Abstract
Microalgae have been reported in many studies with high biomass, high carbohydrate content, fast growth rate and multiple bio-activities, while Dictyosphaerium sp. was rarely studied. Polysaccharide prove to be remarkable in many biological activities. To optimize the effects of the ultrasonic-assisted extraction parameters on the yield of polysaccharide from Dictyosphaerium sp., a single-factor experiment and response surface methodology (RSM) with a central composite rotatable design were employed. Four independent variables were investigated: X1 (ratio of raw microalgal powder to water), X2 (extraction temperature), X3 (extraction time), X4 (extraction power). The optimal conditions obtained via single-factor experiment were: X1 (1:25), X2 (50 °C), X3 (50 min), X4 (500 W), while according to the analysis of RSM, the optimum conditions were: X1 (1:26), X2 (58 °C), X3 (55 min), X4 (525 W). The refined polysaccharide were further prepared and characterized using SEM, FT-IR, XRD and TGA/DSC. Five kinds of antioxidant assays were carried out and the results indicated that the refined polysaccharide had strong scavenging ability to DPPH· and OH·. This study provides theoretical and experimental basis for the development and utilization of microalgal resources.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Polysaccharides are known as the most plentiful form of carbohydrate materials in organism, and have remarkable biological activity due to their diverse structures [2]. Antiviral activity, antitumor activity, antioxidant activity, anti-inflammatory activity, skin whitening capacity, moisturizing capacity, etc. have been reported in various polysaccharides. Besides, because of the advantages of easy availability in nature, nontoxicity, cheapness, safeness, biodegradation and biocompatibility, polysaccharides have gained interesting and wide application in food, medicine, material and cosmetics industries [11]. Polysaccharides extracted from Dendrobium have been attested to have great antitumor activity and antioxidant activity [9, 26]. Xanthan gum, also known as a kind of extracellular polysaccharide secreted from Xanthomonas campestris, has been widely used as emulsifier, stabilizer, thickener, sizing agent, etc. by the reason of its suspension property, emulsibility, water-solubility, pseudoplastic property, thermostability, salt stability, etc. [13].
Currently, Microalgae, which are heterotrophic/autotrophic photosynthesizing organisms that are allowed to use solar energy to combine water with CO2 to produce biomass, have been paid much attention on account of their higher growth rates, higher photosynthetic efficiency, easier cultivation, higher biomass and more bioactivity compounds [1, 16]. Most polysaccharides applied in industrial production are extracted and purified from terrestrial plants and microorganisms. Many macroalgal or microalgal polysaccharides are also discovered to have great bioactivity. According to the previous studies, polysaccharides in algae is to protect algae from extreme environmental stress, such as drought, high salt stress, high light intensity, etc. [25]. Three high molecular weight polysaccharides isolated from three food-grade microalgae (Spirulina platensis, Aphanizomenon flos-aquae and Chlorella pyrenoidosa) possessed strong immunostimulatory activity, which could be potent activators of human monocytes/macrophages [20]. The polysaccharides from Porphyridium sp. showed potent antioxidant activity, which could efficiently eliminate reactive oxygen species (ROS) in a dose-dependent manner [4, 25].
Numerous studies have reported that oxide stress is closely related to many diseases, like leukemia, angiocardiopathy, aging, etc. [6, 7]. Current lifestyle causes the over-production of oxidant factors, resulting in oxide damage in our bodies [30]. Considering the toxicity of many synthetic antioxidants, it is imperative to seek natural, safe, effective, biocompatible alternatives.
In this study, Dictyosphaerium sp. was selected as tested microalga due to its high biomass, high content of carbohydrate, fast growth rate and strong antioxidant capacity [27, 31]. Now, there are scarce literatures about Dictyosphaerium, most of them are about taxonomy and combustion kinetics as biofuel [31]. Therefore, it is significant to broaden new useful biomass resources to alleviate the current raw material crisis. The UAE parameters were optimized using the single-factor and RSM experiments. Based on the optimal extraction conditions, the polysaccharides were refined, and the microstructure and functional groups were characterized by SEM and FTIR. DPPH and ABTS radicals as synthetic radicals, and hydroxyl and superoxide radical as natural radicals were used to test the antioxidant capacity of refined polysaccharides. This paper will provide an useful information for the future utilization of microalgal resources, and potential raw material for human health in modern food, medical and cosmetic industries.
Materials and methods
Microalgal collection and cultivation
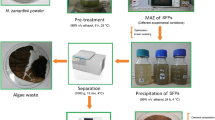
The tested microalgal strain was collected from natural fresh water in Hainan Island, China, and was repeatedly purified and activated. The strain was well characterized using 18 s rDNA sequence in Biological Engineering Lab (Haikou, Hainan, China) and identified as Dictyosphaerium sp. [31]. The microalgae was first cultivated using a 1-L flask with 500-mL BG-11 medium. After 14-day pre-cultivation, Dictyosphaerium sp. was cultivated in in a 5-L circular cylindrical laboratory scale luminescent bubble column photobioreactors (PBRs) with 3-L BG-11 medium, and operated at 200 rpm with inoculation of 10% (v/v) under 27 ± 1 °C with a light density of 360 μm/sm2. The initial pH of medium was 8.0 ± 0.1. Aeration of 100 L/h with filtered sterile air was provided through a 0.25-μm polytetrafluoroethylene membrane which could keep the bacteria out. After 7-day cultivation, the microalgae were harvested under centrifugation of 8000 rpm for 3 min and washed thrice using deionized water. Finally, the microalgal pastes were lyophilized using a vacuum freeze drier (Scientz 10 N, Ningbo, China), and stored under − 40 °C for further experiments.
Polysaccharide extraction with single-factor experiment
Water-soluble polysaccharide from Dictyosphaerium sp. was extracted using a digitally controlled ultrasonic device (DCUD) with an ultrasonic probe (Hongxianglong sonicator SY-1000E, 250 w, 20 kHz, Beijing, China). Before extraction, dried microalgal pastes were grinded to powder using liquid nitrogen and then passed through 60-mesh sieve. The powdered samples of Dictyosphaerium sp. were degreased using petroleum ether in a Soxhlet apparatus for 12 cycles. The degreased samples were placed in a fume cupboard to volatilize residual petroleum ether for 16 h. Afterwards, degreased samples were suspended in 95% ethanol (w/v: 1/40) and allowed to stand for 16 h to remove the residual lipid and other small molecular substances (e.g. monosaccharide and oligosaccharide). Then, the mixture was centrifuged under 5000 rpm for 5 min and the supernatant was removed. The sediment was dried under 50 °C for further extraction.
Pre-treated microalgal powder (1 g) were precisely weighted and mixed with different volume deionized water (w/v = 1/10, 1/15, 1/20, 1/25, 1/30) for 2 min using vortex mixer and kept in DCUD at various temperatures (30, 40, 50, 60, 70 and 80 °C) for different time periods (10, 20, 30, 40, 50 and 60 min). The ultrasonic extraction was performed using different powers (100, 200, 300, 400, 500, 600, 700, 800 and 900 W). The extracts after UAE were centrifuged and filtrated, and the filtrates were then evaporated using rotary evaporator (IKA RV10, Germany) to reduce the solvent (the volume of extracts < 25 mL) and prepared to a constant volume (25 mL) using deionized water for determination of the content of polysaccharides. The final extraction yield of polysaccharide was calculated as the quotient of the content of polysaccharide in extracting solution and the mass of microalgae.
Determination of content of polysaccharide
The content of polysaccharide was determined using a classic sulfuric acid-phenol method. Briefly, 1.0-mL sample solution was placed in a 20-mL test tube, and 1.0-mL 5% phenol solution was added, then, 5.0-mL sulfuric acid was rapidly added into former solution. After 10-min standing, the solution was fully mixed using vortex mixer for 30 s. The test tube was subsequently put into a water bath at temperature of 30 °C for 20 min. The absorbance of reacted mixture was immediately measured at 490 nm using ultraviolet and visible spectrophotometer (Puxi, Beijing, China). A calibration curve was prepared using glucose and the results were expressed as mg glucose per mL of solvent.
where C is the content of polysaccharide in Dictyosphaerium sp., ψ the concentration of the extracts, m the mass of untreated microalgal powder (dry weight), 0.9 the correction coefficient.
Experimental design using the RSM
Based on the single-factor experiment, the ratio of raw microalgal powder to water (X1), extraction temperature (X2), extraction time (X3) and extraction power (X4) were obtained, and a four-variable, three-level Box-Behnken design (BBD) was established. As shown in Table 1, the whole design consisted of 29 experimental runs performed in random order, and the experimental design contained five central points to evaluate the repeatability of the experiments. The following second-order polynomial regression equation was used to express the relationship between the independent variables and the response value (Y):
where Y is the dependent variable (extraction rate), α0, αii and αij the regression coefficients for the linear, quadratic and interaction terms, Xi and Xj the independent variables (ratio of raw microalgal powder, extraction temperature, extraction time and extraction power).
Refinement of water-soluble polysaccharide
The classic ethanol subsiding method was carried out to refine polysaccharide from Dictyosphaerium sp. Briefly, 10-g dried microalgal powders were weighted and extracted based on the optimized extraction conditions. The activated carbon (2%) was added into extracts, and the mixture was heated at 30 °C. The mixture was filtrated to remove pigments. A Savag regent (chloroform: n-butyl alcohol = 4:1) was added into pre-treated extracts (1:4), and the mixture was well shaken for 1 h to remove protein. This procedure was repeated for 4 times. Anhydrous ethanol was then added into discolored extracts (4:1) and the mixture was stored at 4 °C for 24 h. The yellow flocks were collected and freeze-dried to obtained crude polysaccharides. The crude polysaccharides were then successively washed using anhydrous ethanol, acetone and ether. After freeze-drying again, the crude polysaccharides were discolored using hydrogen peroxide then dialysed for 72 h. Finally the polysaccharide was stored at − 20 °C for further use.
Characterization of water-soluble polysaccharide
The microstructure of refined polysaccharides was observed using SEM (S-3000 N, Hitachi, Japan). The accelerating voltage in this experiment was 10 kV.
FTIR was carried out using a Fourier transform infrared spectrometer (TENS0R27, Bruker, Germany) with 4000–400 cm−1 spectral scanning range. Before determination, a small amount of polysaccharide powders were added into the powder of KBr (v/v = 1/50), then after grind in an agate mortar, the mixed powder were then pressed into tablet.
Thermogravimetric analysis of polysaccharide was performed using TG–DTG–DSC thermal analysis technology. TG, DTG and DSC analyses were respectively performed using a thermogravimetric analyzer (Q600, TA Instruments, USA) and a differential scanning calorimeter (Q100, TA Instruments, USA). Before determination, polysaccharide powders were kept at 105 °C for 24 h to remove the moisture. The dehydrated polysaccharide powders were then loaded into an Al2O3 ceramic curable, and the temperature was controlled ranging from 35 to 800 °C under nitrogen atmosphere with gas flux of 20 mL/min and heating rate of 10 °C/min.
XRD analysis for polysaccharide was performed using a polycrystal X-ray diffraction (D8 Advance, Bruker AXS, Germany) with a Cu K α target at 40 kV and 30 mA wand a scan rate of 4°/min. The diffraction angle ranged from 2θ = 0.5° to 2θ = 180°.
Antioxidant activity of refined polysaccharide analyses
DPPH radical (DR) scavenging capacity
DR scavenging capacity assay was performed based on the method adapted from reported method with some modifications [21]. Refined polysaccharide was firstly dissolved in deionized water for preparation of a series of concentrations (0.5, 1.0, 2.0, 4.0, 6.0, 8.0, 10.0 mg/mL). VC was also dissolved in deionized water with a series of concentrations of 2.0, 5.0, 10.0, 20.0, 40.0, 60.0, 80.0, 100.0 μg/mL, while BHT was dissolved in methyl alcohol with the same serial concentrations. The sample solution (100 μL) was added into 100-μL 0.1 mmol/L DPPH solution in a 96-well microplate. After well blending and 30-min reaction in the dark at room temperature, the absorbance was measured at 517 nm using a microplate reader (xMark, BIO-RAD, USA). Deionized water was used as blank control, while VC solution and BHT solution were used as positive control. A lower absorbance of the reaction mixture indicates higher free radical scavenging capacity and vice versa for higher absorbance. The ability to scavenging DR was calculated as follows:
where IDPPH is DR scavenging capacity, A0 the absorbance of blank control, Ai the absorbance of sample group, Aj the absorbance under identical conditions, Ai with 100-μL deionized water instead of DPPH solution.
The 50% inhibition (IC50) of sample was calculated from the graphic plotting inhibition percentage against sample concentrations.
ABTS radical (AR) scavenging capacity
AR scavenging capacity assay was performed based on the method adapted from reported method with some modifications [3]. Briefly, ABTS radicals were generated by mixing a 7 mmol/L ABTS solution and a 2.45 mmol/L K2S2O8 solution in the dark at room temperature for 12 h. Before determination, the ABTS radical solution was diluted about 20 times to meet an absorbance of 0.700 ± 0.050 at 730 nm. The sample solution (10 μL) was added into 200 μL diluted ABTS radical solution in 96-well microplate and incubated for 5 min in the dark at room temperature. The absorbance was measured at 730 nm using a microplate reader. The ability to scavenging AR was calculated as follows:
where IABTS is AR scavenging capacity, A0 the absorbance of blank control, Asample the absorbance of sample group.
Ferric reducing antioxidant power (FRAP)
The reaction was carried out in a 96-well microplate, each well of which contained 150 μL of freshly prepared FRAP reagent (2.5 mL of 10 mmol/L 2,4,6-Tris (2-pyridyl)-s-triazine, 2.5 mL of 40 mmol/L HCl, 2.5 mL of 20 mmol/L FeCl3·H2O and 25 mL of 300 mmol/L acetic acid-sodium acetate buffer solution, pH 3.6), and was warmed to 37 °C. The absorbance of warmed reagent (A1) was measured using a microplate reader at 593 nm. The reagent was then mixed with 20 μL of sample solution, and after 10-min incubation, the absorbance (A2) was measured at the same conditions. The A1 was calculated for each sample and related to the A2 of a standard Fe2+ solution tested in parallel. Different concentrations of FeSO4 solution were used as standard solution to obtain a calibration curve (y = 0.0023x + 0.191, R2 = 0.9996). Results (x) are expressed as μmol FeSO4/g.
Hydroxyl radical (HR) scavenging capacity
HR scavenging capacity assay was performed based on the method adapted from reported method with some modifications using a Bruker EMX EPR spectrometer (Bruker-Biospin, Germany) [5]. A spin-trapping reaction mixture consisted of 40 μL of 0.2 mmol/L FeSO4 solution, 40 μL of deionized water, 40 μL of 0.1 mol/L 5,5-dimethyl-1-pyroline N-oxide (DMPO) solution and 50 μL of sample solution. Then, 40 μL of 10% H2O2 solution was added to trigger the reaction. The operation was performed under ambient temperature, and the EPR parameters were as follows: central field (CF), 3510 G; modulation amplitude (MA), 1.0 G; time constant (TC), 635; amplification factor (AF), 2.0 × 104; microwave power (MP), 20 mW; sweep width (SW), 100G; modulation frequency (MF), 100 kHz; conversion time (CT), 200 s. The ability to eliminate HR was calculated as follows:
where I0 and I are the peak heights of the control group and sample group, respectively.
Superoxide radical (SR) scavenging capacity
SR scavenging capacity assay was performed based on the method adapted from reported method with some modifications using a Bruker EMX EPR spectrometer [5]. A spin-trapping reaction mixture consisted of 40 μL of 0.5 mmol/L diethylenetriamine pentaacetic acid solution, 40 μL of 25 mmol/L xanthine solution, 80 μL of 0.1 mol/L DMPO solution and 50 μL of sample solution. Then, 40 μL of 0.25 U/mL xanthine oxidase solution was added to trigger the reaction. The operation was performed under ambient temperature, and the EPR parameters were as follows: CF, 3510 G; MA, 1.0 G; TC, 635; AF, 3.00e + 004; MP, 20 mW; SW, 100G; MF, 100 kHz; CT, 200 s. The ability to eliminate SR was calculated as follows:
where I0 and I are the peak heights of the control group and sample group, respectively.
Statistical analysis
All the experiments were carried out in triplicate and the results are expressed as mean ± standard deviation (SD; n = 3). Data were analyzed using SPSS Statistics 17.0 and ANOVA (p < 0.05).
Results and discussion
Single-factor experiments
The effect of the ratio of raw microalgal powder to water on the extraction rate of polysaccharides
Different ratios of raw material to extraction solvent, seen as one of the most important variable closely related to the extraction cost, was applied and extraction temperature, time, and power were set as 50 °C, 30 min, and 400 W, respectively. The effect of the ratio of raw microalgal powder to water on extraction rate of polysaccharide is shown in Fig. 1a. As the ratio increased from 1:10 to 1:30, the extraction rate of polysaccharide was increased firstly then slightly decreased. The final extraction rate of polysaccharide was maximized at the ratio of 1:25 with extraction rate of 6.82 ± 0.12%, while the lowest extraction rate (5.58 ± 0.08%) was found at the initial rate (1:10). From whole tendency, higher ratio contributed to higher extraction rate. After ratio of 1:25, the extraction rate was slightly decreased, however, there was no significant difference (p > 0.05) ratio of 1:25 and ratio of 1:30, and the extraction rate caused by highest ratio (1:35) was still notably higher (p < 0.05) than the extraction rates caused by the ratios of 1:10, 1:15 and 1:20. There are two reasons for these results. The first one was due to calculation formula mentioned above in “Polysaccharide extraction with single-factor experiment” section. When the extraction solvent was increased to a certain extent, the polysaccharide in the microalgae had been completely dissolved, and the amount of polysaccharide would not be increased by increasing the solvent. And another reason could be explained by the driving force of mass transfer theory [12, 32]. A higher water and material ratio rendered to greater concentration difference between the exterior water and the microalgal cells, with the higher driving force of mass transfer, the polysaccharide could be prominently dissolved from microalgal cells. Nevertheless, with the increase of ratio, the viscosity of the solvent was also increased, which could cause an extension of diffusion distance for the microalgal cells [29], consequently, leading to the slow increase of extraction of polysaccharide. Besides, excessive water would weaken cavitation effect from the extraction process, which could also reduce the extraction of polysaccharide. Considering the cost saving and reducing consumption, the ratio of 1:25 was chosen as the central point of BBD experiment.
The effect of extraction time on the extraction of polysaccharide
The effect of extraction time on the extraction of polysaccharide was studied with the ratio of raw microalgal powder to water of 1:25, extraction temperature of 50 °C, and extraction power of 400 W. As described in Fig. 1b, the effect of extraction time on the polysaccharide was investigated in the range from 10 to 60 min, and the extraction time showed significant effect (p < 0.05) on the extraction of polysaccharide. The whole tendency was similar to the ratio of material to solvent. The extraction rate was increased at the initial stage (10–50 min) then reduced with the increase of extraction time, and reached to maximum when extraction time was 50 min (7.11 ± 0.03%). After maximum, the extraction rate was slightly decreased with further extraction, and there was no significant difference (p > 0.05) between 40 min and 60 min. This situation may be attributed to the dissolution and diffusion of some time required polysaccharide from microalgal cell membrane, but the positive effect may not last for long with prolonged extraction time, because the cell wall and membrane have already destroyed under the ultrasonic cavitation effect, and the polysaccharide may have completely dissolved in the solvent. Further ultrasonic cavitation may cause apparent reduction and rapid degradation of polysaccharide, leading to decline of extraction rate [22, 33]. Prolonged extraction time also brings energy waste, therefore, the extraction time of 50 min was chosen as the central point of BBD experiment.
The effect of extraction temperature on the extraction of polysaccharide
Heat can efficiently release large amounts of polysaccharide from cells in many cases. Here, extraction temperatures for polysaccharide recovery were between 30 and 80 °C (ratio of raw microalgal powder to water of 1:25, extraction time of 30 min, and extraction power of 400 W). A direct relationship was observed in Fig. 1c. The extraction temperature was optimal at 50 °C with extraction rate of 7.02 ± 0.06%, while the extraction temperature was worst at 80 °C with extraction rate of 4.89 ± 0.04%. The extraction rate of polysaccharide was significantly increased (p < 0.05) along with the increase of temperature from 30 to 50 °C, then rapidly decreased (p < 0.05) over 50 °C.
Ultrasonic cavitation bubble number and surface contact area can be increased and the viscosity and density of solvent can be decreased due to the increase of extraction temperature. Those factors contribute to the enhancement of solubility and diffusion coefficients, leading to the accelerating release of polysaccharide [33]. In addition, according to the equilibrium concentration theory, increased temperature could accelerate the transfer of polysaccharide. However, excessive temperature will also lead to the denaturation of some thermo-sensitive polysaccharide that could only be mobilized under low temperature. In this study, a rapid decline was found after 50 °C, and the extraction rate at 80 °C was even lower than that at 30 °C, which means temperature have prominent impact on the stability of polysaccharide from Dictyosphaerium sp., and the polysaccharide of Dictyosphaerium sp. needs to be treated under relatively lower temperature. Therefore, the extraction temperature of 50 °C was selected as the central point of BBD experiment.
The effect of extraction power on the extraction of polysaccharide
Ultrasonic power is believed as the main driving force. On one hand, at the existence of acoustic cavitation and acoustic waves diffuse, the bubble will be formed and collapsed rapidly, which can generate high-speed liquid jets. These jets will shock the surface of cell membranes, leading to the destruction of cell structure and the increase of the release of polysaccharide [15]. On the other hand, stronger ultrasonic power may, to some extent, result in heating. Heating also provides energy and encourages molecules in the system to vibrate strongly, thus, breaking the bonds among compounds and cell membranes and liberating compounds into the solvent [17]. However, stronger ultrasonic power and higher temperature may enhance the oxidation reaction and thermal degradation of polysaccharide, leading to a fall in polysaccharide yield [19]. To investigate the effect of extraction power on the extraction rate of polysaccharide, other variables were set as follows: ratio of raw microalgal powder to water of 1:25, extraction temperature of 50 °C and extraction time of 30 min. As shown in Fig. 1d, the extraction rate of polysaccharide was sharply increased along with the increase of ultrasonic power varied from 100 to 500 W and reached the peak value (7.12 ± 0.11%) at ultrasonic power of 500 W. However, when the ultrasonic power was over 500 W, a drastic decline (p < 0.05) of extraction rate was revealed. With the enhancement of ultrasonic power, the bubble generated by acoustic cavitation was increased, which could efficiently make solvent penetrate the cell membrane and destroy the cell structure, accordingly, resulting in the increase of extraction rate. However, excessive ultrasonic power might cause structural failure of polysaccharide, degradation and viscosity reduction of polysaccharide would also occur. These factors would lead to the decline of extraction rate [28]. Therefore, the extraction power of 500 W was selected as the central point of BBD experiment.
Optimization of UAE operational parameters for polysaccharide
Based on the single-factor experiments, the ratio of raw microalgal powder to water ranged from 1:20 to 1:30, the extraction time ranged from 40 to 60 min, the extraction temperature ranged from 40 to 60 °C and the extraction power ranged from 400 to 600 W were adopted for RSM experiments.
Fitting the model
The operational parameters were optimized using BBD. The extraction rates of polysaccharide under different experimental conditions are shown in Table 1. The maximum extraction rate (7.88 ± 0.05%) was recorded at Run 18, while the lowest extraction rate (6.03 ± 0.08%) was recorded at Run 17. Multiple regression analysis was carried out based on the experimental data, and a second order polynomial equation was established to reveal the relationship between extraction rate of polysaccharide and coded parameters, as shown in Eq. (8):
The performance of the fitting models was expressed as R2 correlation and significant analyses were performed using F-test. The analysis of variance for response surface quadratic model for extraction of polysaccharide from Dictyosphaerium sp. is presented in Table 2, showing a good fit of model to the experimental data. The model F-value of 12.62 and p value of < 0.0001 implied the model was highly significant. There was a 0.01% chance that a “Model F-value” this large could occur due to noise. Values of “p-value” less than 0.05 indicated model terms were significant. In this case, the linear coefficients of X1 and X2, cross product coefficients of X1X3, and quadratic coefficients of X21, X22, X23 and X24 had prominent impacts (p < 0.05). Therefore, The “Lack of fit F-value” of 2.63 implied there was no significant relation between the “Lack of fit F-value” and pure error. There was a 18.25% chance that a “Lack of fit F-value” this large could occur due to the noise. Meanwhile, non-significant lack of fit was good. Besides, the determination coefficient (R2) was 0.9266, revealing that there was only 7.34% chance that the independent parameters were not occurred by the model. The value of coefficient of variation (C.V.) was 3.05, which was quite low, attesting to have a relative accuracy of the model and good reliance of the experimental data.
Analysis of response surfaces
Three-dimensional (3D) response surface plots and two-dimensional (2D) contour plots (Fig. 2) were established to illustrate the predicted optimal extraction parameters for maximizing the yield of Dictyosphaerium sp. polysaccharide. According to the Fig. 2, the shapes of contour plots are differently, which means the different mutual interactions between different individual variables. Based on the previous study, elliptical contour plot means that the corresponding individual variables are significant, whereas rounded contour plots manifest the insignificant effects of relevant variables [28]. Among the variables, the interactions between ratio of raw microalgal powder to water and extraction time was significant, while other interactions were not significant, which were consistent with Table 2.
The response surface plot for extraction of polysaccharide versus ratio of raw microalgal powder to water and extraction temperature is shown in Fig. 2a, where extraction rate was higher at a ratio of raw microalgal powder to water of 1:25.82 and extraction temperature of 58.14 °C. The response surface plot for extraction of polysaccharide versus ratio of raw microalgal powder to water and extraction time is shown in Fig. 2b, where extraction rate was higher at a ratio of raw microalgal powder to water of 1:25.82 and extraction time of 54.78 min. The response surface plot for extraction of polysaccharide versus ratio of raw microalgal powder to water and extraction power is shown in Fig. 2c, where extraction rate was higher at a ratio of raw microalgal powder to water of 1:25.82 and extraction power of 523.49 W. The response surface plot for extraction of polysaccharide versus extraction temperature and extraction time is shown in Fig. 2d, where extraction rate was higher at an extraction temperature of 58.14 °C and extraction time of 54.78 min. The response surface plot for extraction of polysaccharide versus extraction temperature and extraction power is shown in Fig. 2e, where extraction rate was higher at an extraction temperature of 58.14 °C and extraction power of 523.49 W. The response surface plot for extraction of polysaccharide versus extraction time and extraction power is shown in Fig. 2f, where extraction rate was higher at an extraction time of 54.78 min and extraction power of 523.49 W. As a result, the best extraction parameters simulated by Design Expert 8.0 were as follows: ratio of raw microalgal powder to water of 1:25.82, extraction time of 54.78 min, extraction temperature of 58.14 °C, and extraction power of 523.49 W. Meanwhile, the predicted polysaccharide extraction rate of 7.92% According to the actual situation, the extraction parameters were modified as ratio of raw microalgal powder to water of 1:26, extraction time of 55 min, extraction temperature of 58 °C, and extraction power of 525 W.
To verify the predicted result, the extraction experiment was carried out based on the modified parameters. The experiments were performed for six times to confirm the result. The final optimized extraction result of 8.02 ± 0.12% suggested that the result was in good agreement with the predicted value and the optimized model fitted well.
Refinement and characterization of water-soluble polysaccharide
The microstructural morphology of microalgal polysaccharide with two sizes was investigated by SEM. The SEM pictures of refined polysaccharide are depicted in Fig. 1. Microalgal polysaccharide with size of 100 μm (Fig. 3a) display a clear irregular flaky structure and with size of 20 μm (Fig. 3b) display a more specific porous structure.
In order to confirm the microstructure of polysaccharide. The FTIR spectra of the microalgal polysaccharide was detected in Fig. 4. We focused on the spectral region from 4000 and 400 cm−1, which contained more relevant information on the main chemical functional groups (Fig. 2). The band in the region between 3600 and 3200 cm−1 was due to the stretching vibration of the hydroxyl groups. The small band at 2932 cm−1 was attributed to the C-H stretching and bending vibrations[28]. The absorption peaks at around 1652 cm−1 and 1550 cm−1 were due to the presence of bound water and the asymmetric stretching vibration of COO− [18]. In addition, a strong band between the region of 1200–1000 cm−1 was dominated by ring vibrations overlapped with stretching vibrations of (C–O–H) side groups and the (C–O–C) glycosidic band vibration. The absorption at 1078 cm−1 and 1032 cm−1 indicated a pyranose form of sugars [14].
TGA and DSC curves of refined microalgal polysaccharide are depicted in Fig. 5. Three different stages were well defined during TGA and DSC analysis. Even though the sample has been dried during refinement, the presence of first stage basically indicated the loss of moisture at around 100 °C due to dehydration. Subsequently, The second stage emerged between 290 and 310 °C and consisted in the devolatilization of the sample, where the polysaccharide lost weight rapidly. The second stage indicated that there was no sulphate in refined microalgal polysaccharide. Finally, the third stage began close to about 600 °C and was maintained up to 800 °C.
The XRD pattern of microalgal polysaccharide is shown in Fig. 6. The sample shows peaks at approximately 8°, 14°, 21.5°, 26° 2θ. However, other peaks are very weak and unresolved or are shoulders on more intense peaks. The result of the XRD confirms that of the DSC, which shows that the polysaccharide exhibit both crystalline and amorphous portions [24].
Antioxidant activity of water-soluble polysaccharide
The antioxidant activity of polysaccharide from many terrestrial plants and some famous algae has been reported, for example, polysaccharide from Brassica rapa L., Ganoderma lucidum, red algae Pterocladia capillacea, Spirulina sp., etc. [8, 10, 12, 28]. However, the antioxidant activity of polysaccharide extracted from Dictyosphaerium sp. has not been studied. The antioxidant activity of 80% ethanol extracts and biomass of Dictyosphaerium sp. were well studied and attested to have great potential using as antioxidant raw material. The investigation of the antioxidant activity of polysaccharide from Dictyosphaerium sp. could further analyze the specific antioxidant component of Dictyosphaerium sp. and broaden available natural resources.
Five kinds of antioxidant assays were carried out for determination of antioxidant capacity of Dictyosphaerium sp.: DR scavenging capacity assay and AR scavenging capacity assay were performed to evaluate the ability to eliminate synthetic free radicals; SR scavenging capacity assay and HR scavenging capacity assay were carried out to estimate the ability to eliminate natural free radicals; FRAP assay was used to determine the reducing ability of the polysaccharide. VC and BHT in this section were used as positive controls, because they respectively represent natural antioxidant and synthetic antioxidant. The result of antioxidant activities are shown in Table 3 and compared with Vc as control standards. The DPPH scavenging ability increased from 42.33 to 84.84% when the concentration of the polysaccharide increased from 4 to 10 mg/mL. Especially in the higher doses (10 mg/mL), the refined polysaccharide exhibited very high radical scavenging activity (84.84%), which was close to that of Vc (20 μg/mL; 90.60%). Similarly, the results of ABTS showed that the refined polysaccharide exhibited high radical scavenging activity (4 mg/mL; 27.93%).
The OH· scavenging ability increased from 44.47 to 86.48%, when the concentration of the refined polysaccharide increased from 1 to 10 mg/mL(Table 3). This showed that the polysaccharide exhibited scavenging activity towards OH· in a concentration-dependent manner and the scavenging effect increased based on the polysaccharide’ concentration. However, the antioxidant activity was detected to be lower than that of VC and BHT. As for O.−2, its scavenging activity followed a dose-dependent manner at all tested concentration (Table 3). Scavenging effects seemed not to be very high, and when the concentration of the refined polysaccharide was 10 mg/mL, the O.−2 scavenging activity was just 9.03%. Compared with the activity of the purified polysaccharide DPP2-1 (the second polysaccharide fraction with the strongest antioxidant activity) extracted from Dendrobium denneanum, a kind of traditional Chinese herb, the effects on DPPH, ABTS and OH· radical scavenging activity were about 89.3%, 19.6% and 78.9%, respectively (2 mg/mL) [9]. The similar results were also showed in Sun et al. [23]. However, with the concentration increasing, the scavenging activity of our polysaccharide was close to that of DPP2-1. The results suggested that the radical scavenging activity of the refined polysaccharide was related to their concentration and their physicochemical properties.
Meanwhile, The logarithmic equations and IC50 of samples have been listed in Table 4.
Conclusion
The polysaccharide extracted from Dictyosphaerium sp. The RSM was successful in establishing mathematical models for predicting polysaccharide extracting yield, used to determine the optimal process parameters that gave a high extraction yield. Among four independent variables, extraction power, temperature, time and the ratio of m/w were not quite significant. However, the interaction of extraction and extraction power was significant to the yield. A The optimal conditions were: extraction power 523 W, extraction temperature 58 °C, extraction time 55 min and m/w ration 1:26. The extraction information on Dictyosphaerium sp. Obtained in this work could also be enlightening in other species. Also, the refined polysaccharides were characterized by SEM, FT-IR, DSC, TGA/XRD, which showed typical chemical structure of polysaccharide. With the exception of ultrasonic power, the other three parameters (UAE temperature, and extraction time sample-to-solvent ratio) statistically affected the content of polysaccharide and their antioxidant activities. The results of antioxidant activities assays demonstrated that the polysaccharide had scavenging activities in vitro on DPPH·, ABTS, OH·, O.−2 and Fe concentration, which showed strong scavenging rates in DPPH· and OH·.
References
R.A.I. Abou-Shanab, I.A. Matter, S.-N. Kim, Y.-K. Oh, J. Choi, B.-H. Jeon, Characterization and identification of lipid-producing microalgae species isolated from a freshwater lake. Biomass Bioenergy 35(7), 3079–3085 (2011)
A. Ahmadi, S. Zorofchian Moghadamtousi, S. Abubakar, K. Zandi, Antiviral potential of algae polysaccharide isolated from marine sources: a review. Biomed. Res. Int. 2015, 825203 (2015)
C. Ao, A. Li, A.A. Elzaawely, T.D. Xuan, S. Tawata, Evaluation of antioxidant and antibacterial activities of Ficus microcarpa L. fil. extract. Food Control 19(10), 940–948 (2008)
T.V. Arutyunyan, A.F. Korystova, L.N. Kublik, M. Levitman, V.V. Shaposhnikova, Y.N. Korystov, Taxifolin and Fucoidin Abolish the Irradiation-Induced Increase in the production of reactive oxygen species in rat aorta. Bull. Exp. Biol. Med. 160(5), 635–638 (2016)
N.A. Azman, S. Peiro, L. Fajari, L. Julia, M.P. Almajano, Radical scavenging of white tea and its flavonoid constituents by electron paramagnetic resonance (EPR) spectroscopy. J. Agric. Food Chem. 62(25), 5743–5748 (2014)
L. Ben Mahmoud, M. Mdhaffar, H. Ghozzi et al., Oxidative stress in Tunisian patients with acute lymphoblastic leukemia and its involvement in leukemic relapse. J. Pediatr. Hematol. Oncol. 39(3), 1 (2017)
S. Caprio, S. Wong, K.G.M.M. Alberti et al., Cardiovascular complications of diabetes. Diabetologia 40, B78–B82 (1997)
R. Chaiklahan, N. Chirasuwan, P. Triratana, S. Tia, B. Bunnag, Effect of extraction temperature on the diffusion coefficient of polysaccharide from Spirulina and the optimal separation method. Biotechnol. Bioprocess Eng. 19(2), 369–377 (2014)
Y. Fan, X. He, S. Zhou, A. Luo, T. He, Z. Chun, Composition analysis and antioxidant activity of polysaccharide from Dendrobium denneanum. Int. J. Biol. Macromol. 45(2), 169–173 (2009)
D. Fleita, M. El-Sayed, D. Rifaat, Evaluation of the antioxidant activity of enzymatically-hydrolyzed sulfated polysaccharide extracted from red algae; Pterocladia capillacea. LWT Food Sci. Technol. 63(2), 1236–1244 (2015)
J.-H. Guo, G.W. Skinner, W.W. Harcum, P.E. Barnum, Pharmaceutical application of naturally occurring water-soluble polymers. Pharm. Sci. Technol. Today 1(6), 254–261 (1998)
Y. Kan, T. Chen, Y. Wu, J. Wu, Antioxidant activity of polysaccharide extracted from Ganoderma lucidum using response surface methodology. Int. J. Biol. Macromol. 72, 151–157 (2015)
N. Latifi, S. Horpibulsuk, C.L. Meehan, M.Z.A. Majid, A.S.A. Rashid, Xanthan gum biopolymer: an eco-friendly additive for stabilization of tropical organic peat. Environ. Earth Sci. 75(9), 825 (2016)
X. Lian, S. Zhi-Wei, L.I. Guo-Liang et al., Spectral properties and monosaccharides compositon analysis of polysaccharide from Potentilla anserina L. Nat. Prod. Res. Dev. 23(3), 453–457 (2011)
J.L. Luque-Garcı́a, M.D. Luque de Castro, Ultrasound: a powerful tool for leaching. TrAC Trends Anal. Chem. 22(1), 41–47 (2003)
S.F. Mohsenpour, N. Willoughby, Effect of CO2 aeration on cultivation of microalgae in luminescent photobioreactors. Biomass Bioenergy 85, 168–177 (2016)
A. Nordiyanah, M.A.A. Faris, S. Naziz et al., Optimization of extraction parameters by using response surface methodology, purification, and identification of anthocyanin pigments in Melastoma malabathricum, fruit. Sci. World J. 2013, 1–10 (2013)
A.S. Orabi, A.M. Abbas, S.A. Sallam, Spectral, magnetic, thermal, and DNA interaction of Ni(II) complexes of glutamic acid schiff bases. Synth. React. Inorg. Met.-Org. Nano-Met. Chem. 43(1), 63–75 (2013)
H.N.T. Pham, Q.V. Vuong, M.C. Bowyer et al., Ultrasound-assisted extraction of Catharanthus roseus, (L.) G. Don (Patricia White cultivar) stem for maximizing saponin yield and antioxidant capacity. J. Food Process. Preserv. 42, e13597 (2018)
N. Pugh, S.A. Ross, H.N. ElSohly, M.A. ElSohly, D.S. Pasco, Isolation of three high molecular weight polysaccharide preparations with potent immunostimulatory activity from Spirulina platensis, Aphanizomenon flos-aquae and Chlorella pyrenoidosa. Planta Med. 67(8), 737–742 (2001)
F. Sharififar, G. Dehghn-Nudeh, M. Mirtajaldini, Major flavonoids with antioxidant activity from Teucrium polium L. Food Chem. 112(4), 885–888 (2009)
G. Spigno, L. Tramelli, D.M. De Faveri, Effects of extraction time, temperature and solvent on concentration and antioxidant activity of grape marc phenolics. J. Food Eng. 81(1), 200–208 (2007)
L. Sun, J. Li et al., Characterization and antioxidant activities of degraded polysaccharide from two marine Chrysophyta. Food Chem. 160, 1–7 (2014)
D. Suvakanta, M.P. Narsimha, D. Pulak, C. Joshabir, D. Biswajit, Optimization and characterization of purified polysaccharide from Musa sapientum L. as a pharmaceutical excipient. Food Chem. 149(8), 76–83 (2014)
T. Tannin-Spitz, M. Bergman, D. Van-Moppes, S. Grossman, S. Arad, Antioxidant activity of the polysaccharide of the red microalga Porphyridium sp. J. Appl. Phycol. 17(3), 215–222 (2005)
J.-H. Wang, J.-P. Luo, X.-Q. Zha, B.-J. Feng, Comparison of antitumor activities of different polysaccharide fractions from the stems of Dendrobium nobile Lindl. Carbohyd. Polym. 79(1), 114–118 (2010)
S. Wang, Z. Zhao, P. Liu, N. Luo, X. Pan, Nutrient components and hydroxyl radicals scavenging capacity research of Dictyosphaerium sp. 1A10. J. Shanghai Ocean Univ. 27(2), 39–48 (2018)
W. Wang, X. Wang, H. Ye, B. Hu, L. Zhou, S. Jabbar, X. Zeng, W. Shen, Optimization of extraction, characterization and antioxidant activity of polysaccharide from Brassica rapa L. Int. J. Biol. Macromol. 82, 979–988 (2016)
Z. Ying, X. Han, J. Li, Ultrasound-assisted extraction of polysaccharide from mulberry leaves. Food Chem. 127(3), 1273–1279 (2011)
Z. Zhao, P. Liu, S. Wang, S. Ma, Optimization of ultrasound, microwave and Soxhlet extraction of flavonoids from Millettia speciosa Champ. and evaluation of antioxidant activities in vitro. J. Food Meas. Charact. 11(4), 1947–1958 (2017)
Z. Zhao, P. Liu, S. Wang, S. Ma, J. Cao, Combustion characteristics and kinetics of five tropic oilgal strains using thermogravimetric analysis. J. Therm. Anal. Calorim. 131(2), 1919–1931 (2017)
Q. Zheng, D. Ren, N. Yang, X. Yang, Optimization for ultrasound-assisted extraction of polysaccharide with chemical composition and antioxidant activity from the Artemisia sphaerocephala Krasch seeds. Int. J. Biol. Macromol. 91, 856–866 (2016)
J. Zhou, X. Zheng, Q. Yang, Z. Liang, D. Li, X. Yang, J. Xu, Optimization of ultrasonic-assisted extraction and radical-scavenging capacity of phenols and flavonoids from Clerodendrum cyrtophyllum Turcz leaves. PLoS ONE 8(7), e68392 (2013)
Acknowledgements
The present article is supported by Hainan Provincial Natural Science Foundation of China (219QN258), Hainan Provincial Scientific Research Program of Higher Education Institutions for China (Hnky2019-110). Zhenyu Zhao acknowledges the China Scholarship Council for financial support.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Chen, C., Zhao, Z., Ma, S. et al. Optimization of ultrasonic-assisted extraction, refinement and characterization of water-soluble polysaccharide from Dictyosphaerium sp. and evaluation of antioxidant activity in vitro. Food Measure 14, 963–977 (2020). https://doi.org/10.1007/s11694-019-00346-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11694-019-00346-7