Abstract
Application of sesame protein concentrate (SPC) is limited because of its weak functional properties. Investigations revealed that the grafting reaction occurred between maltodextrin and SPC, as supported by the results of differential scanning calorimetry (DSC) analysis. Circular dichroism (CD) and fluorescence emission spectra confirmed that the treatment altered the molecular structure of SPC. XRD analysis and scanning electron microscopy (SEM) revealed changes in the structural properties of the powders after the graft treatment. Conjugation treatment improved the emulsifying properties which analyzed by different techniques including particle size measurement, ζ-potential, and rheological analysis of emulsions. In addition, the emulsions prepared by the conjugated protein exhibited higher values of viscosity and consistency coefficient. This research showed that conjugation with maltodextrin improved the functional and emulsifying properties of SPC and this product can be introduced as a novel ingredient to develop different food emulsions.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Many food products such as ice cream, milk, salad, soft drinks, desserts, and sauces are based on emulsion structures [1]. Emulsions that have major functions in different food formulations are labile to destabilization because of thermodynamic instability issues. On the other hand, application of emulsifiers could improve the stability of O/W emulsions. Emulsifier absorption at oil–water interfaces can decrease interfacial tension, produce steric and/or electrostatic repulsion between the droplets, and therefore, reduce droplets coalescence or flocculation [2].
Most of food proteins include both charged hydrophilic and hydrophobic regions, and therefore can reduce the surface tension of solutions and relieve at the emulsion interfaces [3]. However, functional and emulsifying properties of proteins are restricted due to different issues in relation to stability, solubility, structure, pH dependence, etc. To overcome the limitations mentioned above, proteins can be conjugated with polysaccharides [4]. Functional properties of proteins is improved through conjugation, and the stability of the compound is boosted [5]. The conjugation between proteins and carbohydrates through the maillard reaction has shown to be a favorable method among different physical, chemical, and enzymatic treatments [6]. Recently, many researchers have investigated the effect of conjugation on the secondary structure, tertiary structure, morphology and functional properties of plant protein isolates. For example, canola protein, oat protein, rice protein, and soy protein were studied [7,8,9,10]. It has been revealed that by-products of sesame processing in many countries compose a high amount of crude protein (50%) which can be regarded as a valuable by-product of the sesame oil industry. Sesame protein can be applied as a possible protein source for human usage. It should be noted that functional characteristics such as solubility, emulsifying, foaming stability, and gelling properties of sesame protein are weak [11], and therefore, there are few industrial applications for sesame protein. Hence, there is a need for the food industry to enhance the desired properties of the protein by its green modification methods such as conjugation with carbohydrates. However, little information on conjugates of SPC–maltodextrin was found in the literature. Previous studies related to this work showed that functional properties of sesame protein could be improved by conjugation with maltodextrin by dry-heating [12].
In this paper the effects of conjugation specifically on emulsifying properties of sesame protein were studied by droplet size analysis (coalescence and flocculation factor), ζ-potential and rheological properties. Furthermore, the conjugation treatment was studied by CD, DSC, SEM, amino acid analysis and XRD for better illustration of the emulsifying characteristics of the conjugated product from structural prospects. The major novelty of this work is selecting sesame protein concentrate as a low expense and nutritious protein source. These investigations were carried out to further grasp the structure and characteristics of sesame protein concentrate-maltodextrin conjugates and explain the potential application of these conjugates in the food industry.
Experimental
Chemicals and materials
Defatted sesame paste (DSP) which is the main by-product of Ardeh (milled roasted sesame) oil processing was provided by Barsam Shirini Salamat Co (Ardakan, Yazd, Iran). Maltodextrin with a dextrose equivalent value of 19, was obtained from Corcoran Chemicals Ltd. (Dublin, Ireland). All other reagents were sourced from Sigma-Aldrich (MA, USA) and Merck (Darmstadt, Germany).
Preparation of sesame protein concentrates–maltodextrin conjugates
SPC powder was obtained from the sesame processing by-products by the acid precipitation method as reported in a previous study. The final product was consisted of 81% protein, < 1.0% moisture content. The SMC powder was produced as described in our former research [12].
Preparation of emulsion
To prepare o/w emulsions, 5 mL of pure sesame oil and 45 mL of SCP or SMC solutions were homogenized at 14,000 RPM for 5 min using an Ultra-Turrax T 25 (IKA, Staufen, Germany). Then the samples were sonicated with an ultrasound probe (CPX, Geneva, Switzerland) at 40 KHz for 5 min to get the final emulsions [7].
Differential scanning calorimetry (DSC) measurements
DSC experiments were carried out with a thermal analyzer (Mettler Toledo Co, Switzerland). The pans containing samples were heated at a rate of 5 °C min−1, in the calorimeter from 20 to 200 °C. Peak or denaturation temperature (Td), denaturation enthalpy change (∆H) and transition glass temperature (Tg) were estimated from the thermogram using Star SW version 9.20 software [13].
Amino acid analysis
Initially, the protein samples were hydrolyzed with 6 N HCl for 24 h at 110 °C in a sealed tube. Then an HPLC system (KNAUER, D-14,163, Germany), using Spheribound 80-5 ODS2 column was applied to study the amino acid composition.
Rheological analysis
Rheological measurements were performed using a Brookfield rheometer (DV-II plus pro, Massachusetts, US). Viscosity testing was performed at a shear rate range of 0–100 s−1 at 25 °C. The connection between apparent viscosity and shear rate has extensively been determined by an empirical power-law model [14].
where η is the viscosity (Pa s− 1), γ the strain rate (s− 1), k the consistency coefficient and n is the flow behavior indices.
Creep test was performed at 2 Pa for 300 s and the following Burger’s model was used to express the rheological properties of the emulsions:
where \({\upgamma }\left(\text{t}\right)\) is total deformation, \({{\upsigma }}_{0}\,(\text{Pa})\) is stress, \({{{\upsigma }}_{0}}/{{\text{G}}_{1}}, {{{\upsigma }}_{0}}/{{\text{G}}_{2}}\) are elastic index, \(\text{M}\text{u}\) is viscose index, and t (s) is deformation time.
X-ray diffraction analysis
An X-ray diffractometer gave X-ray diffraction (XRD) patterns (XRD-6100, Shimadzu, Tokyo, Japan) with scanning rate of 1°/min, step size of 0.02 and 2θ from 5° to 60° [15].
Determination of coalescence and flocculation
To measure the coalescence index (Ci) and flocculation factor (Ff) samples in the presence and absence of 1% Sodium dodecyl Sulfate (SDS) containing 1% SPC and SMC, were diluted with distilled water and the (Eqs. 3, 4) were used [16].
where d43 + SDS, d43-SDS, d43 + SDS,in, and d43 + SDS,t are the volume-weighted mean particle diameter of the emulsion droplets in the presence and absence of 1% SDS, the volume weighted mean particle diameter of the emulsion droplets in the presence of 1% SDS at time 0 and the determined storage time (24 h), respectively.
Analysis of ζ-potential
ζ-potential was determined by a Zetasizer at 25 °C (Brookhaven Ltd., USA) for all emulsion samples which were diluted with deionized water (1:100) before examination [16].
Circular dichroism (CD) analysis
Circular dichroism analysis was carried on by an Avive- model 215 circular dichroism spectrometer (Lakewood, NJ, USA). Quartz cuvette (10 mm) with a protein concentration of approximately 0.5 mg mL−1 in phosphate buffer (pH 7.6) by scanning over a wavelength range from 250 to 320 nm were used to carry out near-UV CD spectral experiment [17].
Fluorescence emission spectrum
The tryptophan emission fluorescence spectra were measured by LS 45 Fluorescence Spectrometer (PerkinElmer, Inc. USA) with 0.04 mg mL−1 protein solution. The florescence intensity of each sample was registered with an excitation wavelength of 280 nm and emission wavelengths of 320–400 nm, with a permanent excitation and emission slit of 5 nm [18].
Scanning electron microscope (SEM) analysis
The structures of sesame protein concentrate and conjugate were investigated by scanning electron microscope (DSM960A; Zeiss Ltd, Germany). Illustration of the samples were gained at an electron hastening voltage of 15 kV and a magnification of 500 × [10].
Statistical analysis
All measurement were repeated at least three times, and the results were represented as means ± standard deviations. Analysis of variance (ANOVA) was performed, and means comparisons were conducted by Duncan’s protected least significant difference (p < 0.05). Data were analyzed with Minitab 17 (Minitab Ltd, Coventry, UK, 2007) statistical analysis package.
Results and discussion
DSC analysis
The results of Differential scanning calorimetry (DSC) are shown in Table 1. This analysis was carried out to examine the thermal characteristics of protein–polysaccharide complexes [13]. As can be seen in Table 1, amorphous fractions of the protein or conjugate experienced a glass to rubber transition (Tg). Afterwards, it revealed a first-order transition depending on the melting of the ordered regions, which was displayed by variation in the enthalpy. SPC and SMC showed semi-crystalline structures according to these thermograms. The glass transition temperatures (Tg) connected to the SMC was much lower (about 74 °C) than that of the SPC. The mobility of the amorphous section in the native protein was more limited and was higher in the native globular protein in comparison to the conjugated structures. Therefore, the glass transition temperature was raised after conjugation treatment [19]. Thermal stability of proteins was displayed by the denaturation temperature (Td) while the energy needed to dominate non-covalent interaction was evaluated by the enthalpy value (∆H) [20]. According to Table 1, the denaturation temperature (Td = 186.55 °C) of conjugate was higher than that observed for native sesame protein (Td = 178.39 °C). It might be assumed that, due to the covalent supplementation of maltodextrin to SPC, molecular net negativity was improved and both steric hindrance and electrostatic repulsion forces were increased [7]. Hence, the conjugated product in hot processing could be more stable against heat- induced aggregation [21]. Denaturation enthalpy change (∆H = 37.54 J g−1) of conjugate was reduced in comparison with SPC. This decline showed partial unfolding of the tertiary structure persuaded by glycosylation. This partial unfolding might partially explain the increase in emulsifying properties of conjugate as compared to SPC. In addition, this observation proposed that a decrease in the secondary structure content (α-helix) and an increase of random coils in SMC occurred. Similar results were gained through the glycosylation of soy protein isolate with dextran [8].
Amino acid composition analysis
The amino acid composition of SPC and SMC are described in Table 2. The reducing free amino group content showed that SPC was success-fully related to maltodextrin under the conditions evaluated. As shown from Table 2, the contents (39.46−38.32%) of hydrophobic amino acids in both SPC and SMC are lower than those (60.80−61.49%) of hydrophilic amino acids. The covalent attachment of carbonyl groups in reducing sugars with free amino groups in proteins produced Schiff base. Therefore, the lack of available primary amino groups displayed the sugar reactivity in the Maillard reaction [22, 23]. In comparison with the sesame protein, conjugation reduced the quantities of Cys and Lys in all conjugates remarkably (72% and 28%, respectively). Therefore, it could be assumed that Cys and Lys were the basic grafting reaction sites of SPC and maltodextrin. The productivity of other amino groups reacting with maltodextrin was affected by conformation changing around Lys residues and, influenced the amount of amino groups [9].
Rheological analysis
The viscosity of emulsions containing sesame protein and conjugate remarkably reduced with gaining the shear rate, explaining a shear-thinning behavior (Fig. 1). According to the results of rheological investigations, emulsion stabilized by the conjugate had higher viscosities than the emulsion containing sesame protein concentrate. This suggested that the solubility of SMC was enhanced, and the tendency of the protein to form aggregates/clusters in the continuous phase was reduced. In other words, emulsions stabilized by conjugates provided higher viscosities in the continuous phase [24].
The flow behavior data of the emulsion samples were successfully fitted to the power law model. Table 3 displays the power law parameters. The power law model revealed n values of 0.85 for emulsions containing SPC and 0.73 for emulsions containing SMC with the regression coefficients (R2) of 0.99 (Table 3). The parameter n provide data about the results of shear on the evaluated system. Both emulsions produced from the protein concentrate and the conjugated substance presented n values lower than one showing the shear-thinning behavior of the system. The n value was lower for SMC. The viscous nature of the system was displayed by the consistency index and was increasing with the application of SMC (Table 3). The colloidal status of the continuous phase was a main factor that influenced the average particle size [24]. The existence of most small molecular weight molecules could increase the apparent viscosity of the emulsion system containing SMC [25].
Results of creep test show G0 and G1 of the emulsion samples with conjugate (0.085, 0.16 Pa) represented a rising values than the emulsions with protein (0.061, 0.12 Pa), which suggested the existence of a weak gel network. Besides, Mu was higher in the emulsion with conjugate (1.9 Pa s) that represents the viscosity of the emulsion system (Fig. 2). This suggested that conjugate and protein solutions had low values of the elastic recovery index, which was a specification of the behavior of a viscous fluid. The creep curve suggests the emulsions prepared from both compounds showed viscous behaviors with little elastic storage and emulsion with conjugate was more viscose than protein system (Fig. 3).
XRD analysis
Figure 4 shows XRD patterns of maltodextrin, SPC and SMC. X-ray diffraction analysis provided determination of amorphous or crystalline nature of molecules and gaining direct structural information [15]. In XRD diffractograms, crystalline fractions related to pointed peaks, while broad and spread background explained amorphous parts [26]. According to the diffractograms in Fig. 4, maltodextrin had the amorphous nature of the material that was evident by the diffused or hollow structure X-ray pattern, both the SMC and SPC had a semi-crystalline structure with different degrees of crystallinity. SMC showed strong reflections at 22°, but the intensity of a strong specification reflection at 2θ about 22° decreased in comparison to the pure SPC. Therefore, the crystalline structure of SPC was gradually demolished by conjugation with maltodextrin and their relative crystallinity significantly decreased from 56.3 to 36.6%. Conjugation with polysaccharides could develop the protein structures and simplify polymeric chain mobility of the protein, therefore reduced the crystalline structures [15].
SEM analysis
Data about the effect of glycosylation on the microstructure of the SPC and SMC was established with scanning electron microscopy. As can be seen in Fig. 5, the particles of maltodextrin have a very high affinity for moisture so there are some particles sticking to each other and composed of coarser different shaped granules, the particles of SPC powder are non-uniform with irregular shapes. After glycosylation, the surface structure altered and became more compressed than those of SPC. It might be because maltodextrin could interfere with the non-covalent bonds of SPC within glycosylation, which diminished the protein molecular aggregation. It could make protein molecular unfold as accepted by the results of CD analysis. These results proposed that conjugation might influence their functionality of the powders formed within freeze-drying of the protein solutions. Other researchers have reported similar results suggesting that surface structure of soy protein isolate and gum acacia conjugates in comparison to soy protein isolate became looser [27, 28].
Effect of conjugation on ζ-potential
As shown in Fig. 6, the ζ-potential (mV) of the samples found out in this study at pH 3–7. The ζ-potential evaluation displayed that at pH 4.2 which was known as the isoelectric point (IEP) of SPC, the emulsions containing SPC had a neutral charge. At this pH, fickle emulsions were got, because emulsions containing SPC did not present enough repulsive forces to overcome droplet aggregation. At higher pH values, the emulsions were not at the IEP of SPC, and therefore, greater ζ-potential of the SPC-stabilized emulsion was obtained, and the emulsion was more stable. It was clear that after conjugation with maltodextrin, positively charged N-termini of the protein fraction involved, so a decline of positive charges occurred. Thus, it was expected a diminishment of ζ-potential of SPC–maltodextrin mixtures when conjugation occurred [29]. Further, the existence of maltodextrin altered the isoelectric point of the emulsions containing the SMC to a lower pH value. According to Fig. 6, emulsions with SMC have an IEP at a pH of about 3.4. These results explained the emulsions containing SMC at pH 4.2 were stable. As expected, the ζ-potential of the sesame protein droplets was higher, while that of the conjugate-coated droplets was powerfully negative so colloidal stability increased because of the conjugation between SPC and maltodextrin (p < 0.05).
CD analysis
Figure 7 presents the near-UV CD spectra calculated in this work. A fingerprint of protein tertiary structure prepared by the near-UV CD spectrum [8]. Aromatic amino acids display CD spectra in the near-UV region (250–320 nm). Every amino acid disposed a specific wavelength outline. Tyrosine residue in near-UV CD spectrum of SPC represented a spectrum with a positive dichroic band around 270 nm. Peak close to 305 nm is referred to as the tryptophan residue; the phenylalanine residue displayed weaker but sharper bands with fine a structure between 255 and 260 nm. This result is similar to other report [7]. The intensity of all bands of SMC was lower than that for SPC, despite SMC showed an indistinguishable spectrum. This is an obvious sign of extremely lack of tertiary conformation of the proteins in SMC (comparative to those in SPC). Also, the pliability of the SMC structure improved, allowing a superior presentation of its functional specifications.
Fluorescence spectral analysis
The fluorescence extent of conjugates was less than the pure protein, which proposed that there was a change for tyrosine or tryptophan residues presence in the environment for sesame protein and conjugate with λmax values of 340, 350 nm, respectively. The movement towards longer λmax explained that the tryptophan residues within the protein molecule were subjected to a more hydrophilic environment (Fig. 8). Because it affected by the conjugation of the maltodextrin, owing to the partial unfolding of the protein [30]. Hence, the disposal of tryptophan to hydrophilic environment might confirm the partial unfolding of the C-terminal domain that was in agreement with the decrease of α-helix described in previous work. Non-polar groups might become exposed to the surrounding aqueous phase if the protein undertook a conformational change, which contributed to a decreasing in fluorescence intensity [31]. It suggested the SMC had undergone a conformational change and had less compact tertiary structure as suggested by near-CD analysis.
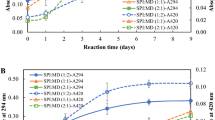
Coalescence and flocculation measurement
Coalescence index (Ci) and flocculation factor (Ff) of SPC and SMC are shown in Table 4. Flocculation factor (Ff), is representative of flocculation destabilization of emulsion within storage time (0–3 days). The major result of conjugate was to lessening the Ci values after 3 days, because of the decreasing in the flocs level (Ff). SMC with lower Ci had a smaller rate of changes in d32 and d43, in comparison to SPC. Repulsive interactions (e.g. steric and electrostatic) between the oil droplets were created by proteins in emulsions which formed the protective obstacle around fat droplets and hampered their coalescence [3]. The results showed the SMC was more impressive than SPC in stabilizing the emulsion versus the coalescence.
Conclusions
In the current research, SPC-maltodextrin conjugates (SMC) were prepared during controlled dry heating and the obtained SMC was proved to exhibit significantly improved emulsifying properties. Smaller particle sizes, higher consistency coefficients, lower flow behavior indices and enhanced emulsifying stabilities were observed for the SMC emulsions when compared to the emulsions stabilized by SPC. Amino acid analysis indicated that cysteine and lysine were the overcoming binding sites of sesame protein concentrate and maltodextrin, as the quantities of Cys and Lys in the conjugated protein was reduced remarkably. Thermal analysis illustrated that protein–polysaccharide cross-linkages established by the Maillard reaction increased the thermal stability of the conjugates. The physicochemical, conformational and functional properties of sesame protein concentrate were changed by conjugation. The XRD profile for SMC showed more crystallinity than sesame protein. Emulsions prepared with SMC during storage explained smaller oil droplets according to optical microscopy evaluations. Improving emulsifying properties with SPC-maltodextrin conjugates was affected by enhanced steric repulsion, limiting the aggregation and coalescence of the droplets. The surface structure of protein powders became weaker and spongier as illustrated by the SEM results. These changes boosted the functionality and thermal stability of SMC that could be partly explained by the protein structure alterations. The investigations confirmed that SMC adopted an extended conformation instead of a folded one with decreased protein tertiary conformation which was confirmed by the near-UV CD spectrum. Overall, the results of this study could be helpful to develop the application of vegetable sources for the preparation of food emulsions through protein–polysaccharide conjugation.
References
A. Yemenicioğlu, S. Farris, M. Turkyilmaz, S. Gulec, A review of current and future food applications of natural hydrocolloids. Int. J. Food Sci. Technol. (2019). https://doi.org/10.1111/ijfs.14363
D.J. McClements, Emulsion stability, in Food emulsions: principles, practices, and techniques. (CRC Press, Boca Raton, London, New York, 2015), pp. 269–340
B. Bi, H. Yang, Y. Fang, K. Nishinari, G.O. Phillips, Characterization and emulsifying properties of β-lactoglobulin-gum Acacia Seyal conjugates prepared via the Maillard reaction. Food Chem. 214, 614–621 (2017)
W. Qu, X. Zhang, X. Han, Z. Wang, R. He, H. Ma, Structure and functional characteristics of rapeseed protein isolate–dextran conjugates. Food Hydrocolloids 82, 329–337 (2018)
L. Chevalier, L. Rioux, P. Angers, S. Turgeon, Study of the interactions between pectin in a blueberry puree and whey proteins: functionality and application. Food Hydrocolloids 87, 61–70 (2019)
A.M. Alashi, C.L. Blanchard, S.O. Agboola, J.A. Mawson, R.E. Aluko, Influence of enzymatic hydrolysis, pH and storage temperature on the emulsifying properties of canola protein isolate and hydrolysates. Int. J. Food Sci. Technol. 53, 2316–2324 (2018)
S. Pirestani, A. Nasirpour, J. Keramat, S. Desobry, J. Jasniewski, Structural properties of canola protein isolate-gum Arabic Maillard conjugate in an aqueous model system. Food Hydrocolloids 79, 228–234 (2018)
L. Zhong, N. Ma, Y. Wu, L. Zhao, G. Ma, F. Pei, Q. Hu, Characterization and functional evaluation of oat protein isolate-Pleurotus ostreatus β-glucan conjugates formed via Maillard reaction. Food Hydrocolloids 87, 459–469 (2019)
Y.H. Cheng, W.J. Tang, Z. Xu, L. Wen, M.L. Chen, Structure and functional properties of rice protein–dextran conjugates prepared by the Maillard reaction. Int. J. Food Sci. Technol. 53, 372–380 (2017)
C. Li Song, J. Ren, J.Peng Cheng, X. Hong Sun, N. Kumar Kopparapu, Y. Guo, Xue, Effect of glycosylation and limited hydrolysis on structural and functional properties of soybean protein isolate. J. Food Meas. Charact. 12, 2946–2954 (2018)
T.O. Fasuan, S.O. Gbadamosi, T.O. Omobuwajo, Characterization of protein isolate from Sesamum indicum seed: in vitro protein digestibility, amino acid profile, and some functional properties. Food Sci. Nutr. 6, 1715–1723 (2018)
A. Saatchi, H. Kiani, M. Labbafi, A new functional protein–polysaccharide conjugate based on protein concentrate from sesame processing by-products: functional and physico-chemical properties. Int. J. Biol. Macromol. 122, 659–666 (2018)
A.M. Hamdani, I.A. Wani, N.A. Bhat, R.A. Siddiqi, Effect of guar gum conjugation on functional, antioxidant and antimicrobial activity of egg white lysozyme. Food Chem. 240, 1201–1209 (2018)
K. Gabsi, M. Trigui, S. Barrington, A.N. Helal, A.R. Taherian, Evaluation of rheological properties of date syrup. J. Food Eng. 117, 165–172 (2013)
C. Sun, B. Liang, H. Sheng, R. Wang, J. Zhao, Z. Zhang, M. Zhang, Influence of initial protein structures and xanthan gum on the oxidative stability of O/W emulsions stabilized by whey protein. Int. J. Biol. Macromol. 120, 34–44 (2018)
Y. Chen, C. Wang, H. Liu, Q. Liu, B. Kong, Enhanced physical and oxidative stability of porcine plasma protein hydrolysates based oil-in-water emulsions by adding oxidized chlorogenic acid. Colloid Surf. A Physicochem. Eng. Asp. 558(5), 330–337 (2018)
K.X. Zhu, X.H. Sun, Z.C. Chen, W. Peng, H.F. Qian, H.M. Zhou, Comparison of functional properties and secondary structures of defatted wheat germ proteins separated by reverse micelles and alkaline extraction and isoelectric precipitation. Food Chem. 123, 1163–1169 (2010)
Y. Wang, J. Gan, Y. Zhou, Y. Cheng, S. Nirasawa, Improving solubility and emulsifying property of wheat gluten by deamidation with four different acids: effect of replacement of folded conformation by extended structure. Food Hydrocolloids 72, 105–114 (2017)
Y. Xu, M. Obielodan, E. Sismour, A. Arnett, S. Alzahrani, B. Zhang, Physicochemical, functional, thermal and structural properties of isolated Kabuli chickpea proteins as affected by processing approaches. Int. J. Food Sci. Technol. 52(5), 1147–1154 (2017)
F. Liu, D. Wang, C. Sun, D.L. McClements, Y. Gao, Utilization of interfacial engineering to improve physicochemical stability of β-carotene emulsions: multilayer coatings formed using protein and protein–polyphenol conjugates. Food Chem. 205, 129–139 (2016)
F. Zha, S. Dong, J. Rao, B. Chen, Pea protein isolate-gum Arabic Maillard conjugates improves physical and oxidative stability of oil-in-water emulsions. Food Chem. 285(1), 130–138 (2019)
P.X. Qi, Y. Xiao, E.D. Wickham, Changes in physical, chemical and functional properties of whey protein isolate (WPI) and sugar beet pectin (SBP) conjugates formed by controlled dry-heating. Food Hydrocolloids 69, 86–96 (2017)
M. Ali, Chemical, structural and functional properties of whey proteins covalently modified with phytochemical compounds. J. Food Meas. Charact. 13(4), 2970–2979 (2019)
Y. T.Sun, J. Qin, H. Xie, J. Xu, J. Gan, J. Wu, X. Bian, X. Li, Z. Xiong, B. Xue, Effect of Maillard reaction on rheological, physicochemical and functional properties of oat β-glucan. Food Hydrocolloids 89, 90–94 (2019)
D.N.A. Zaidel, I.S. Chronakis, A.S. Meyer, Stabilization of oil-in-water emulsions by enzyme catalyzed oxidative gelation of sugar beet pectin. Food Hydrocolloids 30, 19–25 (2013)
L. Chen, Y. Tian, B. Sun, C. Cai, R. Ma, Z. Jin, Measurement and characterization of external oil in the fried waxy maize starch granules using ATR-FTIR and XRD. Food Chem. 242, 131–138 (2018)
J.H. Chiang, A.K. Hardacre, M.E. Parker, Extruded meat alternatives made from Maillard-reacted beef bone hydrolysate and plant proteins. Part II—application in sausages. Int. J. Food Sci. Technol. (2019). https://doi.org/10.1111/ijfs.14362
F. Xue, C. Li, X. Zhu, L. Wang, L.S. Pan, Comparative studies on the physicochemical properties of soy protein isolate-maltodextrin and soy protein isolate-gum acacia conjugate prepared through Maillard reaction. Food Res. Int. 51, 490–495 (2013)
H. Salminen, J. Weiss, Electrostatic adsorption and stability of whey protein–pectin complexes on emulsion interfaces. Food Hydrocolloids 35, 410–419 (2014)
R. Li, X. Wang, J. Liu, Q. Cui, X. Wang, S. Chen, L. Jiang, Relationship between molecular flexibility and emulsifying properties of soy protein isolate-glucose conjugates. J. Agric. Food Chem. 67, 4089–4097 (2019)
M. Ma, Y. Ren, W. Xie, D. Zhou, S. Tang, M. Kuang, Y. Wang, S.K. Du, Physicochemical and functional properties of protein isolate obtained from cottonseed meal. Food Chem. 240, 856–862 (2018)
Acknowledgements
The authors would like to thank Vice-chancellor of research and technology of the University of Tehran. We also grateful to The University of Tehran Science and Technology Park for financial support (Grant no.332082/180). We are also thankful of Barsam Shirini Salamat Co. for supporting this study.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Saatchi, A., Kiani, H. & Labbafi, M. Structural characteristics and functional properties of sesame protein concentrate–maltodextrin conjugates. Food Measure 15, 457–465 (2021). https://doi.org/10.1007/s11694-020-00655-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11694-020-00655-2