Abstract
Several species of microalgae have been known to produce exopolysaccharides (EPS) with potential immune activity. In the present investigation, ethyl acetate fraction of crude EPS secreted by Dunaliella salina was explored for immunomodulatory activity against peripheral blood mononuclear cells (PBMC) and RAW 264.7 macrophages. Effect of EPS on cell growth and cytokines production were measured using MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) assay and ELISA respectively. Griess reagent was used for measuring the nitric oxide production in RAW 264.7 macrophages. FTIR analysis and mass spectroscopy were carried out for the characterization. Ethyl acetate fraction exhibited dose dependent increase in proliferative index and cytokines production (IFN-γ, TNF-α, TGF-β). At low concentration (250 and 500 µg/mL), it showed growth inhibition and at higher concentration (1000 and 1500 µg/mL), it enhanced the cell growth. Interestingly, the pronounced increased TNF-α production was observed in ethyl acetate fraction treated PBMC cells at higher concentration (750 and 1000 µg/mL) indicating the immunostimulatory effect. In RAW cells, concentration dependent diminished cell growth (IC50 = 691 µg/mL) and nitric oxide production (IC50 = 630 µg/mL) was observed. FTIR analysis showed the presence of polysaccharides due to the detection of hydroxyl (–OH), Carbonyl (C–O) and alkyl (C–H) groups. Mass spectroscopy results revealed ethyl acetate fraction as penta-saccharide (m/z = 887.56 and 886.54) which are confirmed to be hetero-polysaccharides consisting of hexoses and pentoses along with association of ions. These results suggest that penta-saccharide (ethyl acetate fraction) isolated from D. salina may have the potential to be used for therapeutic purpose as immunomodulatory agent.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Exopolysaccharides (EPS) produced by microorganisms are widespread throughout the microbial world, from algae, fungi and yeasts to prokaryotes and often show advantages over the polysaccharides that are currently in use [1]. These are mainly referred as secondary metabolites produced by microorganisms during the growth process and secreted into the extracellular broth. EPS have emerged as an important class of bioactive natural compounds and potential role in human health. Some of the biological activities of EPS reported are antioxidant, immunomodulatory, hepatoprotective, anticoagulant, antithrombotic and gastroprotective [2, 3].
Several species of microalgae such as Chlorella, Spirullina, Nostoc, Rhodella, Ulva have been known to produce polysaccharides extracellularly [4] and have been reported to act as potential immunomodulators. Sulphated polysaccharides isolated from Chlorella stigmatophora have showed immunosuppressive effect on macrophage cells whereas the immunostimulant effect was observed for the exopolysaccharides isolated from Phaeodactylum tricornutum [5]. These polysaccharides act on innate as well adaptive immune system. They affect the lymphocytes by inducing proliferation and enhanced cytokines productions [6,7,8]. Apart from lymphocytes, the interaction with other immune cells such as macrophages, dendritic cells and neutrophils with polysaccharides have been reported to enhance the immune activity [6].
Dunaliella salina (D. salina) is a unicellular, biflagellate, green, halotolerant microalga known to survive in the wide range of salts [9]. The algae is known to possess various bioactive properties such as anti-viral, anti-bacterial, anti-cancer and immunomodulatory activities [10]. Dunaliella salina is a well-known edible green algae and is marketed as a whole organism under a trade name of Algotene® by Inter Clinical laboratories Pty Ltd. Australia [11]. Dunaliella salina is mostly studied for producing bioactive compounds such as trans and cis-carotene, fatty acids (oleic acid, linolenic acid and palmitic acid) and glycerol which are mostly produced intracellularly and have gained commercial importance [12, 13] but exopolysaccharide (EPS) of D. Salina exhibiting bioactive properties are yet to be explored. There were studies for characterization of EPS isolated from D. Salina [14, 15] but bioactive properties with focus on immunomodulatory activity of EPS of D. Salina is not reported yet to the best of our knowledge.
In the present study, the EPS isolated from D. salina was characterized and tested for its biological activity such as proliferation effect on peripheral blood mononuclear cell (PBMC) and RAW 264.7 mouse macrophage cell lines as well as cytokines release in PBMC and nitric oxide production in RAW 264.7.
Methods
Growth Conditions and EPS Extraction
The culture of Dunaliella salina was obtained from Birla Institute of Scientific Research, Jaipur, India and was maintained on AS100 medium [16, 17]. For extraction of EPS, the culture was grown at 25 ± 2 °C with 12/12 h light and dark period for 20 days. After 20 days of growth, the culture was centrifuged (10,000 rpm; 30 min) for eliminating the debris and cells. The supernatant was collected, filtered and heated at 60–70 °C till the volume reached to 1/5th of the original volume. Organic precipitation was carried out where equal volume of cold methanol was added in the concentrated supernatant and left it for 16–18 h at 4 °C to precipitate extracellular polysaccharides (EPS). The contents were then centrifuged (10,000 rpm; 10 min) and the methanol was discarded. The obtained pellet was washed with absolute ethanol (two times) and redissolved in Milli Q water. Dialysis was performed to remove salts against double distilled water for 48 h [14]. The dialysed solution was then subjected to trichloroacetic acid (TCA) precipitation to remove proteins [18]. TCA (20%) was added to the dialysed liquid and incubated for at least 1 h. Then, it was centrifuged (14,000 rpm; 10 min) and the pellet of proteins was discarded. The supernatant was dialysed again prior to lyophilisation to remove TCA and the powder was obtained. Phenol sulphuric assay was carried out to assess the sugar content by using glucose as standard [19]. Proteins were estimated by Bradford assay with bovine serum albumin (BSA) as standard [20]. Flavonoids, alkaloids, tannins, cardiac glycosides and quinones as phytochemicals were also assessed based on standard procedure [21].
Fractionation of EPS
EPS was fractionated in a sequential fashion [22] with ethyl acetate, 20% methanol: dichloromethane (DCM): 50% methanol: DCM and 100% methanol by column chromatography. Three-fourth of the column was packed with slurry of silica gel in hexane and hexane was run through the column for packing. Initially, 400 mg lyophilised EPS powder taken in a small round bottom flask and the slurry was made with ethyl acetate and silica in minimum amount just for the adsorption of extracted crude sample. Then, the solvent was dried completely by adding more silica and the dried powder was loaded in the column. After loading the sample, 100 ml of ethyl acetate solvent was run through the column and fraction was collected in test tubes. To increase the polarity, 20% methanol: DCM was run and collected in test tubes. Elution was followed by further increasing the polarity of the solvent to 50% methanol: DCM and finally with 100% methanol.
Isolation of Peripheral Blood Mononuclear Cells (PBMC)
Blood (5 mL) of the healthy volunteers was drawn via veni-puncture by the trained technicians from Nitin Nursing home and Rajendra Hospital, Patiala, India in blood collection EDTA coated tubes. The informed consent was given by all the donors for the experiments. The study obtained the approval from the institutional ethical committee (TU/IEC/DBT/07-07). Peripheral blood mononuclear cells (PBMC) were isolated by ficoll density gradient method as earlier described [23]. The pellet containing cells was dissolved in 1 ml RPMI 1640 media (Sigma–Aldrich) containing 10% (v/v) fetal bovine serum (FBS) (Gibco, Waltham, MA), 100 IU/mL penicillin, 100 µg/mL streptomycin and 2.5 µg/mL amphotericin (Sigma–Aldrich).
Maintenance of Cell Lines
Mouse leukemic monocyte macrophage cell line (RAW 264.7) was procured from National Centre for Cell Sciences (NCCS), Pune, India. The cell line was maintained in DMEM medium supplemented with 10% (v/v) FBS, 100 IU/mL penicillin, 100 µg/mL streptomycin and 2.5 µg/mL amphotericin in the humidified incubator with 5% CO2 in 37 °C in T25 flasks. Pure culture of RAW cell lines was maintained by routine monitoring with inverted microscopes for any cross contamination and only culture not having any kind of contamination was used for the experiments.
Cell Proliferation Assay
The growth effect of EPS on PBMC and RAW 264.7 cells was assayed by 3-(4,5-dimethylthiazol-2-yl)-2-5 diphenyl tetrazolium bromide (MTT assay) [23, 24]. Freshly isolated PBMC cells were seeded in 96 well microtiter plate at the density of 2 × 105cells/well. In case of RAW 264.7 cells, prior to the assay, the culture flask with 70–80% confluency was trypsinised and cells were counted. Then, the microtiter plate was seeded at the density of 1 × 104 cells per well. The seeding was followed by overnight incubation before adding the sample. Crude and fractionated EPS dissolved in sterile water were added in the wells containing PBMC or RAW 264.7 cells and experiments were performed in triplicates. Concanavalin A (10 µg/mL; Sigma–Aldrich) and lipopolysaccharides (2 µg/mL; Sigma–Aldrich) for PBMC and RAW 264.7 cells, respectively served as the positive control. After 48 h of incubation at 37 °C in CO2 incubator with 5% CO2, 20 µL MTT (5 mg/mL) was added in each well. Following the addition, the plate was again incubated for 4 h. The plate was then centrifuged (10 min, 2000 rpm) to settle down the crystals. 170 µL of media was discarded and 100 µL of DMSO was added in each well. The absorbance was recorded at 570 nm taking reference wavelength at 620 nm on ELISA plate reader (Tecan Infinite Pro ELISA reader).
The cell growth was calculated in terms of proliferative index with the help of equation given below:
where A is absorbance of sample (cells treated with concanavalin A/lipopolysaccharides/exopolysaccharides) and B is absorbance of untreated cells (cells only).
Cytokine Estimation
PBMC were cultured in the density of 2 × 105 cells/well and samples were added in varying concentration (250–1000 µg/mL). After the incubation of 48 h at 37 °C in CO2 incubator with 5% CO2, 100 µL supernatant was collected in the micro-centrifuge tubes for estimation of three different cytokines (IFN-γ, TGF-β and TNF-α). Cytokines secretion in the culture supernatants was measured by ELISA kits (IFN-γ and TNF-α: PeproTech, Rocky Hill, NJ, USA and TGF-β: Raybiotech, Norcross, GA, USA) in 96-well ELISA plates as per the manufacturer’s instructions. Cytokine production was expressed as fold change with the help of equation given below:
where A is absorbance of sample (cells treated with concanavalin A/exopolysaccharides) and B is absorbance of untreated cells (cells only).
Nitric Oxide Estimation Assay
The release of nitric oxide (NO) was determined in mouse macrophage cell line (RAW 264.7) with the help of griess reagent (1% sulphanilamide in 5% H3PO4 and 0.1% N-napthyl ethylene-diamine-di-hydrochloride in 5% H3PO4) (Sigma–Aldrich). Cells were seeded at the density of 2.5 × 105 cells/well and incubated overnight for attachment. Next day, medium was changed and sample in the concentration range of 250–1500 µg/mL was added in the well. Lipopolysaccharides (10 µg/mL) served as positive control. The sample addition was followed by 48 h of incubation at 37 °C in CO2 incubator. NO production in the supernatant was measured in terms of nitrite production which is the stable product. 100 µL of the supernatant was aspirated and mixed with 100 µL of griess reagent at room temperature for 10 min. The absorbance was recorded at 570 nm [25]. NO production was expressed as fold change with the help of equation given below:
where A is absorbance of sample (cells treated with lipopolysaccharides/exopolysaccharides) and B is absorbance of untreated cells (cells only).
Mass Spectroscopy and FTIR Analysis
The EPS fraction was dissolved in 80% methanol and MS was recorded on Waters, Micromass Q-TOF micro instrument using electron spray ionisation with the collision energy of 4 eV. IR spectra were recorded on Perkin Elmer Spectrum Two FTIR spectrometer, Platelet made in KBR, and peaks are reported in cm−1.
Statistical Analysis
The data were expressed as the mean ± standard error of mean. All experiments were carried out in triplicates. Data were analysed using analysis of variance (ANOVA) and the means were compared using Tukey’s test at p < 0.05. IC50 was calculated by choosing parameters of nonlinear regression {log (inhibitor) vs. response − Variable slope} using GraphPad Prism.
Results
Biomolecules Content and Fractionation of EPS
The extracted EPS was analysed for the presence of different biomolecules. Phenol sulphuric assay revealed the presence of sugar in EPS. Bradford method and spectrophotometric analysis (A260/A280) confirmed the absence of proteins and nucleic acids, respectively in the isolated EPS. Phytochemical analysis revealed the absence of flavonoids, alkaloids, tannins, cardiac glycosides and quinones in EPS confirming the presence of only polysaccharides in it. The four fractions were prepared from EPS by column chromatography by increasing the polarity of mobile phase. First fraction was obtained by running ethyl acetate in column. Subsequently, the increase of polarity of mobile phase resulted into three different fractions; 20% methanol: dichloromethane (DCM): Methanol, 50% methanol: DCM and 100% methanol.
Glucose Content and Proliferation Effect of EPS
Glucose content in ethyl acetate fraction was significantly higher than crude and other fractions of EPS followed by 20% DCM: Methanol and 50% DCM: Methanol (Fig. 1). Proliferation experiment showed that ethyl acetate have proliferative index more than one which is close to con A (positive control) representing cell growth promoting effect on PBMC. Proliferative index close to 1 was observed with other fractions indicating no effect on PBMC (Fig. 2). In case of RAW 264.7 cells, crude and all fractions have shown inhibition in cell growth while ethyl acetate fraction exhibited significantly higher cell growth inhibition (Fig. 2).
Cell growth effect of different fractions and crude EPS on PBMC and RAW 264.7 macrophages. Bars sharing a common upper and lower case letter among the treatments of PBMC and RAW cells respectively are not significantly different at p < 0.05. Proliferation index is the ratio of absorbance of the ConA/LPS/crude and fractionated exopolysaccharides treated and untreated cells. Con A: Concanavalin A, positive control for PBMC, LPS: Lipopolysaccharide, positive control for RAW 264.7 cell lines. ETAC: (Ethyl acetate), MeOH (Methanol), DCM (Dichloromethane)
Effect of Ethyl Acetate Fraction on PBMC
Proliferation of PBMC significantly increased with increase in concentration of ethyl acetate fraction. At higher concentrations (1000 and 1500 µg/mL), the proliferative index was more than one indicating the enhancement in cell proliferation (Fig. 3). On the hand, the proliferative index is less than one at lower concentration which showed the inhibition in cell growth (Fig. 3). In order to assess the pattern of cytokines production, three different cytokines (IFN-γ, TGF-β and TNF-α) levels in PBMC culture were estimated by ELISA. The production of all three cytokines have shown a trend of increase with concentration (Fig. 4). The trend of IFN-γ was similar like cell growth of PBMC which showed decreased production at lower concentration. On the other hand, ethyl acetate fraction treatment appears to have no effect on TGF-β and TNF-α at lower concentration as fold change is close 1. The effect on TNF-α was most promising as it has significantly increased production at higher concentrations (750 and 1000 µg/mL) (Fig. 4) as compared to other two cytokines (TGF-β and TNF-α). In all experiments of cytokines, Con A was used as positive control and the fold change was always more than one.
Effect of Ethyl Acetate Fraction on RAW 264.7
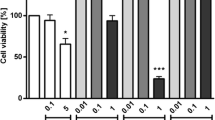
Ethyl acetate fraction treated RAW 264.7 resulted decrease in proliferative index with dose (Fig. 5). Apart from 250 µg/mL, all four concentrations of ethyl acetate fraction have proliferative index less than 1 showing cell growth inhibition effect and the IC50 value was 691 µg/mL. The production of nitric oxide from RAW 264.7 macrophage cell lines was assessed with the help of griess reagent. NO production was observed to be decreased as concentration increased from 250 to 1500 µg/mL where IC50 was 630 µg/mL (Fig. 5).
Cell growth and NO release in ethyl acetate fraction treated RAW 264.7. Bars sharing a common lower and upper case letter among the treatments for cell proliferation and nitric oxide production respectively are not significantly different at p < 0.05. Proliferation index and Fold change is the ratio of absorbance of the LPS/ethyl acetate treated and the untreated cells. LPS: Lipopolysaccharides, positive control
FTIR and Mass Spectroscopy Analysis of Ethyl Acetate Fraction
The broad spectrum at 3400 cm−1 is identified as hydroxyl (–OH) stretching vibration of the polysaccharide (ESM_1) [14]. The strong peak with multiple splits in the range (2800 and 3000 cm−1) represents C-H stretching. The stretching vibration of C=O (1633 cm−1), and the spectrum of C–H (1384 cm−1) is typical characteristics of polysaccharides [26]. The peak of 833 and 684 representing the presence of halo group and the band in between 1350 and 1342 appears to show the presence of sulphur group. The m/z ratio represented the molecular weight of the compounds present in the sample whereas intensity may indirectly correspond to the amount of the particular fraction present in any compound. The positive ion reflector mode represents a series of masses m/z 181.56 (hexose), 163.58 (methyl pentose), and 224.60 (ion associated hexose) (ESM_2). The obtained spectrum showed that ethyl acetate fraction as penta-saccharide (886.54 and 887.56) in very high intensities (> 10,000) which are hetero-oligosaccharides of hexose and pentose sugar (ESM_2). Ethyl acetate fraction have shown m/z of tri-saccharide and tetra-saccharide which may be due to fragmentation of penta-saccharide. The mass spectrum of these hetero-oligosaccharides appears to be associated with different ions such as sulphur, sodium, magnesium, and chlorine.
Discussions
Several microalgae such as Chlorella, Ascophyllum, Palmeria, Dunaliella and Porphyra are reported to produce polysaccharides extracellularly with antioxidant, anti-tumour, anti-glycemic, anti-lipidimic, anti-viral, anti-bacterial and immunomodulatory activities [4]. D. salina is also reported to produce polysaccharides extracellularly but their applications are mainly focused in food industry [27]. Studies on the exploration of EPS isolated from D. salina for immunomodulatory properties are not being reported. In this study, penta-saccharide (Ethyl acetate fraction) obtained by column chromatography exhibited immunomodulatory effect against PBMC and RAW 264.7 cells.
Immunomodulators are the response modifier bio-compounds known to act either as a stimulator or suppressor of immune system and have been widely used for several therapeutic purposes. The possibility of natural products to be used as immunomodulators opens new horizons for the immunotherapeutics. Glucans, fucoidans, fructans and xylans isolated from natural sources were reported to interact directly or indirectly with different immune cells (monocytes, macrophages, T cells and neutrophils) that lead to activation of immune system [6]. EPS isolated from Porphyridium tricornutum and Chlorella stigmatophora have shown immunostimulatory and immunosuppressant activity against PBMC and macrophages, respectively [5]. The sulphated polysaccharides from Ulva rigida (pKG 03) activated the production of nitric oxide and stimulated the production of cytokines from mouse macrophage cell lines [28]. Previously, the size exclusion chromatography has been used to separate the fraction of polysaccharides isolated from Taxillus chinensis and Uncaria rhyncophylla to obtain three and two fractions, respectively reporting the enhancement of NO and TNF-α production in J774 A.1 mouse macrophages [29]. In one study, EPS from D. salina was fractionated using DE-52 ion exchange chromatography to obtain two fractions [30]. Here, the crude EPS was fractionated by silica gel chromatography to obtain four different fractions. Comparative analysis was first carried out of crude and four different fractions for glucose content and cell growth (PBMC and RAW 264.7). Ethyl acetate fraction was found to have higher sugar content, significantly high cell growth promotion (PBMC) and inhibition (RAW 264.7) as compared to crude and other three fractions of EPS, thus ethyl acetate fraction was selected for immunological assessment.
Cytokines plays key role in regulating immune system hence, the production of two pro-inflammatory (IFN-γ, and TNF-α) and regulatory (TGF-β) cytokines was estimated in PBMC culture in addition to cell proliferation assessment. IFN-γ and TNF-α are pro-inflammatory cytokines which provide immunity against viral, bacterial and parasitic infections [31,32,33]. IFN-γ secreted from cytotoxic T cells and Natural killer cells plays key role in intracellular pathogens and cancer [33]. TNF-α is produced by monocytes/macrophages and induces apoptotic or necrotic cell death to infected cell and cancer [31]. TFG-β is a regulatory cytokines produced by CD4 + CD25 + regulatory T cells, macrophages and monocytes and exhibit different immunological phenomenon including anti-inflammation and immunosuppression to maintain immune homeostasis and promote immune tolerance [32, 34]. Ethyl acetate fraction has shown concentration dependent increased proliferation index and cytokines production in PBMC culture. Enhanced cell proliferation was observed at higher concentration while growth inhibition was observed at lower concentration. The effect of Ethyl acetate fraction on IFN-γ and TGF-β release was not much promising but the most remarkable result was observed for TNF-α. Hence, it may be enhanced cell proliferation is associated with production of TNF-α, an inflammatory cytokines.
RAW macrophage cell line is most commonly used to assess the effect of bioactive natural products for different immunomodulatory activities which includes cell proliferation, nitric oxide production and phagocytosis [35, 36]. Thus, RAW cells were used for evaluating the cell proliferation in addition to PBMC. Lipopolysaccharide (LPS) was found to inhibit and enhance the cell growth of macrophages which may be dependent on concentrations and time of exposure [37, 38]. Wu et al. [36] has used LPS as positive control where it was found to be enhanced cell proliferation in RAW cells. In another report, LPS was studied for their growth in RAW cells till 21 days and cells was found to grow with a doubling time of 35 h [39]. LPS is commonly used for stimulation of the RAW cells to produce NO, hence in the current study LPS was used as a common positive control for both NO production and cell proliferation. In case of RAW cells, ethyl acetate fraction was found to exhibit dose dependent inhibitory effect on cell growth and nitric oxide production. The variation in immunological effect on PBMC and RAW cells may be explained by the fact of that they are different types of cells. PBMC is a mixture of mononuclear cells which consists of lymphocytes and monocytes while RAW is single type of immortal macrophages cell lines. It appears that ethyl acetate fraction lead to diminish in macrophage growth while it may increase the proliferation of other immune cells present in PBMC.
In experimental model of mice treated with CT26 mouse colon cancer cell line, exopolysaccharide isolated from Rhizopus nigricans have shown enhanced production of IL-2 and TNF-α in serum [40]. In other study, exopolysaccharide of Pediococcus pentosaceus have shown concentration dependent enhanced splenocytes proliferation in anti-CD3/CD28-primed primary splenocytes [41]. These reports are in accordance with current study where EPS treatment have shown the enhanced cell proliferation and TNF-α production in PBMC. Further, there is need to validate these immunomodulatory effect of D. Salina EPS in some experimental model.
The FTIR spectra are interpreted by correlating the absorption spectra of unknown compound with the known absorption peaks for different functional groups. Previously, FTIR spectroscopy in D. salina has been performed for the crude EPS isolated from D. salina and peaks of –NH, C–O, C–X, C–N, N=C=O and C–C groups were observed [14]. In contrast to this study where crude EPS was analyzed, current study deal with fractionated EPS. Ethyl acetate fraction showed the presence of –OH, C=O, and C–H, group which are characteristic of polysaccharides. The presence of halo and sulphur group band may indicate that ethyl acetate fraction may be sulphated or halogenated.
MALDI-TOF analysis of crude EPS in D. salina represented the m/z ratio corresponding to the deprotonated hexose sugar, disaccharide (hexoses and pentoses), Mg associated disaccharide (hexoses and pentoses) and tetra-saccharides in previous study [42]. Current mass spectrum results revealed the presence of two types of monosaccharides which includes hexose (glucose/galactose/fructose) and methyl pentose (Rhamnose). It is in agreement with previous report where the presence of four monosaccharides (glucose, galactose, fructose and xylose) has been confirmed through HPLC in the crude EPS of D. Salina [14]. Previous studies indicated the presence of sugar associated with ions like Mg, S, Na, and P in crude EPS of D. salina. In accordance with this report, ethyl acetate fraction also appear to contain different ions associated sugar. Apart from that, m/z corresponding to the polysaccharides with up to five monosaccharide units (m/z = 887.56 and 886.54) in very high intensities (> 10,000) indicating the penta-saccharides nature. Thus, ethyl acetate fraction appears to be penta-saccharide (886.54 and 887.56) which are hetero-oligosaccharides of ions associated hexose and pentose sugar.
Conclusion
In summary, ethyl acetate fraction (higher concentration) showed immunostimulatory effect by enhancing the cell growth and TNF-α production in PBMC. Inhibitory effect was observed in RAW 264.7 cells as it diminished the cell growth and NO production. The FTIR spectrum of OH, C=O, and C–H confirmed the presence of polysaccharides. Ethyl acetate fraction of EPS appears to be penta-saccharides consisting of hexoses and pentoses with association of ions. Hence, these data indicates that penta-saccharide (ethyl acetate fraction) isolated from D. salina have the potential to improve the immune dysregulation.
References
Sutherland IW (1996) Extracellular polysaccharides. Biotechnol Prod Prim Metab. https://doi.org/10.1002/9783527620999.ch16f
Borchers AT, Krishnamurthy A, Keen CL, Meyers FJ, Gershwin ME (2008) The immunobiology of mushrooms. Exp Biol Med 233:259–276. https://doi.org/10.3181/0708-MR-227
Zhang Q, Li N, Zhou G, Lu X, Xu Z, Li Z (2003) In vivo antioxidant activity of polysaccharide fraction from Porphyra haitanesis (Rhodephyta) in aging mice. Pharmacol Res 48:151–155. https://doi.org/10.1016/S1043-6618(03)00103-8
de Jesus Raposo MF, de Morais AMB, de Morais RMSC (2015) Marine polysaccharides from algae with potential biomedical applications. Mar Drugs 13:2967–3028. https://doi.org/10.3390/md13052967
Guzman S, Gato A, Lamela M, Freire-Garabal M, Calleja J (2003) Anti-inflammatory and immunomodulatory activities of polysaccharide from Chlorella stigmatophora and Phaeodactylum tricornutum. Phytother Res 17:665–670. https://doi.org/10.1002/ptr.1227
Ferreira SS, Passos CP, Madureira P, Vilanova M, Coimbra MA (2015) Structure–function relationships of immunostimulatory polysaccharides: a review. Carbohydr Polym 132:378–396. https://doi.org/10.1016/j.carbpol.2015.05.079
Zhao G, Kan J, Li Z, Chen Z (2005) Characterization and immunostimulatory activity of an (1 → 6)-aD-glucan from the root of Ipomoea batatas. Int Immunopharmacol 5:1436–1445. https://doi.org/10.1016/j.intimp.2005.03.012
Nosáľová G, Prisenžňáková L, Paulovičová E, Capek P, Matulová M, Navarini L, Liverani FS (2011) Antitussive and immunomodulating activities of instant coffee arabinogalactan-protein. Int J Biol Macromol 49:493–497. https://doi.org/10.1016/j.ijbiomac.2011.06.004
Borowitzka MA (1990) The mass culture of Dunaliella salina. In: Regional workshop on the culture and utilization of seaweeds, Cebu City (Philippines), 27–31 Aug 1990
de Morais MG, Vaz BdS, de Morais EG, Costa JAV (2015) Biologically active metabolites synthesized by microalgae. Biomed Res Int. https://doi.org/10.1155/2015/835761
Bobrov Z, Tracton I, Taunton K, Mathews M (2008) Effectiveness of whole dried Dunaliella salina marine microalgae in the chelating and detoxification of toxic minerals and heavy metals. J Altern Complement Med 14:S8–S9
Ben-Amotz A (1980) Glycerol production in the alga Dunaliella. In: Pietro AS (ed) Biochemical and photosynthetic aspects of energy production. Academia Press Inc, New York, pp 191–208
Ben-Amotz A (1995) New mode of Dunaliella biotechnology: two-phase growth for β-carotene production. J Appl Phycol 7:65–68
Mishra A, Jha B (2009) Isolation and characterization of extracellular polymeric substances from micro-algae Dunaliella salina under salt stress. Bioresour Technol 100:3382–3386. https://doi.org/10.1016/j.biortech.2009.02.006
Dai J, Wu Y, Chen S-w, Zhu S, Yin H-p, Wang M, Tang J (2010) Sugar compositional determination of polysaccharides from Dunaliella salina by modified RP-HPLC method of precolumn derivatization with 1-phenyl-3-methyl-5-pyrazolone. Carbohydr Polym 82:629–635. https://doi.org/10.1016/j.carbpol.2010.05.029
Singh P, Baranwal M, Reddy SM (2016) Antioxidant and cytotoxic activity of carotenes produced by Dunaliella salina under stress. Pharm Biol 54:2269–2275. https://doi.org/10.3109/13880209.2016.1153660
Starr RC (1987) UTEX-The culture collection of algae at the University of Texas at Austin. J Phycol 23:1–47
Link AJ, LaBaer J (2011) Trichloroacetic acid (TCA) precipitation of proteins. Cold Spring Harbor Protoc 2011:pdb. prot5651
Masuko T, Minami A, Iwasaki N, Majima T, Nishimura S-I, Lee YC (2005) Carbohydrate analysis by a phenol–sulfuric acid method in microplate format. Anal Biochem 339:69–72. https://doi.org/10.1016/j.ab.2004.12.001
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254. https://doi.org/10.1016/0003-2697(76)90527-3
Aziz MA (2015) Qualitative phytochemical screening and evaluation of anti-inflammatory, analgesic and antipyretic activities of Microcos paniculata barks and fruits. J Integr Med 13:173–184. https://doi.org/10.1016/S2095-4964(15)60179-0
Andriamanantoanina H, Chambat G, Rinaudo M (2007) Fractionation of extracted Madagascan Gracilaria corticata polysaccharides: structure and properties. Carbohydr Polym 68:77–88. https://doi.org/10.1016/j.carbpol.2006.07.023
Lohia N, Baranwal M (2017) Immune response of highly conserved influenza A virus matrix 1 peptides. Microbiol Immunol 61:225–231. https://doi.org/10.1111/1348-0421.12485
Vasundhara M, Baranwal M, Kumar A (2016) Fusarium tricinctum, an endophytic fungus exhibits cell growth inhibition and antioxidant activity. Indian J Microbiol 56:433–438. https://doi.org/10.1007/s12088-016-0600-x
Pandey R, Maurya R, Singh G, Sathiamoorthy B, Naik S (2005) Immunosuppressive properties of flavonoids isolated from Boerhaavia diffusa Linn. Int Immunopharmacol 5:541–553. https://doi.org/10.1016/j.intimp.2004.11.001
Guo X, Wang X, Liu J (2016) Composition analysis of fractions of extracellular polymeric substances from an activated sludge culture and identification of dominant forces affecting microbial aggregation. Sci Rep 6:28391. https://doi.org/10.1038/srep28391
Ben-Amotz A, Avron M (1989) The biotechnology of mass culturing Dunaliella for products of commercial interest. Algal Cyanobacterial Biotechnol 91–114
Ray B, Lahaye M (1995) Cell-wall polysaccharides from the marine green alga Ulva “rigida”(Ulvales, Chlorophyta). Chemical structure of ulvan. Carbohydr Res 274:313–318. https://doi.org/10.1016/0008-6215(95)00407-6
Zhang L, Koyyalamudi SR, Jeong SC, Reddy N, Bailey T, Longvah T (2013) Immunomodulatory activities of polysaccharides isolated from Taxillus chinensis and Uncaria rhyncophylla. Carbohydr Polym 98:1458–1465. https://doi.org/10.1016/j.carbpol.2013.07.060
Xu X, Tong W, Lei Y, Yao S (2007) Separation and purification of extracellular polysaccharides from Dunaliella salina. J Food Sci Biotechnol 26:28–33
Idriss HT, Naismith JH (2000) TNFα and the TNF receptor superfamily: structure-function relationship (s). Microsc Res Tech 50:184–195. https://doi.org/10.1002/1097-0029(20000801)50:3%3c184:AID-JEMT2%3e3.0.CO;2-H
Maspi N, Abdoli A, Ghaffarifar F (2016) Pro-and anti-inflammatory cytokines in cutaneous leishmaniasis: a review. Pathog Glob Health 110:247–260. https://doi.org/10.1080/20477724.2016.1232042
Schoenborn JR, Wilson CB (2007) Regulation of interferon-γ during innate and adaptive immune responses. Adv Immunol 96:41–101. https://doi.org/10.1016/S0065-2776(07)96002-2
Huber S, Schramm C (2006) TGF-beta and CD4+ CD25+ regulatory T cells. Front Biosci 11:1014–1023
Li M, Yan YX, Yu QT, Deng Y, Wu DT, Wang Y, Ge YZ, Li SP, Zhao J (2017) Comparison of immunomodulatory effects of fresh garlic and black garlic polysaccharides on RAW 264.7 Macrophages. J Food Sci 82:765–771. https://doi.org/10.1111/1750-3841.13589
Wu J, Li M, Liu L, An Q, Zhang J, Zhang J, Li M, Duan W, Liu D, Li Z (2013) Nitric oxide and interleukins are involved in cell proliferation of RAW 264. 7 macrophages activated by viili exopolysaccharides. Inflammation 36:954–961. https://doi.org/10.1007/s10753-013-9626-y
Chang C-Y, Tucci M, Baker RC (2000) Lipopolysaccharide-stimulated nitric oxide production and inhibition of cell proliferation is antagonized by ethanol in a clonal macrophage cell line. Alcohol 20:37–43. https://doi.org/10.1016/S0741-8329(99)00054-3
Moore RN, Steeg PS, Männel DN, Mergenhagen SE (1980) Role of lipopolysaccharide in regulating colony-stimulating factor-dependent macrophage proliferation in vitro. Infect Immun 30:797–804
Zhuang JC, Wogan GN (1997) Growth and viability of macrophages continuously stimulated to produce nitric oxide. Proc Natl Acad Sci 94:11875–11880. https://doi.org/10.1073/pnas.94.22.11875
Zhu L, Cao J, Chen G, Xu Y, Lu J, Fang F, Chen K (2016) Anti-tumor and immunomodulatory activities of an exopolysaccharide from Rhizopus nigricans on CT26 tumor-bearing mice. Int Immunopharmacol 36:218–224. https://doi.org/10.1016/j.intimp.2016.04.033
Shin JS, Jung JY, Lee SG, Shin KS, Rhee YK, Lee MK, Hong HD, Lee KT (2016) Exopolysaccharide fraction from Pediococcus pentosaceus KFT 18 induces immunostimulatory activity in macrophages and immunosuppressed mice. J Appl Microbiol 120:1390–1402. https://doi.org/10.1111/jam.13099
Mishra A, Kavita K, Jha B (2011) Characterization of extracellular polymeric substances produced by micro-algae Dunaliella salina. Carbohydr Polym 83:852–857. https://doi.org/10.1016/j.biortech.2009.02.006
Acknowledgements
The author would like to acknowledge Dr. M. Krishnamohan, Birla Institute of Scientific Research, Jaipur, India for providing the culture of Dunaliella salina and Dr. Akshey Jain from Nitin Nursing Home and Dr. Vandana Goyal, from Rajendra Hospital, Patiala for providing us the blood needed for carrying out the experiments. The authors also would like acknowledge Mr. Anoop Patiyal from SAIF, Punjab University, Chandigarh for mass spectroscopic analysis.
Funding
Not applicable.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
No competing financial interests exist.
Informed Consent
The informed consent was given by all the donors for the experiments.
Research Involving Human Participants and/or Animals
The study obtained the approval from the institutional ethical committee (IEC).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Goyal, M., Baranwal, M., Pandey, S.K. et al. Hetero-Polysaccharides Secreted from Dunaliella salina Exhibit Immunomodulatory Activity Against Peripheral Blood Mononuclear Cells and RAW 264.7 Macrophages. Indian J Microbiol 59, 428–435 (2019). https://doi.org/10.1007/s12088-019-00818-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12088-019-00818-w