Abstract
Cold press oils are value-added food ingredients that are increasingly produced. Due to the removal of oil, protein content in cold press deoiled meals substantially increase. Here, we made an attempt to manufacture protein concentrates from cold press meals of black cumin and studied the influence of aqueous (alkali extraction-isoelectric precipitation, AE-IP) and organic (n-hexane) extraction conditions on the structure and functionality of these concentrates. To determine the basic structural attributes, SDS-PAGE and 2D-electrophoresis, DSC, rheological and FT-IR analysis were utilized. Black cumin proteins contained both α- and β-secondary structural elements, and the proteins were potentially glycosylated. This in turn affected their gel-like behavior prior to heating. The presence of a number of high pI proteins was also detected via 2D-electrophoresis. Functional characteristics of the concentrates were investigated based on solubility, water and oil holding capacities, and dynamic surface tension analysis. Deoiled meals contained 22.3% oil, which was only slightly affected by aqueous extraction. Protein content in the meals was approx. 26.5% which increased to 57.7 and 65.8%, after aqueous (AE-IP) extraction and both aqueous (AE-IP) and hexane extraction, respectively. Solubility, WHC, OHC were found to be improved by hexane extraction. Based on DSC analysis, presence of black cumin oil in the concentrates enhanced the thermal stability of the proteins, while black cumin proteins demonstrated considerable surface activity at the air–water interface. Finally upon enyzmatic proteolysis, protein hydrolyzates demonstrated slight angiotensin-converting enzyme (ACE)-inhibitory activity. Based on the current findings, deoiled black cumin meals represent a viable source of biologically and technically functional proteins.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Plants represent an alternative source of proteins available for utilization in commercial applications. Currently, there is a variety of commercial plant protein products manufactured from legumes, cereals and oilseeds [1]. Due to the rapidly increasing global demand, the exploration of alternative sources is necessary. The drawbacks of using plant proteins include sulfur amino acid deficiency of some plants and presence of antinutritive agents in the final products. However, supplementation with other resources could solve these problems [1].
Raw materials used in plant protein production are renewable and current demand of the food industry heavily consumes the commercially available plant proteins, therefore there is a potential to generate market share for novel products. Since plant proteins can be converted to animal proteins at fairly low efficiency levels (i.e., approx. 15%), plant protein production is both sustainable and cost-efficient [2].
Oilseeds contain considerably higher amounts of protein compared to cereals [3]. After oil extraction, proteins are highly concentrated in the meals, and plant protein concentrates can be produced from these inexpensive by-products, where protein content could account for up to 60% [4]. In addition to economic reasons, manufacture of seed protein products also bring in environmental advantages such as waste reduction [5].
Black cumin (Nigella sativa) is a valuable and annually flowering medicinal plant from Ranunculaceae family [6] which is native to the East Mediterranean countries, South Europe and Asia Minor [6]. Currently, black cumin is also cultivated in the Middle East, North Africa and Asia [7]. According to Commodity Trade Statistics Database, global consumption of black cumin was estimated to be 187,000 tonnes. India cultivates more than 85% of the global production of black cumin, whereas approximately 3.5% and 2.8% are generated by Syria and Turkey, respectively [8].
Black cumin seeds are composed of approximately 21% protein, 35% carbohydrates and 35–38% oil by weight [6]. In the deoiled meal, protein content can be anticipated to be > 30%. Black cumin seeds can be utilized in medicinal applications or nutritional supplements. Further utilization in industrial applications are unknown to us. Due to the difficulty of consuming black cumin seeds, protein products generated from this valuable resource could increase its global manufacture and consumption.
Proteins are functional biomolecules in the sense of both technical and biological functionality. Technical functionality of proteins are related to their hydration, structural/rheological and interfacial/surface related characteristics. Once utilized in food formulations, novel protein products will be anticipated to be compatible with other ingredients [1]. Consequently functional properties such as water and oil holding capacities, solubility, and capabilities in lowering surface/interfacial tension are usually monitored.
The aim of this study was to investigate the structural and functional properties of black cumin protein concentrates manufactured using an aqueous protein isolation methodology with or without the application of an organic extraction step. Firstly, in order to preserve the structural attributes, organic extraction was avoided. After further removal of oil by hexane extraction, structural and functional characteristics of both sets of samples were compared. Finally, based on partial proteolysis of proteins, ACE-inhibitory activity was tested. The simple methodologies utilized here are fully applicable to industrial settings constructed for the valorization of industrial by-product streams. This study presents novel insights on both the technical and biological attributes of black cumin proteins. Consequently, the novelty of the current work is primarily related to the industrial valorization potential of an alternative resource. Cold press meals are comparatively less utilized in protein research and in the following years, we anticipate that their eminence in the manufacture of proteins and protein hydrolyzates will rise. Secondly, since black cumin is a physiologically active but relatively difficult to consume food, its applicability to novel and/or functional food products require extended studies on its technically and biologically active ingredients. Here, using a wide variety of analytical tools, we made a first attempt to investigate certain physicochemical, structural and biological properties of these proteins.
Materials and methods
Materials
Cold press deoiled black cumin meals were donated by a local manufacturer (Neva Foods Ltd., İstanbul, Turkey). The maximum temperature observed by the cold press samples was lower than 40 °C. All chemicals were reagent grade and purchased from Sigma-Aldrich, except for sodium dodecyl sulfate (SDS) (Millipore Corp., Germany).
Determination of amino acid composition
Amino acid composition of the samples was determined using a Shimadzu LC-20AD HPLC unit (Shimadzu Corporation, Kyoto, Japan). For separation, a 250 × 4.6 mm column packed with 5 μm Inertsustain C18 (GL Science, Tokyo, Japan) was used. The method of Gonzalez-Castro et al. [9] was utilized.
Prior to derivatization, proteins were subjected to acidic hydrolysis. 0.1 g lyophilized sample was weighed into screw cap Pyrex tubes and 15 mL of 6 N HCl was added. The tube was throughly flushed with nitrogen gas, quickly capped, and kept in an oven (40 °C) for 24 h. After the completion of hydrolysis, the tube contents were vacuum filtered to remove solids, and the filtrate was brought to 25 mL with water, and an aliquot of this solution was further filtered through a 0.45 μm membrane (Millipore). A standard solution containing 1.25 μmol mL−1 of each amino acid in 0.1 N hydrochloric acid was prepared.
Protein dispersions were dried under vacuum (65 °C). To enable the derivatization, 30 μL methanol–water-TEA (2:2:1 [v/v]) was added to the residues, and vacuum drying was carried out. 30 μL of the derivatizing reagent methanol–water-TEA-PITC (7:1:1:1 [v/v]) was added to the dried sample, and the tube was kept shaken for 20 min. Finally, the solvents were evaporated under nitrogen gas flow, and the samples were stored at 4 °C until further analysis. Prior to injection, the samples were reconstituted in 150 μL 5 mM sodium phosphate (pH 6.2) containing 5% acetonitrile. The analysis was carried out at 30 °C using a gradient elution. Eluent A was a 0.5 mL L−1 TEA in 0.14 M sodium acetate brought to pH 6.2 with glacial acetic acid; eluent B was acetonitrile–water (60:40 [v/v]). Gradient elution of B (4–100%) was carried out.
Preparation of protein concentrates
Protein manufacture was based on alkali extraction-isoelectric precipitation method. Solvent extraction was utilized to remove remaining black cumin oil from the samples.
Alkali extraction-isoelectric precipitation method (AE-IP)
AE-IP technique was based on the solubilization of protein molecules at basic pH, which was followed by isoelectric precipitation at acidic pH values. Protein concentrates were produced from deoiled black cumin meals using the method of Boye et al. [10]. 50 g of deoiled meal was dispersed in water (1:15, w/v) and the pH of the medium was adjusted to pH 9.5 using 1.0 N NaOH. The dispersions were stirred at 500 rpm for 1 h. Immediately afterwards, the dispersions were centrifuged at 13500×g for 15 min at 4 °C using a CR22 N high-speed centrifuge (Hitachi Koki Co., Ltd., Tokyo, Japan). The supernatant containing the solubilized proteins was collected and the medium pH was adjusted to pH 4.5 to induce isoelectric precipitation. To ensure the completion of separation, the supernatant was centrifuged under identical conditions as before. The pellet was collected and immediately frozen at − 20 °C. Frozen samples were lyophilized using a TRS 2/2 V freeze drier (Teknosem Corp., İstanbul, Turkey).
Solvent extraction
Soxhlet extraction system was used for the removal of black cumin oil from meals (Behr Labortechnik, R106S, Germany). Firstly, the samples were treated with hexane for 7 h at a sample to hexane ratio of 1:50. In order to remove hexane, the samples were dried at 55 °C until constant weight was reached.
Physicochemical analyses of meals and protein concentrates
The protein, moisture and ash contents of meals and protein concentrates were determined according to AOAC Official Methods 920.87 (%N × 6.25), 925.10 and 923.03 respectively [11]. Fat content analysis was based on NMKL 960 [12].
Structural analyses on the black cumin protein concentrates
Sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE)
SDS-PAGE analysis was carried out based on Laemmli [13] under reducing conditions using a Mini Protean Tetra Cell System (Bio-Rad Laboratories Inc., USA). Firstly, protein concentrates (1%) were dispersed in deionized water. Immediately afterwards, protein samples and 2 × Laemmli loading buffer containing 0.004% Bromophenol blue, 10% 2-mercaptoethanol, 20% glycerol, 4% SDS and 0.125 M Tris–HCl (pH 6.8) were mixed 1:1 in Eppendorf tubes. Samples were heated 5 min at 100 °C, cooled, and loaded on a TGX Stain-Free Precast Gel (12%). Precision Plus protein standards from the same manufacturer were used as the reference. Gel electrophoresis was carried out for 45 min using Tris/Glycine/SDS running buffer at 200 V (constant). Imaging was carried out by transferring the gel to a stain-free tray and using Gel Doc EZ System. The images were analyzed using Image Lab Software.
Two dimensional (2D) gel electrophoresis
240 µg protein was loaded onto immobilized 11 cm, pH gradient strips (IPG) (pH 3–10) by passive rehydration. Isoelectric point based separation was achieved using a Protean isoelectric focusing cell (IEF) (Bio-Rad). The following conditions were used for IEF: 20 min at 250 V with rapid ramp, 2 h at 4000 V with slow ramp and 2.5 h for 4000 V with rapid ramp until a total of 32.000 V h−1 was reached (20 °C). After isoelectric focusing, strips were washed with buffer I (6 M Urea, 375 mM Tris–HCl pH 8.8, 2% SDS, 20% glycerol, 2% (w/v) DTT) for 30 min and with buffer II (6 M Urea, 375 mM Tris–HCl pH 8.8, 2% SDS, 20% glycerol, 2.5% iodoacetamide (w/v)) for 30 min and subjected to SDS-PAGE for the second dimension. After the separation, the gels were fixed in 40% methanol, 10% acetic acid and stained with Colloidal Coomassie Blue G250 (Bio-Rad, USA).
Differential scanning calorimeter (DSC) analysis
Thermal behaviour of protein dispersions was analyzed using a differential scanning calorimeter system (DSC 60 Plus, Shimadzu Instruments, Japan). Approx. 10 mg of samples were placed into aluminum pans and heated from 30 to 125 °C at a heating rate of 10 °C min−1.
Rheological analysis
An Anton Paar rheometer (MCR 302, Austria) fitted with temperature controlled Peltier system was used to monitor temperature dependence of the rheological characteristics of black cumin protein concentrates (10%, pH 7). Approximately 1 mL protein dispersion was placed on the lower plate of the parallel-plate geometry. The diameter of upper parallel plate was 25 mm. A solvent trap cover was used, and light silicon oil was applied to the exposed part to minimize evaporation during heating. The head was charged with water to saturate the medium with water vapor. The rheometer was operated at constant angular frequency of 1 Hz and strain range of 1–10% depending on compliance to the linear viscoelastic behavior. The heating protocol involved a linear temperature ramp from 25 to 85 °C at a heating rate of 5 °C s−1, holding at 85 °C for 2 min and thereafter cooling to 25 °C at 5 °C s−1. Shear strain and modulus values (G′ and G″) were recorded as a function of time and temperature.
Fourier transform infrared (FT-IR) spectroscopy analysis
FT-IR analysis of protein concentrates was carried out using an IRTracer-100 FT-IR spectrophotometer (Shimadzu, Japan) equipped with a DLATGS detector system, and MIRacle ATR module with a resolution of 2 cm−1 (Pike Technologies, USA). FT-IR absorption spectra were collected from 4000 to 650 cm−1.
Functional properties of protein concentrates
Functional attributes of the protein concentrates were tested and where necessary, compared to that of a commercial soy protein isolate under identical conditions (Jem Nutrimax, Sonic Biochem, A Matlani Group Company, India).
Solubility
Protein solubility (%) was determined by dispersing 0.2 g protein (w/v) in 19 ml of 0.1 N NaCl solution, adjusting pH to 7 using 0.5 N HCl or NaOH, and keeping the dispersion stirred (500 rpm) for 1 h at 50 °C. Total solution volume was brought to 20 ml with 0.1 N NaCl. The mixtures were left to stand for 10 min and then centrifuged at 4200×g for 10 min. Solubility (%) was determined using an appropriate protein analysis kit based on a modified version of Lowry method (TP0300, Sigma Aldrich Corp.). For all samples, absorbance was measured at 750 nm.
Water or oil holding capacity
One gram protein concentrate was added to 10 mL of distilled water (or soy oil, S7381, Sigma Aldrich Corp.) in a 15 ml centrifuge tube. Every 5 min, the contents were stirred for 30 s on a vortex stirrer (Vortex, Genie 2-Mixer, Scientific Industrial Inc., Bohemia, NY, USA) and after 30 min the tubes were centrifuged at 3000×g for 20 min. Once the free water or oil portion was withdrawn, water/oil holding capacity was calculated using the increase (%) in sample weight [14].
Drop shape tensiometry
The surface tension at the air-aqueous solution interface was determined using drop shape tensiometry (25 °C) (Biolin Scientific, Attension Theta, Finland). An air bubble was automatically formed at the tip of an inverted syringe immersed in a quartz cuvette containing the protein dispersion (0.1%) prepared in 100 mM sodium phosphate buffer (pH 7). The droplet shape was automatically recorded over time, as the cuvette and syringe assembly were monitored by a CCD (charge coupled device) camera and high quality image acquisition was utilized [15]. Surface tension was calculated based on the Young–Laplace equation using OneAttension 2.6 software provided by the manufacturer. All the measurements were carried out in triplicate. The surface pressure (π) was calculated as the difference in the surface tension of the buffer (approx. 72.3 mNm−1) and the protein solution at the air–water interface.
ACE-inhibitory activity of black cumin protein hydrolyzates
In vitro proteolysis
Trypsinolysis or chymotrypsinolysis was carried out based on the previous literature [16]. Protein concentrate (1%) was dissolved in 50 mM sodium phosphate buffer (pH 7) by stirring for 1 h. Enzymatic digestion was carried out for 4 h at an enzyme to protein ratio of 1:1000 on a thermomixer (MIULAB Thermo Shaker Incubator, 37 °C, 1000 rpm). Immediately afterwards, the digest was heated to 95 °C and held for 5 min to stop enzymatic activity. The samples were rapidly cooled using ice. Upon reaching ambient temperature, the samples were centrifuged for 30 min at 5000×g. Prior to chromatographic analysis, all samples were filtered through 0.45 µm PTFE syringe filters (Isolab, Germany).
Measurement of ACE inhibitory activity
All the solutions and reactants used here were prepared in 100 mM sodium borate buffer (pH 8.3). Angiotensin I-converting enzyme (ACE) inhibition assay was carried out based on the method described by Sheih et al. [17]. 200 µL 5 mM HHL was mixed with hydrolyzates and the mixture was incubated at 37 °C for 10 min. Immediately afterwards, 20 µL of concentrated ACE solution was added to generate an enzyme concentration of 1.68 mU in the mixture. The incubation was carried out for 0–4 h at 37 °C and stopped by the addition of 250 µL HCl (1 M). The final mixture was injected into the HPLC device in order to determine hippuric acid content [17]. Ascentis C18 Column (4.6 mm ID × 250 mm, 5 µm particle size, Supelco) was used in the analysis. The mobile phase was composed of 1:1 mixture of ethanol and water by volume, which also contained 1 mL L−1 TFA. The absorbance was recorded at 228 nm. The analysis was carried out at 40 °C at a flow rate of 1 mL min−1. In order to determine % ACE inhibition, the performance of the hydrolyzates was compared to the positive control samples, which did not contain any hydrolyzates. 0.02% sodium azide was added as a bacteriostatic in all cases.
Results and discussion
Amino acid composition of deoiled meals
Amino acid composition of the deoiled meals were determined using an HPLC method (Table 1). Based on the current findings, approx. 33% of the meals accounted for essential amino acids. These findings were also comparable to that of Babayan et al. [18], where the extent of essential amino acids was found to be 37.85%, although the histidine content was not specified by these authors. Part of the differences in the results could be attributed to processing related losses or changes in amino acid structures during sample preparation, since sample preparation techniques differed between the two studies (immediate acid hydrolysis vs. treatment of cold press deoiled samples).
Compositional characteristics of black cumin protein concentrates
Using an AE-IP technique, protein concentrates were prepared from the deoiled meals. Consequently, protein, moisture and ash contents of meal and concentrates were determined immediately after lyophilization (Table 2). Protein, fat, ash and moisture contents (%) of black cumin meals were found to be 26.5, 22.3, 7 and 6%, respectively. Although the oil content of the meals was significantly lowered during the cold press treatment, the meal samples still contained appreciable amounts of oil. Based on AE-IP method, the protein content of the concentrate was 54.7% (Table 2) prior to the further extraction of remaining oil. Consequently, the extent of improvement in the protein content of black cumin meals was approximately 107% due to the aqueous extraction. Once solvent extraction was carried out, a further 20% improvement in protein content took place.
Based on Table 2 data, ash and moisture contents of AE-IP samples generally improved compared to the meal samples. It was indicated that strong alkali or acids used in isoelectric precipitation of proteins may result in salt formation and thus, yield higher ash levels in the protein isolate relative to the meal or flour [14]. Since no dialysis was applied after the AE-IP treatment, a certain extent of increase in ash content may be expected. The influence of hexane extraction on moisture and ash contents was less clear.
Structural attributes of the protein concentrates
Electrophoretic analysis of protein concentrates
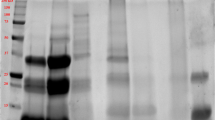
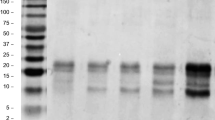
Molecular weight distribution of protein concentrates was analyzed by SDS-PAGE before and after hexane extraction (Fig. 1a). First of all, the major bands were found to lay between 15 and 40 kDa. For the hexane treated samples (Lane 1), there were also other faint bands between 10 and 15 kDa and > 40 kDa. Previously Haq et al. [19] reported that Nigella sativa proteins ranged between 10 and 200 kDa. These investigators further fractionated the proteins by Rotofor technology and mostly found the bands around 25, 40, 65 and 200 kDa. Since the major bands in the current study were located around 15 kDa and 40 kDa in most cases, the current SDS-PAGE findings were generally coherent with the previous findings. After the oil extraction, broadening of the bands were observed. This could imply that broader bands represented less hydrophobic proteins, for which solubility was improved as oil was removed. In general, the influence of solvent extraction on the variety of bands was mostly weak.
Using similar samples, 2D-electrophoresis was also carried out in order to further clarify the distribution of molecular weights and isoelectric points of the protein molecules (Fig. 1b). The pH and molecular weight range was pH 3–10 and 20–200 kDa, respectively. Similar to the 1D-gel (Fig. 1a, SDS-PAGE), there were some protein spots between 20 and 25 kDa and also between 25 and 40 kDa. Since there were spots around 65 kDa and larger and finally around 200 kDa, some of these findings were also comparable to the previous literature [19]. The pI values of most proteins were ≤ pH 7; however, a variety of protein spots were also visualized for alkaline proteins. Based on Table 1 data, amino acids with high pI such as arginine (pI 10.76), lysine (pI 9.47) and histidine (pI 7.59) account for approx. 17.2% of all the amino acids in the deoiled meals. The presence of high pI proteins in food systems is relatively less frequent compared to acidic or neutral pI proteins, while in food processing and nanoparticle based delivery systems, positively charged proteins provide new opportunities [20].
Differential scanning calorimeter (DSC) analysis
Thermal denaturation characteristics of black cumin protein concentrates were analyzed both in the presence and absence of hexane treatments (Fig. 2). The denaturation temperatures for black cumin protein concentrates were approx. 71.9 and 57.6 °C before and after hexane extraction, respectively. The remaining fatty acids potentially formed hydrogen bonds with the protein molecules, which in turn, have altered the thermal stability of the system. Similar results were obtained previously by Guimarães et al. [21]. Here, in both cases, although broad, unimodal denaturation peaks were observed. It is possible that black cumin proteins underwent partial denaturation during extraction processes, which also lowered the enthalphy of denaturation. This value (19.71 J g−1) was comparable to the literature data on the denaturation enthalpies for plant proteins [21].
Rheological characteristics
Temperature dependence of rheological characteristics were investigated using 10% aqueous protein dispersions (pH 7). Prior to heating, black cumin protein concentrates already exhibited gel like character (G′ > G″) (Fig. 3). When the temperature increased to approximately 60 °C, which was comparable to the denaturation range, phase angle was slightly larger than 45°. During 80 °C holding, phase angle was approximately 69°. Immediately afterwards, during the cooling run, the sample returned to gel character around approximately 52 °C. During 25 °C holding, phase angle was approximately 15°. These findings indicated that although the changes in temperature had a bearing on the rheological characteristics of the protein dispersions, gel-like characteristics of the dispersions were not necessarily thermally induced. During the heating and cooling experiments, loss moduli values were larger than or roughly equal to the storage moduli which indicated the absence of gel formation or the formation of weaker gels. Loss modulus measures the viscous contribution representing the molecular interactions that do not lead to formation of 3D-gel networks [22].
We hypothesize that rheological characteristics may be in part attributed to the presence of glycoproteins in the protein concentrates (see FT-IR data). Based on an α-amylase treatment, gel-like characteristics of protein concentrates were considerably reduced in the absence of heating. While, in another qualitative experiment, starch content in the samples were found to be minimal, if any, based on a simple iodine test (data not shown). Consequently gel-like behaviour observed prior to heating could be attributed primarily to protein molecules and their water holding capacities.
Although it is not further discussed here, current protein concentrates were shown to form self-supporting gels at pH 9. Similar results were previously obtained by Kim et al. [23] for canola protein isolates, while these authors commented that alkaline pH limited their extent of utilization in foods.
FT-IR analysis
FT-IR spectroscopy is a vibrational technique that is used to monitor protein structural characteristics and any changes to them regardless of the physical state of the sample [24]. In the utilization of FT-IR data with respect to secondary structural characteristics of the proteins, Amide I and Amide II bands are commonly evaluated [24]. The Amide I band is due to carbonyl stretching vibrations (around 1700–1600 cm−1) while the Amide II band (around 1600–1500 cm−1) is due primarily to NH bending vibrations. In this study hexane treated samples demonstrated both Amide I and II bands (Fig. 4a), where prior to hexane treatment only Amide I was observed with a lesser extent of detail (Fig. 4b).
The FT-IR analysis of protein concentrates was carried out using an FT-IR spectrophotometer equipped with an ATR module. FT-IR absorption spectra were collected from 4000 to 650 cm−1. Only the necessary portions were plotted. FT-IR spectrum for a hexane treated AE-IP sample, b similar sample that was not treated with hexane, c second derivative spectrum for a. d Represents the normalized absorbance spectrum for the glycosylated proteins, specifically between 1200 and 1000 cm−1. Representative runs
Some of the major peaks in the second derivative FT-IR spectra of were labelled on Fig. 4c and attributed to secondary structural elements according to Kong and Yu [25]. Consequently the peaks at 1631, 1641 and 1692 cm−1 represented β-sheet elements, whereas 1666 and 1680 cm−1 peaks demonstrated the presence of β-turn structure in the protein concentrates. Finally 1658 cm−1 peak was attributed to the presence of α-helices.
In the FT-IR spectra, intensities of specific components are related to certain structural attributes, which could largely vary between proteins [26]. Since the current samples are concentrates rather than purified proteins, it is not possible to identify and relate structural characteristics directly to certain components. However, current data clearly demonstrated that black cumin protein concentrates contained both α- and β-structures. Finally based on normalized absorbance data between 1000 and 1200 cm−1 (Fig. 4d), a minor peak was detected around 1050 cm−1 possibly indicating partial glycosylation of the proteins. This assignment was based on the work of Khajehpour et al. [27] and is coherent with the current rheological observations.
Influence of solvent extraction on the functionality of the protein concentrates
Various functional properties of the protein concentrates were tested including solubility, water and oil holding capacities. In addition, surface activity at the air–water interface was also monitored.
Functionality tests
Amino acid composition and distribution of their hydrophilic/hydrophobic characteristics throughout the molecules influence aqueous solubility of proteins. Consequently protein solubility also has a bearing on the other functional properties such as foam and emulsion formation, gelation and thickening in food dispersions. Solubility (pH 7.0) characteristics of the protein concentrates were listed on Table 3. Solvent extraction clearly increased the solubility of concentrates. Prior to solvent extraction, AE-IP concentrates demonstrated approximately 12.1% aqueous solubility, while after hexane extraction this number rose to 32.2%. In the previous literature, Karaca et al. [28] reported that extraction method significantly affected pea protein isolate solubility, which was attributed to the differences in surface characteristics of the proteins that were induced by the extraction methods. In most cases, changes in solubility may also be attributed to conformational changes [29] and the stability/destabilization of the native structure [30].
Water holding capacity (WHC) is among the most critical characteristics of food proteins. A pronounced WHC will inhibit the separation of water due to gravitational forces. Environmental conditions as well as amino acid content and conformational characteristics determine the interactions between water and proteins, which in turn influence sensory and textural attributes of foods [31].
Water holding capacity (WHC) data for the current samples were also presented on Table 3. Once again, solvent extraction was shown to enhance the WHC of the protein concentrates. Prior to solvent extraction AE-IP samples demonstrated a WHC value of 119.2 (g/100 g), whereas after the extraction this value increased to 131.3 (g/100 g). In the literature, extraction technologies were shown to affect the WHC values of proteins [32]. Both the oil and water absorption capacity of the proteins in the oilseed meals were shown to increase due to deoiling of seeds [1]. Proteins with higher amounts of hydrophilic groups near the surface potentially hold increasing amounts of water [33].
The oil holding capacity (OHC) values for concentrates were presented on Table 3. Extraction techniques were previously shown to have a bearing on the OHC values of protein concentrates [32]. Prior to hexane extraction, OHC for AE-IP samples were 162 (g/100 g). After hexane extraction, this value increased to 232 (g/100 g). Consequently hexane extraction enhanced all the functional characteristics investigated here.
Drop shape tensiometry
The surface tension at the air-protein solution surface was determined by drop shape tensiometry (Fig. 5). The surface tension values for black cumin protein concentrates before and after oil extraction were approximately 37.6 and 37.5 mN m−1 respectively, at a protein concentration of 0.1% after 10,000 s. The surface pressure exerted by black cumin proteins were superior to that of a commercial soy protein sample (γ = 49 mN m−1) at the same protein concentration after 10,000 s. Although the equilibrium values for the samples before and after hexane extraction was comparable, hexane extracted sample reached to this level of surface pressure sooner, which could imply differences in adsorption behavior as well as concentration dependence of the plateau value. Interactions of the remaining oil might negatively increase the extent of interactions between the proteins and the air phase. These findings demonstrated that black cumin protein concentrates demonstrated significant technical functionality including surface activity as also demonstrated by functionality tests. The size range of the proteins investigated here (Fig. 1a, b) was mostly comparable to that of highly surface active food proteins. To the best of our knowledge, there were no previous studies that measured the surface tension values for black cumin proteins. As the long-term kinetics of the concentrates were investigated (i.e., ∆Π vs. t−0.5) [15], maximum surface pressure values of 36.7 (no hexane extraction) and 35.8 (hexane extraction) mN m−1 were predicted.
The initial stages of protein adsorption are generally limited by diffusional characteristics. Following adsorption, proteins unfold and rearrange at the interfaces. This process is characterized by rapid increase in surface pressure. In order for surface tension to reach the steady state, penetration, unfolding and molecular rearrangements in the adsorbed film have to be completed [15]. Tsoukala et al. [34] measured surface pressure for broad bean legumin protein dispersions (0.05% w v−1) as approx. 15 mN m−1 and for lupin protein isolate solutions (0.05% w v−1) as aprox. 22 mN m−1 at 10 min. Our results were comparable to these findings.
ACE-inhibitory activities of black cumin protein hydrolyzates
ACE converts angiotensin I to angiotensin II, and hydrolyzes bradykinin, a vasodilator peptide. Due to both actions, ACE has the potential to elevate blood pressure in humans [35]. ACE-inhibitory peptides could reduce blood pressure [36] without demonstrating pronounced side effects of synthetic drug molecules. Food-grade ACE-inhibitory peptides may be utilized in functional foods or food supplements. Black cumin protein concentrates were treated with trypsin or chymotrypsin to determine whether they demonstrated ACE-inhibitory activity (Fig. 6). Although slight, in both cases, ACE-inhibition was observed and the extent of ACE-inhibition was found to be minimally dependent on proteolysis duration. ACE-inhibitory characteristics of chymotryptic peptides were more pronounced than tryptic peptides. Based on further purification or utilization of other enzymes [37], the potential to generate ACE-inhibitory peptides might be enhanced.
In summary, in this study, technical and biological activities of black cumin protein concentrates were investigated. Although some analyses on the proteomic and genomic characteristics of black cumin proteins were previously published, here we report new information on the thermal, structural, rheological and ACE-inhibitory characteristics. Black cumin proteins contained both α- and β-secondary structural elements, part of which were potentially glycosylated, which potentially controlled the gel-like behavior of these proteins at ambient temperature, whereas thermally induced gelation was not observed at neutral pH. A number of high pI proteins were also detected via 2D-electrophoresis. Technical functionality of the concentrates including solubility, WHC, and OHC were improved by hexane extraction, while the presence of black cumin oil in the concentrates enhanced the thermal stability of the proteins. Black cumin proteins also demonstrated considerable surface activity at the air–water interface. Finally upon enyzmatic proteolysis, protein hydrolyzates demonstrated slight angiotensin-converting enzyme (ACE)-inhibitory activity.
Conclusion
The global protein demand is constantly increasing, which in turn requires a sustainable supply. Cold press deoiled meals represent a viable and economic source of plant protein manufacture. Here, based on a simple methodology which can conveniently be scaled up, black cumin protein concentrates were manufactured. Hexane extraction clearly enhanced the functional characteristics in most cases, due to the enhancement of protein-solvent interactions in the absence of oil and also partial denaturation of the proteins during hexane treatments.
Secondary structural attributes of these proteins were analyzed based on FT-IR data and a number of peaks were reported for both α- and β-elements. Black cumin proteins potentially contained some glycosylated proteins, which explained their gel-like behavior prior to heating. Alkaline gelation was observed, whereas self-supporting gels did not form at pH 7. Findings on the structure and bioactivity of protein concentrates could enhance the utilization potential of this otherwise difficult to consume seed, which has a balanced amino acid content and a relatively high concentration of essential amino acids.
References
A. Moure, J. Sineiro, H. Dominguez, J.C. Parajo, Functionality of oilseed protein products: a review. Food Res. Int. 39, 945–963 (2006)
L. Day, Proteins from land plants—potential resources for human nutrition and food security. Trends Food Sci. Technol. 32, 25–42 (2013)
N. Potter, J. Hotchkiss, Food Science, 5th edn. (Springer Sciences Business Media, New York, NY, 1995)
C. Radha, P.R. Kumar, V. Prakash, Preparation and characterization of a protein hydrolysate from an oilseed flour mixture. Food Chem. 106, 1166–1174 (2008)
A. Tekeli, Nutritional value of black cumin (Nigella sativa) meal as an alternative protein source in poultry nutritıon. J. Anim. Sci. Adv. 1(4), 797–806 (2014)
H. Baydar, Science and Technology of Medicinal and Aromatic Plants, 3rd edn. (Süleyman Demirel University, Faculty of Agriculture, Isparta, 2009)
F.R. Durani, N. Chand, K. Zaka, A. Sultan, F.M. Khattak, Z. Durrani, Effect of different levels of feed added black seed on the performance of broiler chicks. Pak. J. Biol. Sci. 10(22), 4164–4167 (2007)
Anon, Proposal for new work on Codex Standart for Brown/Black Cumin (Whole and Ground), (prepared by India). Joint FAO/WHO Food Standards Programme, (2014)
M.J. Gonzalez-Castro, J. Lopez-Hernández, J. Simal-Lozano, M.J. Oruña-Concha, Determination of amino acids in green beans by derivatization with phenylisothiocianate and high-performance liquid chromatography with ultraviolet detection. J. Chromatogr. Sci. 35(4), 181–185 (1997)
J.I. Boye, S. Aksay, S. Roufik, S. Ribereau, M. Mondor, E. Farnworth, S.H. Rajamohamed, Comparison of the functional properties of pea, chickpea and lentil protein concentrates processed using ultrafiltration and isoelectric precipitation techniques. Food Res. Int. 43(2), 537–546 (2010)
AOAC, Official Methods of Analysis of AOAC International, 17th edn. (Association of Official Analytical Chemists Inc., Gaithersburg, MD, 2003)
NMKL 960, Nordic committee on food analysis, (1968)
U.K. Laemmli, Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 5259, 680–685 (1970)
M. Hadnađev, T. Dapčević-Hadnađev, A. Lazaridou, T. Moschakis, A.M. Michaelidou, S. Popović, C.G. Biliaderis, Hempseed meal protein isolates prepared by different isolation techniques part I. Physicochemical properties. Food Hydrocoll. 79, 526–533 (2018)
İ. Gülseren, D. Güzey, B.D. Bruce, J. Weiss, Structural and functional changes in ultrasonicated bovine serum albumin solutions. Ultrason. Sonochem. 14(2), 173–183 (2007)
İ. Gülseren, M. Corredig, Storage stability and physical characteristics of tea-polyphenol-bearing nanoliposomes prepared with milk fat globule membrane phospholipids. J. Agric. Food Chem. 61(13), 3242–3251 (2013)
I.C. Sheih, T.J. Fang, T.K. Wu, Isolation and characterisation of a novel angiotensin I-converting enzyme (ACE) inhibitory peptide from the algae protein waste. Food Chem. 115(1), 279–284 (2009)
V.K. Babayan, D. Kootungal, G.A. Halaby, Proximate analysis, fatty acid and amino acid composition of Nigella sativa L. seeds. J. Food Sci. 43(4), 1314–1315 (1978)
A. Haq, N. Remo, S.T. Al-Sedairy, Fractionation of black seed (Nigella sativa Linn) proteins by using rotofor. J. Liq. Chromatogr. Relat. Technol. 19(4), 593–599 (1996)
F. Liu, S. Zhang, J. Li, D.J. McClements, X. Liu, Recent development of lactoferrin-based vehicles for the delivery of bioactive compounds: complexes, emulsions, and nanoparticles. Trends Food Sci. Technol. 79, 67–77 (2018)
R.D.C.A. Guimarães, S.P. Favaro, A.D.V.D. Souza, C.M. Soares, Â.A. Nunes, L.C.S.D. Oliveira, M.R. Honer, Thermal properties of defatted meal, concentrate, and protein isolate of baru nuts (Dipteryx alata Vog). Food Sci. Technol. 32(1), 52–55 (2012)
M. Davidovich-Pinhas, S. Barbut, A.G. Marangoni, The gelation of oil using ethyl cellulose. Carbohydr. Polym. 117, 869–878 (2015)
J.H.J. Kim, N.V. Varankovich, M.T. Nickerson, The effect of pH on the gelling behaviour of canola and soy protein isolates. Food Res. Int. 81, 31–38 (2016)
R. Kizil, J. Irudayaraj, Spectroscopic Technique Fourier Transform Raman (FT-Raman) Spectroscopy, Modern Techniques for Food Authentication (Academic Press, London, 2018), pp. 193–217
J. Kong, S. Yu, Fourier transform infrared spectroscopic analysis of protein secondary structures. Acta Biochim. Biophys. Sin. 39(8), 549–559 (2007)
P. Jaiswal, S.N. Jha, A. Borah, A. Gautam, M.K. Grewal, G. Jindal, Detection and quantification of soymilk in cow–buffalo milk using attenuated total reflectance Fourier transform infrared spectroscopy (ATR–FTIR). Food Chem. 168, 41–47 (2015)
M. Khajehpour, J.L. Dashnau, J.M. Vanderkooi, Infrared spectroscopy used to evaluate glycosylation of proteins. Anal. Biochem. 348(1), 40–48 (2006)
A.C. Karaca, N. Low, M. Nickerson, Emulsifying properties of chickpea, faba bean, lentil and pea proteins produced by isoelectric precipitation and salt extraction. Food Res. Int. 44, 2742–2750 (2011)
Y.A. Adebowale, U. Schwarzenbolz, T. Henle, Protein concentrate from Bambara groundnut (Voandzeia subterranean L.): chemical characterization and functional properties. Int. J. Food Prop. 14, 758–775 (2011)
H. Fuhrmeister, F. Meuser, Impact of processing on functional properties of protein products from wrinkled peas. J. Food Eng. 56, 119–129 (2003)
O.S. Lawal, Functionality of African locust bean (Parkia biglobossa) protein isolate: effects of pH, ionic strength and various protein concentrations. Food Chem. 86, 345–355 (2004)
A.K. Stone, A. Karalash, R.T. Tyler, T.D. Warkentin, M.T. Nickerson, Functional attributes of pea protein concentrate prepared using different extraction methods and cultivars. Food Res. Int. 76, 31–38 (2015)
J. Yao, Y. Zhou, X. Chen, F. Ma, P. Li, C. Chen, Effect of sodium alginate with three molecular weight forms on the water holding capacity of chicken breast myosin gel. Food Chem. 239, 1134–1142 (2018)
A. Tsoukala, E. Papalamprou, E. Makri, G. Doxastakis, E.E. Braudo, Adsorption at the air–water interface and emulsification properties of grain legume protein derivatives from pea and broad bean. Colloids Surf. B 53, 203–208 (2006)
R. Hartmann, H. Meisel, Food-derived peptides with biological activity: from research to food applications. Curr. Opin. Biotechnol. 18(2), 163–169 (2007)
V. Vermeirssen, J. Van Camp, W. Verstraete, Bioavailability of angiotensin I converting enzyme inhibitory peptides. Br. J. Nutr. 92, 357–366 (2004)
İ. Gülseren, In silico methods to identify ACE and DPP-IV inhibitory activities of ribosomal hazelnut proteins. J. Food Meas. Charact. 12(4), 2607–2614 (2018)
Acknowledgements
This study was funded by a grant from TÜBİTAK 3501 Programme (Grant No. 115O569). The authors would like to express their gratitude to Neva Foods Ltd. (İstanbul, Turkey) for the donation of deoiled meals. The analytical costs for 2D-electrophoresis were covered by direct support from İstanbul Sabahattin Zaim University (İZÜ). We would like to thank Prof. Murat Kasap from Kocaeli University DEKART Proteomics Laboratory, Turkey. Anton Paar, Turkey is also acknowledged for support in rheological analysis.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Coşkun, Ö., Çakır, B., Vahapoğlu, B. et al. Influence of extraction conditions on structural and functional characteristics of black cumin protein concentrates and ACE-inhibition in their hydrolyzates. Food Measure 13, 2328–2338 (2019). https://doi.org/10.1007/s11694-019-00152-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11694-019-00152-1