Abstract
Response surface methodology was employed to optimize the ultrasound-assisted extraction of crude polysaccharides from the cell wall of Ulva intestinalis. The impacts of four independent variables including extraction temperature (X 1 50–90 °C), extraction time (X 2 20–40 min), the ratio of water to raw material (X 3 50–70), and pH (X 4 7–9) were investigated through Box–Behnken experimental design. The experimental data were adequately fitted into a second-order polynomial model, and optimized conditions were as follows: extraction temperature 66 °C, extraction time 40 min, water to raw material ratio 50, and pH 7.0. Fourier transform infrared (FT-IR) results revealed the presence of sulfate esters indicated by the peaks at 850 and 1256 cm−1 derived from the bending vibration of C–O–S and stretching vibration of S–O of sulfate. The experimental yield achieved under these conditions was 8.30 %, which was well in close agreement with the value (8.36 %) predicted by the response surface methodology (RSM) model. The extracted polysaccharides exhibited appreciable 2,2-diphenylpicrylhydrazyl (DPPH) radical scavenging and reducing power as well as macrophage-stimulating capacity indicating their potential value for health and food industry.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Naturally occurring polysaccharides are generally considered as being biocompatible and biodegradable. Many of these polysaccharides have had the chance to find their way into food and pharmaceutical industries due to their specific properties. Anionic polysaccharides, for instance, are the predominant constituents found in the cell wall matrix of marine algae which are widely used in food industry (Li et al. 2014; Knudsen et al. 2015). The cell walls of brown algae are major sources of sulfated fucans, consisting mainly of α-l-fucopyranose residues (Adhikaria et al. 2006). Sulfated galactans, such as agarans and carrageenans, are predominantly found in red algae and contain l- and d-galactopyranose residues, respectively (Pomin 2010). Green algae are common sources of aldobiuronans called ulvan which have α-l-rhamnose as the major monosaccharide unit (Robic et al. 2009). Ulvan polysaccharides have recently garnered attention, as they also exhibited various health-improving properties. These biomacromolecules are known to possess various biological activities including anti-inflammatory, antivirus, antitumor, immunomodulatory, and anticoagulant activities (Tabarsa et al. 2013; Arata et al. 2015; Ropellato et al. 2015; Hwang et al. 2015; Briseno et al. 2015).
In general, the biological activities of the sulfated polysaccharides are thought to be closely in correlation with their chemical structure and molecular properties such as sugar composition, molecular weights, sulfate contents, etc. (Qi et al. 2005a, b; Tabarsa et al. 2015). On the other hand, the structural characteristics of the sulfated polysaccharides may differ due to intrinsic and extrinsic determinants such as phylogenetic differences and growing conditions of the algae (Lahaye and Robic 2007). Besides, an overview of recent studies reveals that different extraction methods are playing a huge role in obtaining polysaccharides with significant discrepancies in structural properties which makes it either impossible or tiresome to relate the bioactivity of polysaccharides with their structures (Ale et al. 2011).
For many years, the focus of researchers was mainly on the extraction efficiency of polysaccharides with the assistance of complementary techniques such as employing quaternary ammonium salts, organic solvents, and pressurized liquid extraction (Reviers and Leproux 1993; Santoyo et al. 2011). A direct result of such approach was to overlook the preservation of polymer structure. Although, later, applying enzyme-based procedures in the isolation of polysaccharides promised higher yields and structural integrity, finding suitable enzymes, release of non-target compounds from the cell walls, and cost-related issues were restrictive in the utilization of biological methods (Athukorala et al. 2006; Li et al. 2013a, b, c).
Lately, ultrasound-assisted extractions have been evaluated as an alternative method for isolating polysaccharides from different plant materials. In comparison with the conventional methods, a combination of ultrasound and traditional solvent extraction facilitates the mass transfer through agitation at low frequency (Vinatoru et al. 1997). Hence, enhancing the extraction efficiency, shortening the extraction time, and reducing the consumed energy along with the main advantage of obtaining intact thermolabile polymers would be achievable (Chen et al. 2012). Ultrasound-assisted extraction of polysaccharides mostly has been investigated on fruits, seeds, and roots of terrestrial plants such as mushrooms and herbs (Zou et al. 2011; Deng et al. 2014). Previous findings revealed that the structural differences of the cell walls of the plant of origin play a key role in reaching the optimal conditions. For instance, while the best extraction condition for pomegranate peel was 114 min at 98 °C, the best result for the roots of Codonopsis pilosula was 44 min at room temperature (Zou et al. 2011; Zhu and Liu 2013). In addition, it has to be noted that the industrial-scale employment of ultrasound waves at high power and intensity is still a great challenge due to high instrumentation and operation costs (Rastogi 2011). To address this concern, we use a common ultrasonic bath generating a moderate power (53 kHz, 180 W) and consider temperature, time, ratio of material to solvent, and pH as key parameters.
Therefore, in this investigation, we aim at exploring the effects of different variables during the ultrasound-assisted extraction of sulfated polysaccharides from Ulva intestinalis using the statistical technique of response surface methodology. In addition, we intend to preliminarily characterize the isolated sulfate polysaccharides and test their antioxidant and macrophage-stimulating activities.
Materials and methods
Reagents and samples
The green seaweed Ulva intestinalis was collected from the coast of Noor, Mazandaran Province, Iran. The raw material was thoroughly washed with tap water and air-dried at 60 °C. The dried raw material was milled using a blender, sieved (<0.5 mm), and stored in plastic bags at −20 °C in a dry environment before extraction of the sample molecules. All of the chemicals and reagents used in this work were of analytical grade.
Isolation of crude molecules
The milled sample (20 g) was treated with 80 % ethanol (200 mL) with constant mechanical stirring overnight at room temperature. The supernatants were then clarified by centrifugation at 10 °C and 6000×g for 10 min. The sediment was rewashed with EtOH under the same conditions, rinsed with acetone, centrifuged at 10 °C and 6000×g for 10 min, and then dried at room temperature in a fume hood. The dried samples (20 g) were extracted with distilled water and sonicated with an ultrasound bath (Soner, 206H, Taiwan; 53 KHz; 180 W) at the temperature of 30–100 °C, the ratio of water to raw material 10:1 to 80:1 (mL g–1) and pH 4–9 for 10–70 min. The extracts were centrifuged at 10 °C and 10,000 rpm for 10 min, and the supernatants were collected. The supernatants were concentrated by evaporation under reduced pressure at 60 °C to approximately 50 mL. EtOH (99 %) was added into the supernatant to obtain a final EtOH concentration of 70 % and then kept at 4 °C overnight. The precipitate was obtained by centrifugation at 10 °C and 9400×g for 10 min, washed with EtOH (99 %), which was followed by acetone, and then dried at room temperature in a fume hood. The polysaccharides were weighted with an analytical balance (AAA 250 L-Adam, UK; Capacity 5 mg–160 g).
Experimental design
After determining the preliminary range of extraction variables through single-factor test, the response surface methodology (RSM) with the Box–Behnken design (BBD) was employed to determine the optimal combination of extraction variables. Extraction temperature (X 1 50–90 °C), extraction time (X 2 20–40 min), the ratio of water to raw material (X 3 50–70), and pH (X 4 7–9) were independent variables selected to be optimized for the extraction of Enteromorpha intestinalis sulfated polysaccharides (ESPs), while the response variable was the extraction yield. The coded and uncoded levels of the independent variables are presented in Table 1. Based on the results of preliminary experiments, a single block of 29 sets of test conditions was designed and carried out with three replications to evaluate the pure error (Table 2). Experimental data showed that response variables were fitted to a quadratic polynomial model. The general form of the second-order polynomial equation is as follows:
where β 0 is a constant coefficient of the models. The regression coefficients (β 1, β 2, β 3, and β 4), (β 11, β 22, β 33, and β 44), and (β 12, β 13, β 14, β 23, β 24, and β 34) respectively represent linear, quadratic, and interaction effects of the model estimated by multiple regression analysis. X 1 (extraction temperature), X 2 (extraction time), X 3 (pH), and X 4 (the ratio of water to raw material) are coded variables ranging from −1 to +1.
Chemical composition of ESP
The moisture and ash contents were determined using AOAC method (1997). The total carbohydrate content was evaluated by the phenol-H2SO4 assay using d-glucose as a standard (Dubois et al. 1956). The sulfate content of the ESP was determined by the BaCl2 gelatin method (Dodgson and Price 1962). Lowry method was employed to measure the amount of protein using bovine serum albumin (BSA) as a standard (1951).
FT-IR spectroscopy
One milligram of sulfated polysaccharide was milled with 300 mg of KBr and pressed into a disk for transmission infrared spectroscopy. Then, Fourier transform infrared (FT-IR) spectrum was recorded between 500 and 4000 cm−1 at a resolution of 2 cm−1 (Tensor 27, Bruker Instruments, USA).
Antioxidant activity
DPPH radical scavenging activity assay
The assay was carried out according to a method developed by Ren et al. (2008) with some modification. Briefly, different volumes of the polysaccharide solution with different concentrations (0.5–5.0 mg mL−1) were added to 2.0-mL 2,2-diphenylpicrylhydrazyl (DPPH) solution and the final reaction volume was made up to 4 mL with 70 % ethanol. After shaking vigorously, the mixture was incubated at 25 °C in the dark for 30 min. The absorbance was measured at 517 nm by ultraviolet–visible spectrometer. The lower absorbance of the reaction mixture indicates higher free radical scavenging activity. The ability to scavenge the DPPH radical was calculated according to the following equation:
where Ac is the absorbance of the control (100 μL of ethanol with 100 μL of the DPPH solution) and As is the absorbance of the sample.
Reducing power
The reducing power of the sample was determined by the method described by Oyaizu (1986). An aliquot of each sample (500 μL) with concentrations ranging from 0.5 to 5.0 mg mL−1 was mixed with 500 μL sodium phosphate buffer (0.2 M, pH 6.6) and 500 μL of 1 % potassium ferricyanide, followed by incubation at 50 °C for 20 min. After the addition of 500 μL of 10 % TCA, the mixture was centrifuged at 8000 for 10 min and the supernatant (1.0 mL) was incubated in the presence of 1.0 mL of distilled water and 200 μL of 0.1 % ferric chloride for 10 min. The absorbance was read at 700 nm. The result was expressed as a percentage of the activity shown by 0.01 of ascorbic acid.
Macrophage proliferation activity and nitric oxide releasing capacity
The proliferation of the macrophage cell line, RAW264.7 cells, obtained from ATCC was determined by the WST-1 colorimetric assay after plating the cells (1 × 104 cells well−1, in a volume of 100 μL) in 96-well microplates and incubating them in RPMI-1640 medium containing 10 % FBS with 100 μL of the samples (1.5–12.0 μg mL−1). Each concentration was tested in triplicate. The immune-enhancing activity of the polysaccharides was determined on the basis of nitric oxide (NO) production in RAW264.7 cell culture supernatants, and the NO concentration was measured using the Griess reaction.
Statistical analyses
Statistical analyses were performed using Design-Expert software (version 7.1.6) and SPSS (version 16.0) statistical software. All experiments were performed in triplicate. Data are presented as mean values ± standard deviations. The comparison of quantitative variables was performed using analysis of variance (ANOVA), and the differences were calculated using Duncan’s test (p < 0.05).
Results and discussion
Single-factor experiments
Primary experiments were conducted in order to understand the effects of key extraction factors including extraction temperature, extraction time, ratio of water to raw material, and pH, independently. As it is presented in Fig. 1, all four factors were found to have significant impact on the extraction yield of ESP.
To investigate the influence of different temperatures on extraction yield of EPS, extraction process was carried out from 30 to 100 °C. Simultaneously, the extraction time, ratio of water to raw material, and pH were fixed at 40 min, 20, and 7, respectively. As shown in Fig. 1a, with the rise of temperature from 30 to 60 °C, the extraction yield started to slowly increase from 2.7 to 3.14 %. Then, a sharp increase was witnessed at 70 °C which continued until it reached the maximum amount of 8 % at 90 °C. The positive effect of temperature on yield can be explained by the enhancement of polysaccharide solubility and the following increase in diffusion coefficient of the molecules (Wang et al. 2014).
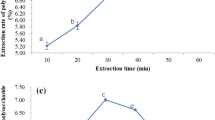
Various extraction times were set from 10 to 50 min to evaluate the effect of time on the extraction yield of ESP while the other factors were set as follows: temperature 60 °C, ratio of water to raw material 20, and pH 7.0. Figure 1b indicates that the extraction yield was correlated with the time, and it reached the highest amount when the extraction time was 30 min. However, running the extraction beyond 30 min was no longer effective and caused a reverse impact on the yield. This tendency is in agreement with other studies in extracting polysaccharides (Li et al. 2013a, b, c). Besides, the current test exhibited that short extraction time is sufficient to obtain polysaccharides, which was less than the conventional heating extraction time (Tabarsa et al. 2013).
To evaluate the effect of water to raw material ratio on extraction yield of polysaccharides, a range from 10 to 70 of water to raw material ratio was examined while the treatment temperature, time, and pH were kept at 60 °C, 40 min, and pH 7. A constant increase of polysaccharide amount was observed with the increase of water to raw material ratio until it reached the highest yield at the ratio of 60. This phenomenon can be explained by the lower concentration of polysaccharides in the water leading to the lower viscosity of the extraction solvent which facilitates solubilization and diffusion of polysaccharide molecules (Xu et al. 2014). A slight decrease of extraction yield was observed at the water to raw material ratio of 70. pH is thought to play a detrimental role in extraction procedure of polysaccharides (Jiao et al. 2009). This effect on the extraction yield of polysaccharides is demonstrated in Fig. 1d. In this set of runs, pH of the extraction water was set from 6 to 9, when the other factors including temperature, time, and ratio of water to raw material kept at 60 °C, 40 min, and 20, respectively. Starting from pH 6 where the lowest amount of polysaccharides was obtained, the extraction yield started rising until it reached its peak at pH 8.
Hence, from the preliminary experiments, three levels of each factor including 50, 70, and 90 °C for temperature; 30, 40, and 50 min for time; 50, 60, and 70 for W/R ratio; and 7, 8, and 9 for pH were chosen for further optimization in BBD design (Table 1).
Statistical analysis and the model fitting
There were a total of 29 runs for optimizing the four individual parameters in the present BBD. The design was applied to the production of ESP by ultrasound-assisted water extraction. The data were analyzed by multiple regression analysis using the Design-Expert 7.1.6, and following polynomial equation was derived to represent polysaccharide yield as a function of the independent variables tested, where Y is the predicted polysaccharide yield and X 1, X 2, X 3, and X 4 are the coded values for the extraction temperature, extraction time, ratio of water to raw material, and pH, respectively. Table 2 shows the process variables and experimental data. The percentage yield ranged from 3.53 to 9.91 % where the maximum yield of ESP was obtained at extraction temperature 90 °C, extraction time 40 min, ratio of water to raw material 60 mL/g, and pH 8. The application of RSM provided an empirical relationship between the response variable and the test variables. By applying multiple regression analysis on the experimental data, the response variable and the test variables are related by the following second-order polynomial equation:
Analysis of variance (ANOVA) was performed to evaluate the predictive model and the variables. As shown in Table 3, the F value is 82.25 and p value is less than 0.0001, indicating that the response surface quadratic model was significant and can be used to optimize the extraction variables. In addition, the linear coefficients (X 1, X 2, X 3, and X 4), quadratic coefficients (X 1 2, X 2 2, and X 3 2), and cross product coefficients (X 1 X 2, X 1 X 3, X 1 X 4, and X 2 X 3) had a significant effect, with small p values (p < 0.05). The lack of fit is an indication of the failure for a model representing the experimental data at which points are not included in the regression or variations in the models cannot be accounted for random error (Pan et al. 2010). In the current study, the lack of fit (0.389) did not result in a significant p value for selected variables in the model, which means that the model was significantly accurate for predicting the relevant response. The determination coefficient (R 2) for model (0.988) was very high indicating a satisfactory correlation between actual and predicted values. The adjusted R 2 was 0.976 which means that most variation of the extraction yield could be predicted by the model. Moreover, the value of the coefficient of variation (CV %) was 3.82, showing a better precision and reliability of the experimental values.
Optimization of extraction conditions of ESP
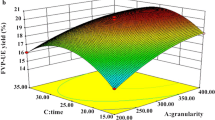
The graphical representations of regression equation are illustrated using 3D response surface and 2D contour plots in Figs. 2 and 3. This provides means to visualize the correlation between responses and experimental levels of each variable and the type of interactions between two test variables. Contour plots appear in different shapes, each implying the type of interaction existing between test variables. Circular contour plot indicates that there is a negligible interaction between corresponding variables while elliptical contour plot reveals significant correlation. In the current study, the extraction yield of ESP was obtained in association with two continuous variables, while the other variables were fixed constant at their zero level.
Figures 2a and 3a, which show the extraction yield of ESP as a function of extraction time and temperature at fixed ratio of water to raw material (60) and pH (8), showed that the extraction yield increased rapidly with increase of extraction temperature from 50 to 90 °C. However, with the extraction time from 20 to 40 min, the extraction yield only had a small increase. The extraction yields of ESP affected by different extraction temperatures and water to raw material ratios are illustrated in Figs. 2b and 3b, when the extraction time and pH were fixed at 30 min and pH 8. Results show that extraction yield of ESP increased evidently with increasing of extraction temperature from 50 to 90 °C. On the other hand, the extraction yield of polysaccharides increased very slowly with the rise in water to raw material ratio. Figures 2c and 3c show a mutual effect of pH and extraction temperature on the yield of polysaccharides at fixed extraction time (30 min) and water to raw material ratio (60). The extraction yield increased with the increasing extraction temperature from 50 to 90 °C and reached the maximum of 8.59 % at 90 °C and pH 7. The 3D and contour plots based on independent variables of water to raw material ratio and extraction time are shown in Figs. 2d and 3d, while the extraction temperature and pH were kept at their zero levels. An increase in the extraction yield of ESP was significantly achieved with the increase of extraction time from 20 to 40 min. The extraction yield of ESP affected by different pH and extraction time is shown in Figs. 2e and 3e, when the water to raw material ratio and temperature were fixed at 60 and 70 °C, respectively. The yield increased rapidly with the extension of extraction time. The maximum extraction yield of ESP achieved when time and pH of extraction were set at 40 min and 7, respectively. While time (30 min) and temperature (70 °C) of extraction are maintained at their zero levels, the 3D response surface and contour plots of the effects of water to raw material ratio and pH on extraction yield are shown in Figs. 2f and 3f. The yield of polysaccharides increased with the water to raw material ratio of extraction from 7 to 9.
Validation of the model
The optimal conditions suggested by the model in order to obtain the maximum ESP yield of 8.369 % were extraction temperature 65.87 °C, extraction time 40 min, water to raw material ratio 50, and pH 7.0 (Table 4). To validate the adequacy of the model equation, verification experiment was carried out under the modified optimal conditions: extraction temperature 66 °C, extraction time 40 min, water to raw material ratio 50, and pH 7.0. The extraction yield of polysaccharides achieved under these conditions was 8.3 %, which was very close to that of corresponding predicted value. The results of current set of experiments indicated that the response model was adequate for reflecting the expected optimization, and the model equation was satisfactory and accurate.
Chemical composition of ESP
As shown in Table 5, the ESP was mostly composed of carbohydrates (64.9 %). Further analyses also revealed a limited amount of protein (11.2 %) and ash (12.5 %) in the ESP. In addition, as shown by the FT-IR, the ESP carries sulfate groups (8.7 %) on its polysaccharide chain.
FT-IR spectroscopy
IR spectrum of the ESP demonstrated several bands corresponding to sulfate ester: the peaks at 850 and 1256 cm−1 derived from the bending vibration of C–O–S of sulfate in axial position and stretching vibration of S–O of sulfate, respectively (Fig. 4) (Gan et al. 2011). The signal at 3445 cm−1 was from stretching vibration of O–H, 2942 cm−1 was due to stretch vibration of C–H, and 1053 cm−1 was from stretching vibration of C–O. Besides, there were signals at 1660 cm−1, related to asymmetric stretch vibration of COO− of uronic acids, and 1446 cm−1, related to symmetric stretch vibration of COO− and stretch vibration of C–O within COOH (Zhao et al. 2010).
Antioxidant activities
DPPH scavenging activity
The antioxidant capacity of the polysaccharides from U. intestinalis was evaluated using their ability to scavenge DPPH radicals (Fig. 5a). DPPH is widely used as a free radical to evaluate antioxidant compounds that have the capability to reduce DPPH radicals by donating hydrogen and produce a stable form. In the current study, the ESP exhibited dose-dependent DPPH radical scavenging capacities at concentrations ranging from 0.5 to 5.0 mg mL−1. The highest scavenging activity was found to be 74 % at the maximum concentration of 5.0 mg mL−1. It has been found that sulfated polysaccharides not only function as dietary fiber, but also incorporate into antioxidant activity of seaweeds. It has been thought that sulfated polysaccharides with higher molecular weights and sulfate contents exhibit potent antioxidant activities (Zhang et al. 2011; Li et al. 2013a, b, c).
Reducing power
The reducing capacity of the polysaccharides was determined by measuring the amount of reductones involved in the sample. Reductones are substances that exhibit antioxidant activities by donating a hydrogen atom and breaking the free radical chains (Qi et al. 2005a). Figure 5b shows a dose-dependent increase in reducing power of extracted ESP over the concentration range of 0.5 to 5.0 mg mL−1. The ESP sample reached its maximum reducing power of 88 % at the highest concentration. The reducing power of sulfated polysaccharides has been previously reported in species including Ulva pertusa, Ulva linza, and Bryopsis plumose (Zhang et al. 2010).
Stimulation and proliferation of RAW264.7 macrophage cells
The NO releasing capacity of the ESP polysaccharide at the concentrations of 1.5 to 12.0 μg mL−1, expressed as the amount of NO released from RAW264.7 cells, is shown in Fig. 6a. The level of NO produced was found to be 27–50 μmol mL−1 which was comparable with that of positive control, LPS (1.0 μg mL−1), indicating the potent immunostimulating activity of ESP. Also, as a standard procedure, the ESP was examined for endotoxin content with Limulus amebocyte lysate (LAL) method using a commercial assay kit (ETOXATE, Sigma, USA) which showed a negative result. Macrophages are known to be closely related to the response of innate immune system and therefore play an essential role in the host defense against pathogenic organisms and invading cells by releasing NO or cytokine release (Ikekawa et al. 1992). The ability of sulfated polysaccharides from green seaweeds to stimulate macrophage cells and release NO at various amounts has been previously reported (Jiao et al. 2009; Tabarsa et al. 2013).
Nitric oxide production (a) and proliferation activity (b) of the RAW264.7 cells following treatment with ESP. The RAW264.7 cells were plated in an RPMI-1640 medium containing 10 % FBS in a 96-well microplate (1 × 104 cells/well, 100 μL) and incubated with 100 μL of ESP or 1 μL LPS (positive control). D ifferent letters indicate a significant difference (p < 0.05) among different concentrations
Subsequently, the effect of ESP polysaccharide on the proliferation of RAW264.7 cells was evaluated using WST-1 and found that RAW264.7 cell proliferation was greatly increased by the presence of ESP polysaccharides (Fig. 6b). This suggested that the extracted polysaccharides were not toxic to RAW264.7 cells over the concentrations tested.
Conclusions
Ultrasound-assisted extraction was primarily performed in order to obtain sulfated polysaccharides from green seaweed U. intestinalis. Under optimum conditions: extraction temperature 66 °C, extraction time 40 min, ratio of water to raw material 50, and pH 7, a maximum yield of 8.3 % was achieved which was agreed closely with the predicted yield of 8.37 %. The variance analysis suggested that the regression model was accurate and adequate for the extraction of ESP. The ESP was then characterized using FT-IR revealing its carboxylic and sulfate groups. At last, the antioxidant results exhibited that ESP had strong DPPH scavenging activity and reducing power of ferric ions. The purification of U. intestinalis sulfated polysaccharides extracted under optimum conditions and determination of their chemical and molecular structures are also in progress.
References
Adhikaria U, Mateub CG, Chattopadhyaya KC, Pujolb A, Damonteb EB, Ray B (2006) Structure and antiviral activity of sulfated fucans from Stoechospermum marginatum. Phytochemistry 67:2474–2482
Ale MT, Mikkelsen JD, Meyer AS (2011) Important determinants for fucoidan bioactivity: a critical review of structure-function relations and extraction methods for fucose-containing sulfated polysaccharides from brown seaweeds. Mar Drugs 9:2106–2130
AOAC (1997) Official Methods of Analysis of AOAC International. AOAC International, Maryland
Arata PX, Quintana I, Canelon DJ, Vera BE, Compagnone RS, Ciancia M (2015) Chemical structure and anticoagulant activity of highly pyruvylated sulfated galactans from tropical green seaweeds of the order Bryopsidales. Carbohydr Polym 122:376–386
Athukorala Y, Jung WK, Vasanthan T, Jeon YJ (2006) An anticoagulative polysaccharide from an enzymatic hydrolysate of Ecklonia cava. Carbohydr Polym 66:84–91
Briseno JAA, Suarez LEC, Sassi JF, Marie DR, Benavides PZ, Gamboa EM, Padilla CR, Trejo-Avila LM (2015) Sulphated polysaccharides from Ulva clathrata and Cladosiphon okamurans seaweeds both inhibit viral attachment/entry and cell-cell fusion, in NDV infection. Mar Drugs 13:697–712
Chen W, Wang WP, Zhang HS, Huang Q (2012) Optimization of ultrasonic-assisted extraction of water-soluble polysaccharides from Boletus edulis mycelia using response surface methodology. Carbohydr Polym 87:614–619
Deng Q, Zhou X, Chen H (2014) Optimization of enzyme assisted extraction of Fructus mori polysaccharides and its activities on antioxidant and alcohol dehydrogenase. Carbohydr Polym 111:775–782
Dodgson KS, Price RG (1962) A note on the determination of the ester sulphate content of sulphated polysaccharides. Biochem J 84:106–110
Dubois M, Gilles KA, Hamilton JK, Rebers PA, Smith F (1956) Colorimetric method for determination of sugars and related substances. Anal Chem 28:350–356
Gan D, Ma L, Jiang C, Xu R, Zeng X (2011) Production, preliminary characterization and antitumor activity in vitro of polysaccharides from the mycelium of Pholiota dinghuensis Bi. Carbohydr Polym 84:997–1003
Hwang PA, Hung YL, Chien SY (2015) Inhibitory activity of Sargassum hemiphyllum sulfated polysaccharide in arachidonic acid-induced animal models of inflammation. J Food Drug Anal 23:49–56
Ikekawa T, Saitoh H, Feng W, Zhang H, Li L, Matsuzawa T (1992) Antitumor activity of Hypsizigus marmoreus. I: antitumor activity of extracts and polysaccharides. Chem Pharm Bull 40:1954–1957
Jiao L, Li X, Li T, Jiang P, Zhang L, Wu M, Zhang L (2009) Characterization and anti-tumor activity of alkali-extracted polysaccharides from Enteromorpha intestinalis. Int Immunopharmacol 9:324–329
Knudsen NR, Ale MT, Meyer AS (2015) Seaweed hydrocolloid production: an update on enzyme assisted extraction and modification technologies. Mar Drugs 13:3340–3359
Lahaye M, Robic A (2007) Structure and functional properties of ulvan, a polysaccharide from green seaweeds. Biomacromolecules 8:1766–1774
Li B, Liu S, Xing R, Li R, Qin Y, Wang X, Wei Z, Li P (2013a) Degradation of sulfated polysaccharides from Enteromorpha prolifera and their antioxidant activities. Carbohydr Polym 92:1991–1996
Li Y, Wang J, Yu Y, Li X, Jiang X, Hwang H, Wang P (2013b) Production of enzymes by Alteromonas sp. A321 to degrade polysaccharides from Enteromorpha prolifera. Carbohydr Polym 98:988–994
Li Q, Yu N, Wang Y, Sun Y, Lu K, Guan W (2013c) Extraction optimization of Bruguiera gymnorrhiza polysaccharides with radical scavenging activities. Carbohydr Polym 96:148–155
Li L, Ni R, Shao Y, Mao S (2014) Carrageenan and its applications in drug delivery. Carbohydr Polym 103:1–11
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275
Oyaizu M (1986) Studies on products of browning reaction. Antioxidative activities of products of browning reaction prepared from glucosamine. Jpn J Nutr 44:307–314
Pan Y, Hao Y, Chu T, Li C, Zhang Z, Zhou Y (2010) Ultrasonic-assisted extraction, chemical characterization of polysaccharides from Yunzhi mushroom and its effect on osteoblast cells. Carbohydr Polym 80:922–926
Pomin VH (2010) Structural and functional insights into sulfated galactans: a systematic review. Glycoconj J 27:1–12
Qi H, Zhang Q, Zhao T, Chen R, Zhang H, Niu X, Zhien L (2005a) Antioxidant activity of different sulfate content derivatives of polysaccharide extracted from Ulva pertusa (Chlorophyta) in vitro. Int J Biol Macromol 37:195–199
Qi H, Zhao T, Zhang Q, Li Z, Zhao Z, Xing R (2005b) Antioxidant activity of different molecular weight sulfated polysaccharides from Ulva pertusa Kjellm (Chlorophyta). J Appl Phycol 17:527–534
Rastogi NK (2011) Opportunities and challenges in application of ultrasound in food processing. Crit Rev Food Sci Nutr 51:705–722
Ren J, Zhao M, Shi J, Wang J, Jiang Y, Cui C, Kakuda Y, Xue SJ (2008) Purification and identification of antioxidant peptides from grass carp muscle hydrolysates by consecutive chromatography and electrospray ionization-mass spectrometry. Food Chem 108:727–736
Reviers BD, Leproux A (1993) Characterization of polysaccharides from Enteromorpha intestinalis (L.) Link, Chlorophyta. Carbohydr Polym 22:253–259
Robic A, Rondeau-Mouro C, Sassi JF, Lerat Y, Lahaye M (2009) Structure and interactions of ulvan in the cell wall of the marine green algae Ulva rotundata (Ulvales, Chlorophyceae). Carbohydr Polym 77:206–216
Ropellato J, Carvalho MM, Ferreira LG, Noseda MD, Zuconelli CR, Concalves A, Ducatti DR, Kenski JC, Nasato PL, Winnischofer SM, Durate WE (2015) Sulfated heterorhamnans from the green seaweed Gayralia oxysperma: partial depolymerization, chemical structure and antitumor activity. Carbohydr Polym 117:476–485
Santoyo S, Plaza M, Jaime L, Ibanez E, Reglero G, Senorans J (2011) Pressurized liquids as an alternative green process to extract antiviral agents from the edible seaweed Himnthalia elongate. J Appl Phycol 23:909–917
Tabarsa M, Karnjanapratum S, Cho ML, Kim JK, You SG (2013) Molecular characteristics and biological activities of anionic macromolecules from Codium fragile. Int J Biol Macromol 59:1–12
Tabarsa M, Park GM, Shin IS, Lee EJ, Kim JK, You SG (2015) Structure-activity relationships of sulfated glycoproteins from Codium fragile on nitric oxide releasing capacity from RAW264.7 cells. Mar Biotechnol 17:266–276
Vinatoru M, Toma M, Radu O, Filip PI, Lazurca D, Mason TJ (1997) The use of ultrasonic for the extraction of bioactive principles from plant materials. Ultrason Sonochem 4:135–139
Wang Y, Liu Y, Hu Y (2014) Optimization of polysaccharides extraction from Trametes robiniophila and its antioxidant activities. Carbohydr Polym 111:324–332
Xu Y, Zhang L, Bailina Y, Ge Z, Ding T, Ye X, Liu D (2014) Effects of ultrasound and/ or heating on the extraction of pectin from grapefruit peel. J Food Eng 126:72–81
Zhang Z, Wang F, Wang X, Liu X, Hou Y, Zhang Q (2010) Extraction of the polysaccharides from five algae and their potential antioxidant activity in vitro. Carbohydr Polym 82:118–121
Zhang Z, Wang X, Yu S, Yin L, Zhao M, Han Z (2011) Synthesized oversulfated and acetylated derivatives of polysaccharide extracted from Enteromorpha linza and their potential antioxidant activity. Int J Biol Macromol 49:1012–1015
Zhao L, Dong Y, Chen G, Hu Q (2010) Extraction, purification, characterization and antitumor activity of polysaccharides from Ganoderma lucidum. Carbohydr Polym 80:783–789
Zhu C, Liu X (2013) Optimization of extraction process of crude polysaccharides from pomegranate peel by response surface methodology. Carbohydr Polym 92:1197–1202
Zou Y, Chen X, Yang W, Liu S (2011) Response surface methodology for optimization of the ultrasonic extraction of polysaccharides from Codonopsis pilosula Nannf. var. modesta L.T. Shen. Carbohydr Polym 84:503–508
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rahimi, F., Tabarsa, M. & Rezaei, M. Ulvan from green algae Ulva intestinalis: optimization of ultrasound-assisted extraction and antioxidant activity. J Appl Phycol 28, 2979–2990 (2016). https://doi.org/10.1007/s10811-016-0824-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-016-0824-5