostimulatory and/or antioxidant effect and, if so, to determine the chemical composition of the extracts. Specifically, we aimed (a) to evaluate and compare the potential effect of IPS extracted with hot water from G. australe growing in three different culture media on the maturation/activation of dendritic cells (DCs), (b) to evaluate and compare the potential antioxidant activity of IPS extracted with hot water from G. australe growing in three different culture media, and (c) to determine the chemical composition (percentage of carbohydrate, protein, phenolic and uronic acids, and monosaccharide composition) of IPS extracted with hot water from G. australe growing in three different culture media, and their possible relation with their biological activity.
Materials and methods
Chemicals, strain, media, sample preparation and culture
Chemicals
Monoclonal antibodies, Brefeldin A, and cytofix/cytoperm and perm/wash were purchased from BD Biosciences, eBioscience, and Biolegend. LPS, free radical 1,1-diphenyl-2-picryl-hydrazyl (DPPH·), 6-hydroxy- 2,5,7,8-tetramethylchroman-2-carboxylic acid (Trolox), 2,2 azino bis (3-etheylbenzothiazoline-6-sulphonicacid) diammonium salt (ABTS) radical cation, and 5,6-bis (4-phenyl-sulfonic acid)-1,2,4-triazine (Ferrozine) were purchased from SigmaAldrich, St. Louis, MO, USA. All other chemicals and reagents used were of the highest commercially available purity.
Fungal strain
A Ganoderma australe strain was obtained from the culture collection of CIEFAP (CIEFAPcc 657). This strain had been isolated in 2016 from Nothofagus obliqua forests in Lanin National Park, Yuco, Neuquén, Argentina (Lat.: − 40.09974; Long.: − 71.31527; Alt.: 657.5 m.a.s.l.). The strain was cultured on MEA (malt extract 2%, agar 1.5%) plates at 23 °C.
Media
To find a suitable culture medium for the production of polysaccharides with potential immunomodulatory and/or antioxidant effect, three culture media were evaluated (M1, M2, and AG). M1 contained 3 g/l glucose, 7 g/l sucrose, 3 g/l yeast extract, 5 g/l meat peptone, 0.5 g/l KH2PO4, 0.1 g/l K2HPO4, and 0.5 g/l MgSO4⋅7H2O. M2 contained 20 g/l maltose and 4 g/l meat peptone. AG contained 10 g/l glucose, 4 g/l asparagine, 0.5 g/l KH2PO4, 0.6 g/l K2HPO4, 0.5 g/l MgSO4⋅7H2O, 0.4 mg/l CuSO4⋅5H2O, 0.09 mg/l MnCl2⋅4H2O, 0.07 mg/l H3BO3, 0.02 mg/l Na2MoO4⋅2H2O, 2.5 mg/l ZnCl2, 1 mg/l FeCl3, 0.1 mg/l thiamine hydrochloride, 0.005 mg/l biotin. The pH values of the media were 6.3, 6.6, and 7 for M1, M2, and AG, respectively.
Sample preparation and culture
A 10-day-old culture of G. australe growing on MEA plates was used as inoculum, and it was transferred by punching out a 10 mm surface agar plug to a 125 ml Erlenmeyer flask containing 50 ml of one of the three liquid culture media. For each culture media, nine Erlenmeyer flasks were used. The cultures were statically grown in the dark, at 20 °C (± 2 °C) for 30 days. Six Erlenmeyer flasks were used to obtain the IPS, while the other three were used to determine mycelia dry weight, by drying the mycelia at 40 °C until constant weight. Uninoculated Erlenmeyer flasks served as controls for possible preexisting polysaccharides contained in the culture media.
Extraction of polysaccharides
Mycelia mats were separated from the culture broth by vacuum filtration using a nitrocellulose filter with a pore size of 5 µm. The extraction procedure of IPS was slightly modified from the method of Berovič et al. [39]. To eliminate low-molecular weight components, mycelia mats were washed with methanol (2 × 1.5 h, with stirring). Then, mycelia mats were cut with a scalpel, and IPS were extracted with hot distilled sterile water (T = 90 − 100 °C, 3 h with stirring). The resulting filtered culture was concentrated by a rotary evaporator at 50 °C and at reduced pressure, and 3 volumes of 96% cold ethanol were added. The solution was mixed and left overnight at 4 °C. The precipitate was centrifuged at 3500 rpm for 20 min, and the supernatant was discarded. Then, the precipitate was washed with acetone and centrifuged at 3500 rpm for 5 min. The precipitated IPS were air-dried to remove residual acetone and re-dissolved in sterile distilled water. To further purify the IPS extracts, a 48 h dialysis against tap water and a 24 h dialysis against distilled water were performed, using a cellulose membrane (cut-off Mw 14,000 Da).
Immunomodulation assays with IPS extracts
Mice
Female C57BL/6 wild-type mice were acquired from Ezeiza Atomic Center (Buenos Aires, Argentina). They were kept under specific pathogen-free conditions and used at 8–10 week of age. Animal protocols complied with the Guide for the Care and Use of Laboratory Animals published by the U.S. National Institutes of Health and local institutional animal care committee guidelines.
Preparation and culture of bone marrow-derived DC
To obtain immature DCs (iDCs), bone marrow was flushed from the femur and tibia of C57BL/6 female mice, and bone marrow progenitors were cultured in RPMI 1640 medium, with 10% fetal bovine serum (FBS), in the presence of granulocyte-macrophage colony-stimulating factor (GM-CSF) obtained from the supernatant of the J558 hybridoma cell line. Cells were fed every 2 days.
To obtain mature DCs (mDCs), at day 8, iDCs (1 × 106) were incubated in 24-well culture plates, with IPS extracts (IPS_M1, IPS_M2, and IPS_AG) previously filtered with 0.2 µm nylon membrane, at different concentrations (30 and 100 µg/ml) at 37 °C in a humidified atmosphere of 5% CO2 in air. Alternatively, iDCs were incubated with Gram-negative bacteria cell-wall agent lipopolysaccharide (LPS: 0.1 µg/ml, Escherichia coli strain 0111:B4) as a positive control [40]; well culture plates with iDCs and without polysaccharides were used as control (basal). After 24 and 48 h, DCs were collected and washed.
Flow cytometric analyses
The ability of IPS extracts to induce the maturation of iDCs was evaluated and compared with the effects achieved in assays without polysaccharides (basal) and with LPS (positive control). The induction of maturation was determined by the quantification of the percentage of DCs, using CD11c as a marker for this specific population, expressing MHC II and CD86 after 48 h or 24 h respectively. The activation status was assessed by the quantification of the percentage of IL-12 producing CD11c + cells.
DCs were washed twice with PBS and resuspended (1 × 106 cells/ml) in 2% FBS-PBS. Then, cells were incubated with the following fluorochrome-conjugated monoclonal antibodies (mAbs) at 4 °C for 30 min: Allophycocyanin (APC)-anti-CD11c, Phycoerythrin (PE)-Texas Red-anti-IA/IE (MHC II), and PECy7-anti-CD86. For intracellular cytokine staining, DCs were treated with Brefeldin A (10 μg/ml) for the last 4 h of cell culture. Subsequently, cells were fixed and permeabilized using Cytofix/Cytoperm and Perm/Wash according to the manufacturer's instructions; after that, cells were stained with PE-conjugated anti-IL-12 mAb. Then, cells were processed in BD FACSCanto II and/or BD LSR Fortessa flow cytometers (BD Biosciences), and data were analyzed using the FlowJo software (Tree Star, Ashland, OR, USA).
In vitro antioxidant activity of IPS extracts
To test the reproducibility of each of the assays, each single sample extract was performed in triplicate. The absorbance in the different assays was measured using a UV–Visible spectrophotometer (Biotraza model 752).
DPPH radical scavenging activity
The assay was carried out according to Blois [41], with modifications. Each polysaccharide extract (0.25–2.5 mg/ml, 1 ml) in distilled water was mixed with 2 ml freshly prepared ethanol solution containing 0.1 mM DPPH. The reaction mixture was vortexed and kept in the dark at room temperature for 24 h. The absorbance of the resulting solution was measured spectrophotometrically at 517 nm against the control or blank. Ascorbic acid (0–30 µg/ml) dissolved in distilled water and TROLOX (0–25 µg/ml) dissolved in ethanol were used as the positive controls. The percentage of scavenging was calculated with the Eq. (1):
where Ac is the absorbance of the control or control at 24 h (1 ml of distilled water mixed with 2 ml of DPPH solution), and As is the absorbance of the sample at 24 h. To discard any possible interference with color development, the absorbance of the sample was measured immediately after the addition of the DPPH and subtracted from the control at 0 h, and the result was then subtracted from the absorbance of the sample at 24 h (Asi). The EC50 value (mg of extract/ml) is an important index to evaluate the antioxidant capacity of substances, because it represents the effective concentration of the antioxidant at which DPPH radicals are scavenged by 50%, and it was obtained by interpolation from linear regression analysis.
ABTS radical cation decoloration assay
The free radical scavenging activity was determined by the ABTS (2,2 azino bis (3-etheylbenzothiazoline-6-sulphonicacid) diammonium salt) radical cation decolorization assay according to Re et al. [42]. ABTS was dissolved in water to a 7 mM concentration. Then, ABTS radical cation (ABTS.+) was generated by mixing 5 ml of the ABTS solution with 88 µl of 140 mM potassium persulfate and allowing the mixture to stand in the dark at room temperature for 16 h. The ABTS.+ solution was diluted with ethanol to an absorbance of 0.70 at 734 nm, and the ABTS radical cation scavenging activity was assessed by mixing 5 ml of this ABTS.+ solution with each polysaccharide extract (0.25–2.5 mg/ml, 1 ml). The reaction mixture was kept in the dark for 6 min, and the absorbance was measured spectrophotometrically against the control. Ascorbic acid dissolved in distilled water and TROLOX dissolved in ethanol were used as positive controls (0–30 µg/ml in both cases). The percentage of scavenging was calculated with the Eq. (2):
where Ac is the absorbance of the control, and As is the absorbance of the sample. The EC50 value was obtained by interpolation from linear regression analysis.
Ferric-reducing antioxidant power assay
Reducing power was determined according to Oyaizu [43]. Each polysaccharide extract (1 mg/ml, 2.5 ml) in distilled water was mixed with 2.5 ml 0.2 M sodium phosphate buffer (pH 6.6) and 2.5 ml of 1% potassium ferricyanide. The mixture was vortexed and incubated at 50 °C for 20 min. Then, 2.5 ml of 10% trichloroacetic acid (w/v) was added, and the mixture was centrifuged at 2000 g for 10 min. The upper layer (5 ml) was mixed with 5 ml of distilled water and 1 ml of 0.1% ferric chloride. The absorbance was measured at 700 nm against a control. The control was the solution with all reagents but without the extract. A higher absorbance indicates a higher reducing power. Ascorbic acid dissolved in distilled water was used as the positive control (0–30 µg/ml).
Chelating ability on ferrous ions
The ability of the extracts to chelate ferrous ions was determined according to the method of Dinis et al. [44] with modifications [28]. Each polysaccharide extract (1 mg/ml, 1 ml) in distilled water was mixed with 3.7 ml of distilled water and 0.1 ml of 2 mM ferrous chloride. The reaction was initiated by the addition of 0.2 ml of 5 mM ferrozine. After 10 min at room temperature, the absorbance of the mixture was determined at 562 nm against the control. The control was the solution with all reagents but without the extract. A lower absorbance indicated a greater chelating ability. EDTA disodium salt was used as the positive control (0–200 µg/ml). Results were expressed as a proportion of the absorbance of the control.
Chemical composition of the different IPS extracts
Carbohydrate content and monosaccharide composition
Total carbohydrate content of the different extracts was determined by the phenol–sulfuric acid method, using glucose as the standard [45]. To determine monosaccharide compositions, the polysaccharides were hydrolyzed with 2 M CF3COOH (90 min, 120 °C) [46]. The hydrolysates were derivatized to the alditol acetates and analyzed by gas–liquid chromatography (GLC) using a capillary column (30 m × 0.25 mm) coated with SP-2330 (0.20 m) on a HP-5890 Gas Chromatograph equipped with a flame ionization detector (FID). Nitrogen was used as the carrier gas, with a head pressure of 15 psi and a split ratio of 100:1. Chromatography runs were isothermal at 220 °C, while the injector and detector were set at 240 °C. When confirmation of identity was needed, the GLC–MS analysis was carried out on a Shimadzu QP 5050 A GC/MS apparatus working at 70 eV, in conditions similar to those described above, but using He as a gas carrier with a split ratio of 60:1. The mole percentages of different monosaccharides were calculated by the area normalization method. Uronic acids were determined using the method of Filisetti-Cozzi and Carpita [47] with glucuronolactone as the standard.
Content of proteins and phenolic compounds
To determine whether the IPS extract contained proteins and/or phenolic compounds, total protein content was determined as described by Lowry et al. [48] using bovine serum albumin as the standard, and total phenolic content was determined according to Heleno et al. [49] with modifications. Each IPS extract (100 µl) was mixed with Folin–Ciocalteu reagent (100 µl, previously diluted 1:5 with distilled water). The mixture was allowed to stand at room temperature for 3 min. Sodium carbonate was added to the mixture (50 µl, 20% w/v), and the total volume was adjusted to 750 µl with distilled water and then mixed gently. After the mixture stood at room temperature for 60 min, the absorbance was measured at 725 nm using a Microplate Reader (Multiskan ™ SkyHigh—Thermo Scientific). The standard calibration curve was plotted using gallic acid (5–150 µg/ml). The total phenolic content was expressed as µg gallic acid equivalents (GAE) per g of mycelia and µg GAE per ml of extract.
Statistical analyses
Three replicates of each assay were performed. All modeling and statistical analyses were performed in InfoStat software [50]. InfoStat implements an interface of the R platform [51] for estimation of linear mixed models through the functions ‘gls’ and ‘lme’ from Nonlinear Mixed-Effects Models library [52, 53]. To create the artworks, Infostat software was used.
To evaluate and compare the effect of IPS extracts, linear mixed models were performed using the mean value of each response variable (percentage of CD11c + cells expressing CD86, MHC II, and IL-12). These mean values were further compared using Fisher's least significant difference (LSD) at p level < 0.05. The factor was ‘treatment’ with eight levels: six corresponded to culture media and two concentrations of IPS extracts (M1_30, M1_100, M2_30, M2_100, AG_30, AG_100), one was the control (BASAL), and another one was the positive control (LPS); the factor was treated as a fixed effect. Normality assumption was evaluated through Shapiro-Wilks tests, and homoscedasticity assumption through residuals. When heteroscedasticity was detected, a second test modeling was carried out. Both models were compared and, if p < 0.05, the one with the lower Akaike (AIC) value was selected.
To compare the chemical composition of the different IPS extracts, linear mixed models were also performed using the mean values of the response variables (percentage of carbohydrates, percentage of proteins, and GAE per ml of extract of phenolic compounds). Assumptions were tested and mean values were compared as described above.
To compare the monosaccharide composition of the different IPS extracts, Pearson square tests were performed. Categorical variables were ‘culture media’ and ‘monosaccharide’, and the percentage of each monosaccharide in each culture medium was used for comparison.
Results and discussion
Our aims were to determine whether IPS isolated from G. australe mycelia had immunomodulatory and/or antioxidant activities and, if so, whether these activities depended on the composition of the IPS extract.
We cultivated G. australe in three different media and extracted the IPS with hot water. The production of IPS was 29 (± 6.5), 45 (± 2.5), and 60 (± 10) µg polysaccharides/mg mycelium dry weight for mycelia growing in M1, M2, and AG media, respectively (n = 3). However, the mycelium dry mass was 1.32- and 2.93- fold higher in M2 than in M1 and AG, respectively, and 2.22- fold higher in M1 than in AG. This could be explained by the fact that we extracted IPS with hot water but, maybe, other fractions of IPS prevail in M1 and M2. We found that a considerable amount of IPS are extracted from Ganoderma sessile using sodium hydroxide or ammonium oxalate (data not shown). Alternatively, growth rates may be different in the different media and, probably, the depletion of the carbon source in M2 occurs earlier than in the other media, leading to the degradation of its own IPS to favor new fungal growth. In this sense, Diamantopoulou et al. [54] stated that the synthesis of IPS seems to be a process related with mycelial mass production, mainly at the early stages of growth in parallel with IPS synthesis, until most of the available sugar is assimilated. Future studies are needed to disentangle these results.
The chemical composition of the three extracts was significantly different (p < 0.0001); while carbohydrates were the main biomolecules composing IPS_M1 and IPS_M2, proteins were the main constituents of IPS_AG. The carbohydrates content was significantly higher in IPS_M1, intermediate in IPS_M2, and lower in IPS_AG. The protein content was significantly higher in IPS_AG, intermediate in IPS_M1, and lower in IPS_M2. The proportion of phenolic compounds was significantly higher in IPS_M2, intermediate in IPS_M1, and lower in IPS_AG (Table 1).
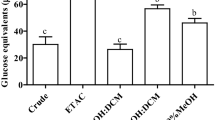
Moreover, the monosaccharide compositions of IPS extracts was also significantly different (p < 0.0001; χ2 58.76, 43.17, and 31.15 for IPS_M1 vs. IPS_M2, IPS_M1 vs. IPS_AG, and IPS_M2 vs. IPS_AG, respectively). In general, mannose, glucose, and galactose were the main constituents of all extracts, although in different proportions; ribose was also an important component of IPS, especially IPS_M2. The IPS_M1 extract was mainly composed of glucose and, to a lesser extent, mannose; no ribose was found, as opposed to IPS_M2, which had almost the same proportion of ribose, glucose, and galactose, and a lower proportion of mannose. The IPS_AG extract was mainly composed of mannose and glucose, like IPS_M1, but unlike the latter, the percentages of these two monosaccharides were similar; galactose was also present in a considerable proportion. None of the extracts presented detectable amounts of uronic acids (Table 2).
Effect of G. australe IPS on DC maturation
Dendritic cells can regulate immune responses through their activation and maturation state [55]; the biological signals that modulate these characteristics are constantly being studied and developed. To evaluate whether IPS influence the phenotypic maturation of DCs, cells were cultured with IPS extracts (30 and 100 μg/ml) or LPS (0.1 µg/ml) for 24 and 48 h for CD86 and MHC II, respectively, and then analyzed for surface expression of these molecules by flow cytometry. DCs treated with IPS_M1 (100 µg/ml) and with IPS_AG (30 µg/ml) exhibited a significantly increased surface expression of MHC II after 48 h (p < 0.0001) (Fig. 1a). Also, DCs treated with IPS_M1 (30 and 100 µg/ml) exhibited a significant increase in the surface expression of CD86 molecules on the surface of DCs after 24 h (p < 0.0001) (Fig. 1b). Moreover, a tendency to increase the production of IL-12 by DCs was found after 24 h of treatment with IPS_M1 and with IPS_AG (Fig. 1c). In contrast, a tendency of IPS_M2 to reduce MHC II levels and IL-12 production was observed in comparison with the control (Fig. 1a, c). As expected, LPS could stimulate the expression of MHC II, CD86, and IL-12 production by DCs. The results showed, at least for IPS_M1, that the immunomodulatory effects promoting the maturation of DC are concentration-dependent, as demonstrated by the increase in CD86 and MHC II expression. (Fig. 1 a,c).
Dendritic cell (DC) maturation and activation by inner-polysaccharide extracts (IPS_M1, IPS_M2, and IPS_AG) of Ganoderma australe at two different concentrations (30 and 100 µg/ml), obtained from mycelia growing in three different culture media (M1, M2, and AG). a MHC II expression after 48 h. b CD86 expression after 24 h. c IL-12 production after 24 h. Results are expressed as percentage of positive cells, media ± SE. Different letters indicate significant differences (p < 0.05)
The increase in the expression of CD86 and MHC II molecules after DC treatment with IPS_M1 extract was the evidence that this extract induces the maturation of DCs (Fig. 1a, b). In addition, an induction of the production of IL-12 by DCs was observed after IPS_M1 stimulation (Fig. 1c). This pro-inflammatory cytokine is related to Th1 immune response polarization [56, 57] and, therefore, it is essential for cell-mediated immunity and inflammation against intracellular bacteria and protozoa [58]. The Th1 cells also promote the production of CD8 + T cells with the achievement of a cytolytic phenotype, required for antitumor immunity [59]. Hence, the induction of IL-12 production by IPS_M1 may have implications in different processes of the adapted immunity. In accordance with our results, Chan et al. [60] demonstrated that a mycelial polysaccharide of G. lucidum extracted with hot water can induce human DC maturation with IL-12 production. In addition, Cao and Lin [23] have shown that a polysaccharide from basidiomata of the same species was able to promote the cytotoxicity of specific T cells induced by DCs pulsed with tumor antigens. In a previous report, these authors demonstrated the maturation of DCs and the secretion of IL-12 of DCs under the same polysaccharide stimulation [61]. Similar results were observed with different polysaccharide extracts (from basidiomata and from the culture broth) in other Ganoderma species [62, 63]. However, Yoshida et al. [64] found that aqueous extracts of mycelia of G. lucidum, cultured in a solid medium, led to the activation of DCs with an increase in IL-23 production and without IL-12 increment, inducing Th17 cell differentiation, which might have implications in the anti-cancer and anti-allergic effects reported for Reishi (G. lucidum). These contrasting results reinforce the fact that the origin of the polysaccharide (basidiomata, mycelia, or culture broth) and the culture conditions are crucial in determining the biological activity of polysaccharides.
The immunomodulatory effects of Ganoderma polysaccharides on DCs were mainly attributed to β-glucans or polysaccharides containing β-glucan regions [64, 65]. However, some protein-bound polysaccharides (5 to 6.5% of proteins) [60, 66] or polysaccharides mainly composed of mannose and galactose with a low proportion of glucose [62] were also effective in activating DCs. The high content of glucose in IPS_M1 (Table 2) suggests that glucans may also be involved in the maturation and activation of murine DCs, although more studies are needed to confirm glycoside-type links, polysaccharide structure, and the role of proteins in the observed effect.
Antioxidant activity of IPS of G. australe
To analyze the in vitro antioxidant activity of IPS extracts, four parameters were evaluated: scavenging ability on DPPH and on ABTS free radicals, ferric-reducing antioxidant ability, and chelating ability on ferrous ions.
The suggested antioxidant mechanisms of polysaccharides involve the termination of radical chain reactions [67]. The DPPH and the ABTS assays are based on the reduction of a radical by a hydrogen donor (DPPH) or by an electron donor (ABTS), which leads to radical scavenging. Of the three fractions analyzed, IPS_M2 was the only extract showing very good DPPH scavenging activity at the concentration of 1 mg/ml (p < 0.001) (Fig. 2). Moreover, our results showed that is an excellent DPPH scavenger antioxidant, as evidenced by its EC50 value (0.46 mg/ml), even better than IPS extracts from G. curtisii and G. applanatum basidiomata [68, 69], and from G. lucidum basidiomata and spores at the same concentration [13, 28].
Antioxidant activity of inner-polysaccharide extracts (IPS_M1, IPS_M2, and IPS_AG) of Ganoderma australe at a concentration of 1 mg/ml, obtained from mycelia growing in three different culture media (M1, M2, and AG). Media ± SE are shown. Different letters (uppercase letters for DPPH, lowercase letters for ABTS) indicate significant differences (p < 0.05)
Regarding the ABTS assay, IPS_M2 also showed significantly higher ABTS free radical scavenging activity (p < 0.001) (Fig. 2), although its EC50 value was much higher than that in DPPH (1.38 mg/ml). Our results are in accordance with those reported by Shi et al. [70]; these authors found approximately 40% ABTS scavenging activity at a concentration of 1 mg/ml for G. lucidum mycelial polysaccharide. They also reported that this activity was concentration-dependent, reaching almost 100% of activity at a concentration of 5 mg/ml. Although IPS_M2 seems to be a good antioxidant, the findings reported above reinforce the importance of taking into account the origin of the polysaccharide, the culture conditions, and the concentration of the extract needed for achieving the desired antioxidant activity.
The IPS_M1 scavenger activity (5.52% at 1 mg/ml) was also better than that of G. tsugae mycelial polysaccharide at a much higher concentration (20 mg/ml) [12]; it is worth noting that Saltarelli et al. [71] did not find scavenger activity in G. lucidum polysaccharide extracted from mycelia. Ascorbic acid and TROLOX were both confirmed as excellent scavengers of DPPH (EC50 = 0.015 and 0.013 mg/ml, respectively) and of ABTS radicals (EC50 = 0.015 and 0.020 mg/ml, respectively).
The reduction in Fe3+ ions is often used as an indicator of electron-donating activity, while iron chelators mobilize tissue iron by forming soluble, stable complexes that are then excreted [72]. In line with the DPPH and ABTS results, in the ferric-reducing power assay, our IPS_M2 extract showed significantly higher reducing power (p < 0.0001) (2.29-fold increase in the activity in comparison with the control at 1 mg/ml), while IPS_M1 and IPS_AG showed no activity (1.11- and 1.08-fold increase respectively in comparison with the control) (Fig. 3). However, at the concentration of 1 mg/ml, none of the extracts showed ferrous ion chelating activity. The proportions obtained, in comparison with the control, were 1.098 (± 0.014), 1.101 (± 0.014), and 1.017 (± 0.013) for IPS_M1, IPS_M2, and IPS_AG, respectively. EDTA showed good chelating ability (EC50 = 0.080 mg/ml).
Ferric reducing power activity of inner-polysaccharide extracts (IPS_M1, IPS_M2, and IPS_AG) of Ganoderma australe at a concentration of 1 mg/ml, obtained from mycelia growing in three different culture media (M1, M2, and AG), and control (without IPS). Media ± SE are shown. Different letters indicate significant differences (p < 0.05)
Polysaccharide extracts from mycelia of G. tsugae, from spores of G. lucidum, and from basidiomata of G. applanatum also showed reducing activity, although a much higher concentration of the extract was needed in all cases [12, 28, 68]; but, contrary to our results, G. lucidum extract from spores and basidiome showed 85% and 25% chelating ability at 1 mg/ml, respectively [28, 68]. In addition, G. applanatum extracts from basidiome exhibited 35% chelating ability approximately at the same concentration but greater ability at a higher concentration [68], and G. tsugae extracts from mycelia showed 5% chelating ability at 20 mg/ml [73]. It seems that IPS_M2 extract is able to donate an electron and, hence, reduce Fe3+, but it is not able to form complexes with iron. Alternatively, the concentrations required to do so may be much higher than the tested one, as found for hot water extracts from mycelia of other species [74]. Moreover, this difference in the biological activity of our extract and the previous studies mentioned above could also be due to the characteristics of the polysaccharides, such as their molecular structure, molecular mass, solubility [74], monosaccharide composition [70, 71], as well as the composition of the extracts [75], especially the content of carbohydrates, phenolic compounds, proteins, and uronic acids.
The extracts obtained from mycelia growing in M1 and M2 were mainly composed of carbohydrates, and, to a lesser extent, proteins (Table 1). These results are in agreement with those reported for Agaricus blazei hot water extract (81% carbohydrates, 16% proteins), which turned out to be an excellent antioxidant [74]. Regarding phenolic content, IPS_M2 showed a greater total phenolic content than the other extracts, and it also showed the highest antioxidant values. Similarly, previous studies showed a linear relationship between total phenolic content and antioxidant properties of mushroom extracts [75] and, particularly, Wei [76] reported a highly significant relationship between the scavenging activity of polysaccharide extracts and their content of phenols. Moreover, Subramaniam et al. [36] found a correlation between the antioxidant activity of G. australe and the total phenolic content of mycelia extracts.
Proteins do not seem to enhance antioxidant activity in proteoglycans, at least for G. lucidum [77]. Our extract obtained from mycelia growing in AG media had a great percentage of proteins and a much lower percentage of carbohydrates (Table 1). This extract did not show antioxidant activity (Figs. 2 and 3) and only increased the expression of MHC II, but it did not increase the other activation parameters of the dendritic cells (Fig. 1). Further studies are needed to disentangle this mechanism, leading to a more accurate use of G. australe IPS extracts as an antioxidant and/or anti-carcinogenic/immunomodulatory agent.
The comparison of the chemical composition of the different extracts and the results of their antioxidant effects led us to hypothesize that the phenolic content of IPS_M2 might be involved in this biological activity. It must be noted that, although mycelia were washed with methanol and IPS precipitated with ethanol, these steps might not be sufficient to remove all phenolic compounds from the extracts; hence, the antioxidant activity could be due to the presence of polysaccharide and phenolic compound complexes, or phenolic compounds that were not removed, which could contribute to the antioxidant activity of the extract [28, 49]. In addition, the presence of ribose in this extract (Table 2) could also enhance its antioxidant activity. There is little available information concerning the role of ribose in antioxidant functions but, in line with our results, previous studies reported that polysaccharides with ribose, among other monosaccharide constituents, had antioxidant activity [78, 79]. It is interesting that this monosaccharide was one of the main constituents of IPS_M2 and that it was not present in IPS_M1 (Table 2). Although ribose is not generally reported in the chemical composition of Ganoderma IPS, it has been found to be a constituent of polysaccharides from aqueous extracts of several basidiomycetes [79,80,81]. Further studies are needed to evaluate the role of this monosaccharide in this biological activity.
Conclusion
To our knowledge, this is the first study to report the immunomodulatory effect of different aqueous extracts of G. australe on dendritic cell maturation/activation and to relate this effect with the composition of the extract. It also highlights the importance of an extract of this species as an antioxidant. In addition, our study shows that culture media influences the production of polysaccharides from the same species and, consequently, the biological activity of these carbohydrates. Indeed, our results showed that IPS_M1 induced the in vitro maturation of mouse bone marrow-derived DCs, while IPS_M2 turned to be an excellent antioxidant. Future studies could aim at deepening the understanding of the key chemical features responsible for the immunomodulatory activity of IPS_M1 and of the antioxidant activity of IPS_M2.
Data availability
Most of the data generated and/or analyzed during this study are included in this published article. The datasets not included here are available from the corresponding author upon reasonable request.
Code availability
Not applicable.
Abbreviations
- APC:
-
Allophycocyanin
- BRMs:
-
Biological response modifiers
- CD:
-
Cluster of differentiation
- DCs:
-
Dendritic cells
- EC50 :
-
Effective concentration of the antioxidant at which radicals are scavenged by 50%
- FBS:
-
Fetal bovine serum
- GAE:
-
Gallic acid equivalents
- GM-CSF:
-
Granulocyte-macrophage colony-stimulating factor
- iDCs:
-
Immature DCs
- IL:
-
Interleukin
- LPS:
-
Lipopolysaccharide
- IPS:
-
Inner-polysaccharides
- IPS_M1:
-
Inner-polysaccharides obtained from mycelia growing in M1 media
- IPS_M2:
-
Inner-polysaccharides obtained from mycelia growing in M2 media
- IPS_AG:
-
Inner-polysaccharides obtained from mycelia growing in AG media
- mAbs:
-
Monoclonal antibodies
- mDCs:
-
Mature DCs
- MEA:
-
Malt Extract Agar media
- MHC II:
-
Major histocompatibility complex class II
- PE:
-
Phycoerythrin
- PBS:
-
Phosphate buffered saline
- ROS:
-
Reactive oxygen species
- T cells:
-
Lymphocytes T
- Th:
-
T helper cells
References
M.L. Gargano, L.J. van Griensven, O.S. Isikhuemhen, U. Lindequist, G. Venturella et al., Plant Biosyst. (2017). https://doi.org/10.1080/11263504.2017.1301590
M.Y.K. Leung, C. Liu, J.C.M. Koon, K.P. Fung, Immunol. Lett. (2006). https://doi.org/10.1016/j.imlet.2006.01.009
S. Patel, A. Goyal, Biotech (2012). https://doi.org/10.1007/s13205-011-0036-2
D. Kothari, S. Patel, S.K. Kim, Biomed. Pharmacother. (2018). https://doi.org/10.1016/j.biopha.2018.05.138
S.T. Chang, K.E. Mshigeni, Mushroom and their human health: Their growing significance as potent dietary supplements, 1st edn. (The University of Namibia, Windhoek, 2001) pp. 1–79
Y. Cao, X. Xu, S. Liu, L. Huang, J. Gu, Front. Pharmacol. (2018). https://doi.org/10.3389/fphar.2018.01217
S. Zhang, S. Nie, D. Huang, W. Li, M. Xie, Food Chem. (2013). https://doi.org/10.1016/j.foodchem.2012.08.090
X.Q. Han, G.L. Yue, R.Q. Yue, C.X. Dong, C.L. Chan et al., PLoS ONE (2014). https://doi.org/10.1371/journal.pone.0100380
C.L. Wang, C.Y. Lu, Y.C. Hsueh, W.H. Liu, C.J. Chen, Appl. Microbiol. Biotechnol. (2014). https://doi.org/10.1007/s00253-014-6027-6
J. Wang, Y. Yuan, T. Yue, Carbohydr. Polym. (2014). https://doi.org/10.1016/j.carbpol.2013.10.087
R.H. de Melo, A.E. do Amaral, R.A. Menolli, T.S. Ayala, R.D.C.G. Simao et al., Int. J. Med. Mushrooms (2016). https://doi.org/10.1615/IntJMedMushrooms.v18.i4.40
Y.H. Tseng, J.H. Yang, J.L. Mau, Food Chem. (2008). https://doi.org/10.1016/j.foodchem.2007.08.073
C. XiaoPing, C. Yan, L. ShuiBing, C. YouGuo, L. JianYun, L. LanPing, Carbohydr. Polym. (2009). https://doi.org/10.1016/j.carbpol.2009.01.009
Y. Gao, S. Zhou, W. Jiang, M. Huang, X. Dai, Immunol. investig. (2003). https://doi.org/10.1081/IMM-120022979
L.X. Sun, W.D. Li, Z.B. Lin, X.S. Duan, X.F. Li et al., Cell Physiol. Biochem. (2014). https://doi.org/10.1159/000356669
L. Ren, J. Zhang, T. Zhang, Food Chem. (2021). https://doi.org/10.1016/j.foodchem.2020.127933
F. Motta, M.E. Gershwin, C. Selmi, J. Autoimmun. (2021). https://doi.org/10.1016/j.jaut.2020.102576
J. Banchereau, R.M. Steinman, Nature (1998). https://doi.org/10.1038/32588
M. Merad, P. Sathe, J. Helft, J. Miller, A. Mortha, Annu. Rev. Immunol. (2013). https://doi.org/10.1146/annurev-immunol-020711-074950
B. Pulendran, Annu. Rev. Immunol. (2015). https://doi.org/10.1146/annurev-immunol-020711-075049
U. Švajger, P. Rožman, Front. Immunol. (2018). https://doi.org/10.3389/fimmu.2018.02482
T. Kaisho, S. Akira, Curr. Mol. Med. (2003). https://doi.org/10.2174/1566524033479726
L.Z. Cao, Z.B. Lin, Acta Pharmacol. Sin. 24(4), 321–326 (2003)
D.T. Førland, E. Johnson, A.M.A. Tryggestad, T. Lyberg, G. Hetland, Cytokine (2010). https://doi.org/10.1016/j.cyto.2009.09.002
M. Valko, D. Leibfritz, J. Moncol, M.T. Cronin, M. Mazur, J. Telser, Int. J. Biochem. Cell Biol. (2007). https://doi.org/10.1016/j.biocel.2006.07.001
B.V. Nemzer, A.Y. Yashin, A.N. Vedenin, Y.I. Yashin, D.V. Yashunsky et al., J. Food Res. (2019). https://doi.org/10.5539/jfr.v8n1p60
B.V. Nemzer, D. Kalita, A.Y. Yashin, N.E. Nifantiev, Y.I. Yashin, J. Food Res. (2019). https://doi.org/10.5539/jfr.v8n6p78
M. Kozarski, A. Klaus, M. Niksic, D. Jakovljevic, J.P. Helsper, L.J. van Griensven, Food Chem. (2011). https://doi.org/10.1016/j.foodchem.2011.06.029
Z.A. Bhat, A.H. Wani, J.M. War, M.Y. Bhat, Asian J. Pharm. Clin. Res. (2021). https://doi.org/10.22159/ajpcr.2021v14i3.40390
Y.J. Tang, J.J. Zhong, Enzyme Microb. Technol. (2002). https://doi.org/10.1016/S0141-0229(02)00066-2
S. Nie, H. Zhang, W. Li, M. Xie, Bioact. Carbohydr. Diet Fibre (2013). https://doi.org/10.1016/j.bcdf.2013.01.001
I.C. Ferreira, S.A. Heleno, F.S. Reis, D. Stojkovic, M.J.R. Queiroz et al., Phytochemistry (2015). https://doi.org/10.1016/j.phytochem.2014.10.011
F. León, M. Valencia, A. Rivera, I. Nieto, J. Quintana et al., Helv Chim. Acta. (2003). https://doi.org/10.1002/hlca.200390251
E.D.F.A. Smania, F. Delle Monache, R.A. Yunes, R. Paulert, A. Smania Jr., Rev. Bras. Farmacogn. (2007). https://doi.org/10.1590/S0102-695X2007000100004
J.M. Moncalvo, P.K. Buchanan, Mycol. Res. (2008). https://doi.org/10.1016/j.mycres.2007.12.001
S. Subramaniam, V. Sabaratnam, U.R. Kuppusamy, Y.S. Tan, Int. J. Med. Mushrooms (2014). https://doi.org/10.1615/IntJMedMushr.v16.i3.60
G.M. Liew, H.Y. Khong, C.J. Kutoi, A.K. Sayok, Int. J. Res. Stud. Biosci. 3, 191–197 (2015)
M. Campi, C. Mancuello, F. Ferreira, Y. Maubet, E. Cristaldo, G. Robledo, Int. J. Med. Mushrooms (2021). https://doi.org/10.1615/IntJMedMushrooms.2021039298
M. Berovič, J. Habijanič, I. Zore, B. Wraber, D. Hodžar et al., J. Biotechnol. (2003). https://doi.org/10.1016/S0168-1656(03)00069-5
Y.C. Wang, X.B. Hu, F. He, F. Feng, L. Wang et al., J. Biol. Chem. (2009). https://doi.org/10.1074/jbc.M901144200
M.S. Blois, Nature (1958). https://doi.org/10.1038/1811199a0
R. Re, N. Pellegrini, A. Proteggente, A. Pannala, M. Yang, C. Rice-Evans, Free Radic. Biol. Med. (1999). https://doi.org/10.1016/S0891-5849(98)00315-3
M. Oyaizu, Jpn. J. Nutr. Diet. 44(6), 307–315 (1986)
T.C.P. Dinis, V.M.C. Madeira, L.M. Almeida, Arch. Biochem. Biophys. (1994). https://doi.org/10.1006/abbi.1994.1485
M. Dubois, K.A. Gilles, J.K. Hamilton, P.T. Rebers, F. Smith, Anal. Chem. (1956). https://doi.org/10.1021/ac60111a017
P. Albersheim, D.J. Nevins, P.D. English, A. Karr, Carbohydr. Res. (1967). https://doi.org/10.1016/S0008-6215(00)80510-8
T.M. Filisetti-Cozzi, N.C. Carpita, Anal. Biochem. (1991). https://doi.org/10.1016/0003-2697(91)90372-Z
O. Lowry, N. Rosebrough, A. Farr, R. Randall, J. Biol. Chem. 193, 265–275 (1951)
S.A. Heleno, L. Barros, A. Martins, M.J.R. Queiroz, C. Santos-Buelga, I.C. Ferreira, Food Res. Int. (2012). https://doi.org/10.1016/j.foodres.2011.12.009
J.A. Di Rienzo, F. Casanoves, M.G. Balzarini, L. Gonzalez, M. Tablada, C.W. Robledo, (2018). InfoStat (Centro de Transferencia InfoStat, FCA, Universidad Nacional de Córdoba, Argentina.) Retrieved November 6, 2021, from http://www.infostat.com.ar
R Core Team, R: a language and environment for statistical computing. R Foundation for Statistical Computing. Vienna, Austria (2018). Retrieved from November 6, 2021, from https://www.r-project.org
J.C. Pinheiro, D.M. Bates, Mixed-Effects Models in S and S-PLUS (Springer, New York, 2004). https://doi.org/10.1007/b98882
J.A. Di Rienzo, R. Macchiavelli, F. Casanoves, Modelos Lineales Mixtos Aplicaciones en InfoStat (Córdoba, Grupo Infostat, 2017)
P. Diamantopoulou, S. Papanikolaou, M. Komaitis, M. Aggelis, A. Philippoussis, Bioprocess Biosyst. Eng. (2014). https://doi.org/10.1007/s00449-013-1112-2
K.L. Hilligan, F. Ronchese, Cell Mol. Immunol. (2020). https://doi.org/10.1038/s41423-020-0465-0
A.C. Mullen, F.A. High, A.S. Hutchins, H.W. Lee, A.V. Villarino et al., Science (2001). https://doi.org/10.1126/science.1059835
N. Mikhalkevich, B. Becknell, M.A. Caligiuri, M.D. Bates, R. Harvey, W.P. Zheng, J. Immunol. (2006). https://doi.org/10.4049/jimmunol.176.3.1553
J. Zhu, W.E. Paul, J. Am. Soc. Hematol. (2008). https://doi.org/10.1182/blood-2008-05-078154
H. Raskov, A. Orhan, J.P. Christensen, I. Gögenur, Br. J. Cancer (2021). https://doi.org/10.1038/s41416-020-01048-4
W.K. Chan, H.K.W. Law, Z.B. Lin, Y.L. Lau, G.C.F. Chan, Int. Immunol. (2007). https://doi.org/10.1093/intimm/dxm061
L.Z. Cao, Z.B. Lin, Immunol. Lett. (2002). https://doi.org/10.1016/S0165-2478(02)00087-1
C.C. Pi, C.L. Chu, C.Y. Lu, Y.J. Zhuang, C.L. Wang et al., Vaccine (2014). https://doi.org/10.1016/j.vaccine.2013.11.027
H. Wang, Q. Yu, S.P. Nie, Q.D. Xiang, M.M. Zhao et al., Food Chem. Toxicol. (2017). https://doi.org/10.1016/j.fct.2017.02.026
H. Yoshida, M. Suzuki, R. Sakaguchi, I. Tani, H. Kotani et al., Biochem. Biophys. Res. Commun. (2012). https://doi.org/10.1016/j.bbrc.2012.04.135
S. Zhang, G. Pang, C. Chen, J. Qin, H. Yu et al., Carbohydr. Polym. (2019). https://doi.org/10.1016/j.carbpol.2018.10.028
Y.L. Lin, S.S. Lee, S.M. Hou, B.L. Chiang, Mol. Pharmacol. (2006). https://doi.org/10.1124/mol.106.022327
J. Lu, R. He, P. Sun, F. Zhang, R.J. Linhardt, A. Zhang, Int. J. Biol. Macromol. (2020). https://doi.org/10.1016/j.ijbiomac.2020.02.035
M. Kozarski, A. Klaus, M. Nikšić, M.M. Vrvić, N. Todorović et al., J. Food Compos. Anal. (2012). https://doi.org/10.1016/j.jfca.2012.02.004
I. Huerta Aguilar, J. Molina Torres, G. Garnica Romo, B. Yahuaca Juárez, J. Med. Plants Stud. 4(4), 136–141 (2016)
M. Shi, Z. Zhang, Y. Yang, Carbohydr. Polym. (2013). https://doi.org/10.1016/j.carbpol.2013.02.081
R. Saltarelli, P. Ceccaroli, M. Iotti, A. Zambonelli, M. Buffalini et al., Food Chem. (2009). https://doi.org/10.1016/j.foodchem.2009.02.023
M.A. Ebrahimzadeh, S.F. Nabavi, S.M. Nabavi, Pak. J. Biol. Sci. (2009). https://doi.org/10.3923/pjbs.2009.447.450
J.L. Mau, S.Y. Tsai, Y.H. Tseng, S.J. Huang, LWT Food Sci. Technol. (2005). https://doi.org/10.1016/j.lwt.2004.08.010
Y.B. Ker, K.C. Chen, C.C. Chyau, C.C. Chen, J.H. Guo et al., J. Agric. Food Chem. (2005). https://doi.org/10.1021/jf0510034
L.M. Cheung, P.C. Cheung, V.E. Ooi, Food Chem. (2003). https://doi.org/10.1016/S0308-8146(02)00419-3
S. Wei, Int. J. Med. Mushrooms (2008). https://doi.org/10.1615/IntJMedMushr.v10.i4.30
X. Zeng, P. Li, X. Chen, Y. Kang, Y. Xie et al., Int. J. Biol. Macromol. (2019). https://doi.org/10.1016/j.ijbiomac.2018.12.222
K. Caicai, H. Limin, Z. Liming, Z. Zhiqiang, Y. Yongwu, Int. J. Biol. Macromol. (2018). https://doi.org/10.1016/j.ijbiomac.2017.10.139
J. Liu, J. Zhou, Q.Q. Zhang, M.H. Zhu, M.L. Hua, Y.H. Xu, Chin. Herb. Med. (2019). https://doi.org/10.1016/j.chmed.2019.05.008
D. Ren, Y. Jiao, X. Yang, L. Yuan, J. Guo, Y. Zhao, Chem.-Biol. Interact. (2015). https://doi.org/10.1016/j.cbi.2015.06.017
Y. Wang, J. Jia, X. Ren, B. Li, Q. Zhang, Int. J. Biol. Macromol. (2018). https://doi.org/10.1016/j.ijbiomac.2018.09.209
Acknowledgements
We are grateful to Administración de Parques Nacionales for kindly allowing us to sample in Lanin National Park, and to Agencia Nacional de Promoción Científica y Técnica of Argentina (ANPCyT, FONCyT), Universidad Nacional de la Patagonia “San Juan Bosco” (UNPSJB), and Centro de Investigación y Extensión Forestal Andino Patagónico (CIEFAP) for making this investigation possible.
Funding
This research was funded by Agencia Nacional de Promoción Científica y Técnica (ANPCyT-FONCyT) PICT-2018-03234, Universidad Nacional de la Patagonia “San Juan Bosco” PI1462/18, and CIEFAP PE-P7A2007.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study’s conception and design. Polysaccharide extracts production and extraction, antioxidant assays, and chemical determinations were performed by ALG and MLV. Immunomodulation assays were performed by FS and ALG. Data analyses were performed by ALG and FS. The first draft of the manuscript was written by ALG and MLV, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Gallo, A.L., Soler, F., Pellizas, C. et al. Polysaccharide extracts from mycelia of Ganoderma australe: effect on dendritic cell immunomodulation and antioxidant activity. Food Measure 16, 3251–3262 (2022). https://doi.org/10.1007/s11694-022-01444-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11694-022-01444-9