Abstract
Purpose
Oil processing generates an increasing extent of deoiled seed meals globally, which are rich in plant proteins. Based on simple aqueous extraction techniques, a variety of technically and biologically active plant protein products can be generated from deoiled meals.
Methods
Pumpkin, pomegranate, and grape seed protein concentrates were prepared using aqueous protein extraction techniques (alkali extraction-isoelectric precipitation (AE-IP), salt extraction (SE), and micellar precipitation (MP)). Protein concentration was determined using a Kjeldahl method. SDS-PAGE was utilized to determine the molecular weight distribution of proteins. Functional characteristics of the isolated proteins were investigated based on solubility, water and oil holding capacities (WHC and OHC), foaming and emulsion forming capacities, drop shape tensiometry, and thermal gelation characteristics.
Results
Protein concentration in the samples was widely distributed between 20 and 86%. Protein contents of AE-IP-treated pumpkin, pomegranate, and grape seed protein concentrates were 82.9, 45.8, and 30.1%, respectively. Although the foam formation and water holding capacities of these protein concentrates were relatively weak, oil holding characteristics were comparable or better than a commercial soy protein isolate. All proteins demonstrated considerable surface activity at the air-water interface. Pumpkin and pomegranate protein concentrates formed weak gels upon heating, whereas the heating of grape seed protein concentrates did not yield thermally induced gels.
Conclusion
Extraction techniques had a clear bearing on both protein concentration and functionality of the current concentrates. While further processing could enhance their functionality, current samples demonstrated considerable functionality after simple extraction techniques that are conveniently adaptable to industrial settings.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Processing of oilseeds generates significant amounts of by-products globally such as oilseed meals. For example, in 2002, thirty to thirty-five million metric tons of oilseeds were processed in the EU, mostly including soybeans (approx. 50%), rapeseeds (approx. 33%), and sunflower seeds (approx. 18%) according to official data from European Commission. While in many cases, it is practical to utilize meals in feed or fertilizer applications, these products are mostly of low commercial value [1]. Furthermore, prior to drying, microbial degradation is also an issue. Their bulk volumes render processing, transportation, and utilization rather difficult. Consequently, value added products are necessary to rationalize their valorization [1].
While oilseeds contain significant amounts of oil (i.e., in most cases, 17–47%), the extent of oil recovery depends on the means of oil manufacture such as cold press technologies vs. solvent extraction. Especially oilseed meals generated via desolventization represent significant sources of protein both due to high protein content and the extent of availability [2]. However, solvent usage and thermal treatments might affect the nutritional properties of protein mixtures [3], especially since meal proteins need to be converted into edible-grade products. The presence of phenolic substances (i.e., chlorogenic, quinic, and caffeic acids), reduced sugars (glucose, fructose), or toxic factors (i.e., gossypol) could limit the potential utilization of meal proteins in foods [4, 5].
Pumpkin seeds can potentially be included in food formulations due to their physiological functionalities, anticarcinogenic activities, and role in the prevention of protein malnutrition and in the inhibition of blood coagulation [6,7,8]. Previously, antidiabetic [9], antifungal [10], antibacterial, anti-inflammatory [11], and antioxidant activities [12] were also documented. In the previous studies, protein content of pumpkin (Cucurbita sp.) seed has been found to be approx. 36% [13], whereas protein concentration significantly increased due to deoiling approx. to a range of 60–65% [14]. Based on recent market analysis by Accuray Research LLP (Anon. 2017), global pumpkin seed market will account for about 1.88 USD by 2025 [15], which emphasized the financial potential of the seeds along with their technical and biological functionalities.
Pomegranate (Punica granatum Linn.) is a shrub belonging to the family Punicaceae and is mainly cultivated in the tropics and subtropical regions such as China, Japan, the USA, and Mediterranean countries. While pomegranate seeds are not of comparable economic importance as grape or pumpkin seeds, cold press processing converts more than 80% of the seed to meal, which signifies the importance of further valorization. Pomegranate seeds contain a large amount of flavonoids and anthocyanins [16], which enable their utilization in the manufacture of medicines, cosmetics, and functional foods. The protein content of pomegranate seeds might be as high as 120 g.kg−1; while biological activities of other seed ingredients have been widely investigated [17,18,19], the information about its protein composition and characteristics are largely unknown [20].
Grape (Vitis vinifera L.) seeds constitute another abundant source of seed proteins, since grapes are among the most heavily cultivated fruits at 69 million tons annually on a global basis [21]. The seeds account for approximately 2–3% of the total grape harvest, and the protein content in the seeds is in the order of 10–13% [22]. Grape pomace constitutes around 13% of the total mass during winemaking, and around 38–52% of the pomace weight is originating from grape seeds [23, 24]. In either during winemaking or cold press oil processing, grape seeds become an abundant resource for plant protein manufacture [25, 26]. The protein content of the grape seed was reported to range widely between 8.4 and 25.9% [25, 27].
In the literature, although quite a bit work on the composition and functional characteristics of pumpkin, pomegranate, and grape seed oils exists [28,29,30,31,32,33,34], the characteristics of these protein concentrates are known to a lesser extent. In summary, various bioactivities of these oils include enhancement of immune functions, demonstration of anticarcinogenic activity, scavenging of free radicals in vivo, protection against prostate and colon cancers, and protection against hepatotoxicity.
The composition of these oils is also well-characterized and features some similar characteristics. For example, grape seed oil is mostly composed of unsaturated fatty acids (88.6%), where linoleic (72.2%) and oleic (15.6%) acids are its major unsaturated fatty acids [35], while significant amounts of carotenoids and tocopherols are also present [30]. Their overall composition has also been reported in the references mentioned herein. Linoleic and oleic acids were also dominant in pumpkin seed oil [36], while pumpkin seed oil was also rich in tocopherols, sterols, and phenolic acids. The major unsaturated fatty acids were identical for pomegranate seed oil as well [37].
In addition to their potential bioactivities, it is the technical characteristics of protein systems that enable their commercial utilization. In that sense, the valorization of deoiled meals requires the comprehensive analysis on the functional characteristics. The major functional properties of proteins are related to their hydration, structural/rheological, and interfacial/surface-related characteristics. Novel protein products should be able to compare favorably with animal proteins to partially or fully replace them [38]. Consequently, functional properties such as water and oil holding capacity, solubility, foam and emulsion formation capacity, and ability in lowering surface/interfacial tension are usually monitored.
Using industrial samples, we made an attempt to manufacture protein concentrates from cold press meals of pumpkin, pomegranate, and grape seeds and characterize their technical functionality without utilizing an organic extraction step. The industrial processing was carried out at < 40 °C which preserved the quality of proteins, after which the meals were immediately collected and processed gently based on aqueous extraction techniques. The physicochemical and functional properties of the protein concentrates were investigated, since technical functionality studies on these protein systems are relatively scarce in this field. The information obtained from this study may be useful in identifying appropriate extraction methods for producing protein concentrates that are best suited for a specific end use.
Materials and methods
Materials
Cold press deoiled meals of pumpkin, pomegranate, and grape seed meals were generously donated by Oneva (Neva Foods Ltd., İstanbul, Turkey), a local manufacturer of cold press oils. In all cases, the maximum temperature observed by the cold press meals was up to 40 °C. All chemicals used were of reagent grade and purchased from Sigma-Aldrich, except sodium dodecyl sulfate (SDS) which was purchased from Merck (Millipore Corp., Germany).
Preparation of seed protein concentrates
In all cases, further solvent-based extraction was avoided in order to preserve the structural and functional characteristics of protein concentrates. Three different aqueous extraction techniques were utilized in order to generate protein concentrates from the deoiled meals.
Alkali extraction-isoelectric precipitation method
Alkali extraction-isoelectric precipitation (AE-IP) technique was based on the solubilization of protein molecules at basic pH, which was immediately followed by the isoelectric precipitation at acidic pH values. Protein concentrates were produced using the method of Boye et al. [39] with modifications [40]. Firstly, 50 g of deoiled meal was dispersed in water (1:15, w/v), and the pH of the medium was adjusted to pH 9.5 using 1.0 N NaOH. The dispersions were stirred at 500 rpm for 1 h at ambient temperature (22 ± 1 °C). Immediately afterwards, the dispersions were centrifuged at a rate of 4200×g (Mixtasel-BL centrifuge, Abrera, Barcelona, Spain) for 30 min. The supernatant containing the solubilized proteins was collected, and the medium pH was adjusted to pH 4.5 using 1.0 N HCl in order to induce isoelectric precipitation. To ensure the complete separation of precipitating proteins, the supernatant was once again centrifuged at 4200×g for 30 min. The pellets were collected and pooled as necessary and immediately frozen at − 20 °C. Frozen samples were lyophilized using a Teknosem TRS 2/2 V freeze drier (Teknosem Corp., İstanbul, Turkey).
Salt extraction method
Salt extraction (SE) methodology detailed in Liu et al. [41] was used with slight modifications. Fifty grams of deoiled meal was mixed with 500 ml of 0.1 M sodium phosphate buffer (pH 8) containing 6.4% KCl. The dispersions were kept stirred (500 rpm, 1 h) at ambient temperature (22 ± 1 °C). Immediately afterwards, dissolved proteins were separated from the non-dissolved matter by centrifugation at a rate of 4200×g for 30 min. The supernatant was collected and diafiltrered using a Sartorius Masterflex Ultrafiltration System (10 kDa cutoff; Sartorius Stedim Biotech GmbH, Goettingen, Germany) against deionized water, until the conductivity decreased approx. to 20 μs.cm−1. The extract was frozen at − 20 °C and kept frozen until lyophilization.
Micellar precipitation method
Micellar precipitation (MP) was performed according to the method of Lampart-Szczapa [42] with slight modifications. Fifty grams of deoiled meal was suspended in 500 ml of 1.0 N NaCl solution and kept stirred for 2 h (500 rpm) at ambient temperature. The suspension was centrifuged at 4000×g for 20 min, and the supernatant was diluted 10x with cold deionized water (4 °C), which was followed by refrigerated storage (4 °C) overnight. Immediately afterwards, the dispersion was centrifuged again under similar conditions. Finally, the pellet was collected and stored at − 20 °C until lyophilization.
Analysis of protein concentrates
The protein, moisture, and ash contents of the protein concentrates were determined according to AOAC Official Methods 920.87 (%N × 6.25), 925.10, and 923.03, respectively [43].
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE)
SDS-PAGE analysis was carried out based on the protocols of Laemmli [44] under reducing conditions using a Bio-Rad Mini Protean Tetra Cell System (Bio-Rad Laboratories Inc., USA). Firstly, lyophilized protein concentrates were dispersed in deionized water (1%). The dispersion pH was adjusted to pH 10 with 0.5 M NaOH solutions in order to enhance separation of proteins. Immediately afterwards, protein samples and 2x Laemmli loading buffer containing 0.004% bromophenol blue, 10% 2-mercaptoethanol, 20% glycerol, 4% SDS, and 0.125 M Tris-HCl (pH 6.8) were mixed 1:1 in Eppendorf tubes (1.5 ml). Samples were heated 5 min at 100 °C, cooled, and loaded on a Mini-Protean TGX Stain-Free Precast Gel (12%). Precision Plus protein dual color standards from the same manufacturer were used as the reference sample. Gel electrophoresis was carried out for 45 min using Tris/glycine/SDS running buffer at 200 V (constant). Imaging was carried out by transferring the gel to a stain-free tray and using Gel Doc EZ system. The images were analyzed using the Image Lab Software (Bio-Rad Laboratories, Inc., USA).
Functional properties of protein concentrates
The functional attributes of the protein concentrates were tested and compared with a commercial soy protein isolate sample (Sigma-Aldrich) under similar conditions.
Solubility
Protein solubility (%) was determined by dispersing 0.2 g sample in 19 ml of 0.1 N NaCl solution, adjusting the pH to 7 using 0.5 N HCl or NaOH as necessary and keeping the dispersion stirred (500 rpm) for 1 h at 50 °C. Total solution volume then was brought to 20.0 g with 0.1 N NaCl. The mixtures were left to stand for 10 min in order to ensure the completion of precipitation, if any. The solution was then centrifuged at 4200×g for 10 min at ambient temperature. Percent solubility was determined in the supernatant using an appropriate protein analysis kit based on a modified Lowry method (TP0300, Sigma-Aldrich). For all the standards and samples, absorbance was measured at 750 nm.
Water or oil holding capacity
One gram of sample was added to 10 ml of distilled water (or oil) in a 15-ml centrifuge tube. The contents were vortexed for 30 s every 5 min (Genie 2 Mixer), and after 30 min, the tubes were centrifuged at 3000×g for 20 min at ambient temperature. Once the free water or oil portion was removed, water/oil holding capacity was calculated from the percentage of increase in sample weight due to water or oil holding [45].
Emulsification activity
Emulsification activity and emulsion stability (o/w) were determined based on the method proposed by Beuschel et al. [46]. Five milliliter of protein concentrate dispersion (1%) at a defined pH value was blended with 15 ml soy oil (S7381, Sigma-Aldrich) for 60 s with an ultrasonic homogenizer (Hielscher Model UP200Ht) at full power, 1:1 pulse with 1-s cycles. Sample temperature was controlled using a water bath (25 °C). A small aliquot from the emulsions (80 μl) was diluted to 10 ml with 0.1% sodium dodecyl sulfate (SDS), and sample absorbance was measured at 500 nm (Optima SB-3000 UV/VIS spectrophotometer). Emulsion activity index (EAI) was calculated [47]:
where A˳ is the absorbance of the diluted emulsion immediately after homogenization, N is the dilution factor, C is the weight of protein per volume (g·mL−1), and φ is the oil volume fraction of the emulsion.
Drop shape tensiometry
The surface tension (mN·m−1) at the air-aqueous dispersion interface was determined using drop shape tensiometry (25 °C) (Biolin Scientific, Attension Theta, Espoo, Finland). An air bubble was automatically formed at the tip of an inverted syringe which was immersed in a quartz cuvette containing the protein dispersion prepared in 100 mM sodium phosphate buffer (pH 7). The shape of the droplet was automatically analyzed to record the changes in the surface tension over time, as the cuvette and syringe assembly were monitored by a CCD (charge coupled device) camera, and high-quality image acquisition was utilized [48]. Surface tension was calculated based on the Young-Laplace equation using Attension Theta OneAttension version 2.6 (r5305) software. All measurements were carried out in triplicate. The surface pressure (π) was calculated as the difference in the surface tension of the buffer (72.3 mN·m−1) and the protein solution at the air-water interface, as a function of time.
Rheological analysis
An Anton Paar rheometer (MCR 302, Austria) fitted with a temperature controlled Peltier system (H–PTD 200) was used to monitor temperature dependence of the rheological characteristics of protein concentrates prepared by the AE-IP method. Ten percent aqueous protein dispersions were adjusted to pH 7 using 1 M NaOH. Immediately afterwards, centrifugation was performed to remove the undissolved matter. Approximately 1 mL of protein dispersion was placed on the lower plate of the parallel plate geometry. The diameter of the upper parallel plate was 25 mm, and the gap distance between the two parallel plates was 1 mm. A solvent trap cover was used, and light silicon oil was applied to the exposed part to minimize evaporation during heating. Furthermore, the head was charged with water so as not to contact the sample and in order to saturate the medium with water vapor. The rheometer was operated at constant angular frequency of 1 Hz and strain range of 1–10% depending on sample behavior within the linear viscoelastic region of the protein dispersions. The heating protocol involved a linear temperature ramp from 25 to 85 °C at a heating rate of 5 °C·s−1, holding at 85 °C for 2 min and thereafter cooling to 25 °C at a cooling rate of 5 °C·s−1, and finally holding at 25 °C for 2 min. Shear strain and modulus values (G′ and G″) of the samples were investigated as a function of time and temperature.
Statistical analysis
Analysis of variance (ANOVA) was carried out to determine whether there were significant differences (p < 0.05) on protein, moisture, and ash contents of different samples as well as their corresponding solubility, water and oil holding capacities, and emulsification activities. This analysis was based on the comparison of the influence of all 3 extraction techniques on each and every sample individually since their composition, and initial protein contents were distinctly different. In all cases, at least three replicates were analyzed, and the sample mean and its corresponding standard deviation were reported. When necessary, a representative run was shown.
Results and discussion
Physicochemical properties of protein concentrates
Prior to all operations, deoiled cold press meals were analyzed for their initial protein content based on the Kjeldahl method. Using 3 different protein extraction techniques, all three meals were subjected to AE-IP, SE, or MP treatments. The protein, moisture, and ash content of all samples were determined immediately after freeze-drying (Table 1). In all cases, pumpkin seed concentrates contained more proteins than the other concentrates. Based on the AE-IP method, the protein contents of the concentrates prepared from deoiled meals were approx. 83%, 45.8%, and 30.1% for pumpkin, pomegranate, and grape seed samples, respectively. In the case of SE method, although the procedure was more labor intensive and possibly less suitable to an industrial scale-up, the extent of protein recovery was considerably lower compared with the AE-IP counterparts (Table 1). Finally, in the case of MP treatment, although the protein recovery efforts for the other samples was unsuccessful, the protein content of the MP pumpkin protein concentrates was slightly higher compared with the AE-IP pumpkin samples. It was possible to generate approx. 86.6% protein containing pumpkin protein concentrate without further purification.
In the previous investigations, protein content in pomegranate seeds was found to vary between approx. 12 and 20% [49]. Similarly, according to Bucko et al. [50] and Rezig et al. [51], protein content in deoiled pumpkin seed flours or cakes was in the order of 43–64%. Protein content of grape seeds was previously found to be approx. 10% [52, 53]. In most cases, aqueous extraction techniques generated a significant enhancement in protein content of the samples.
In most cases, SE-treated samples demonstrated higher moisture and ash contents compared with AE-IP-treated samples (Table 1). Sosulski and McCurdy [54] indicated that strong alkali or acid used in isoelectric precipitation methods could result in salt formation and a subsequently high ash level in the protein concentrates relative to the meal or flour. Although extensive diafiltration was carried out, salts remaining in the system contributed to higher ash contents in the concentrates based on SE treatment.
Statistical analysis was carried out to determine whether significant differences occurred in protein, moisture, or ash contents of each sample between different preparation methods (p < 0.05). The corresponding findings were also indicated on Table 1. In general, moisture and ash values were more significantly affected by the protein concentrate preparation methods when compared with protein content measurements.
The samples utilized here were obtained from an industrial process, and the cold press operation was optimized by the manufacturer to enhance the efficiency of oil production capacity. Consequently, the oil content of all the meal samples were < 10% (p < 0.05). For example, grape seed AE-IP sample was shown to contain approx. 5.4% oil. To ensure minimal processing and industrial applicability, no further oil extraction process was administered. Our previous work on black cumin also demonstrated that the oil extraction processes (both the sheer presence of oil and the methodologies to extract oil) affect the concentrate performance [54]. The compositions of the corresponding seed oils are mostly similar based on the previous data available from the literature. These oils are primarily composed of linoleic, oleic, and palmitic acids, all of which form at least 80–90% of the oil phases [30, 35,36,37].
SDS-PAGE analysis of protein concentrates
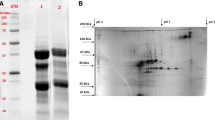
Molecular weight distribution of protein concentrates from deoiled pumpkin, pomegranate, and grape meals was analyzed by SDS-PAGE (Fig. 1). In the loading of the gel lanes, identical amounts of protein (1%) were administered considering the protein concentration in every concentrate. However, since there are three different meals samples and three different extraction technologies applied in this experiment, it is difficult to ensure about identical solubility in the samples, especially once they are mixed with the electrophoresis chemicals. Consequently, although the level of loading is identical, some differences might occur due to the differences in solubility.
SDS-PAGE analysis of pumpkin seed (lane numbers: 1-AE-IP; 2-SE; 3-MP), pomegranate seed (lane numbers: 4-AE-IP; 5-SE), and grape seed (lanes number 6-AE-IP; 7-SE) protein concentrates (1%) manufactured by alkali extraction-isoelectric precipitation (AE-IP), salt extraction (SE), or micellar precipitation (MP) methods
In the previous literature, pumpkin seed proteins were shown to be represented by a 12S globulin (325 kDa), called cucurbitin [50, 51] which contains six 54 kDa subunits. These subunits are linked by disulfides, which are approx. 33 kDa and 22 kDa in size [55]. Some studies also demonstrated the presence of 4.8 kDa, 7.9 kDa, and 12.5 kDa proteins [56]. In our analysis, for pumpkin seed protein concentrates, the major bands laid between 25–37 kDa, 20–25 kDa, and finally around 10 kDa (especially AE-IP and SE), which were mostly coherent with the previous literature. There were some faint bands around 50–75 kDa for SE- and MP-treated pumpkin seed protein concentrates.
Although more faint, for pomegranate seed protein concentrates, the major bands laid around 15, 25, and 35 kDa. There were faint bands and some smearing at lower and higher molecular weights (Fig. 1). The protein concentrate obtained by AE-IP method had more intense bands than the SE sample. Yang et al. [20] reported that water soluble storage proteins of pomegranate seed protein concentrate had the highest amount of polypeptides in a molecular weight range of 10–25 kDa, which are comparable to the current findings.
For the grape seed samples, although quite faint, the bands were located mostly between 20 and 40 kDa. Manufacturing techniques affected the composition of samples in all cases (Fig. 1). Previously, Gazzola et al. [57] showed that under non-reducing conditions, there were various proteins/polypeptides in the size range of 25 to 65 kDa. Under reducing conditions, however, the bands changed significantly. According to Zhou et al. [26, 58], there were two main bands around 160 and 300 kDa. In addition, these investigators demonstrated the presence of other bands between 20 and 43 kDa. Consequently, our findings were partly coherent with both sets of investigations. Some of the discrepancies in the results might be attributed to the cold press processing vs. other processing techniques and relative solubility of the proteins in each treatment. Furthermore, since the administration of an organic extraction step was avoided here and cold press processed samples were utilized, some differences might have occurred in the native structure of the proteins. Both the processing and extraction techniques had a bearing on sample composition and performance in all cases.
Functionality of protein concentrates
The functional attributes of the protein concentrates were tested and, when necessary, compared with a commercial soy protein reference under similar conditions.
Solubility
The solubility characteristics of proteins are among the most important functional properties since many functional performances of proteins depend upon their capacity to go into solution [59]. Therefore, solubility of pumpkin, pomegranate, and grape seed protein concentrates were evaluated (Table 2). The solubility (%) of AE-IP, SE, and MP pumpkin seed concentrates were found to be aprox. 5, 40, and 12.3%, respectively. Bucko et al. [8, 50] reported that solubility of pumpkin seed protein isolate (PSPI) was ~ 60% at pH 7. While they have utilized an alkali extraction-isoelectric precipitation method, differences in solubility could be attributed to extraction procedures since in our samples, and no organic extraction was carried which could imply the presence of higher extents of residual oil, which in turn might influence both the structure and functionality of proteins [40].
In the case of AE-IP and SE methods, the solubility of grape seed protein concentrates was found to be approx. 92.5 and 31.7%, respectively. In the previous literature, Zhou et al. [26] reported that grape seed protein concentrate solubility was approx. 18% at pH 7. Finally, AE-IP and SE pomegranate protein concentrate solubility was found to be 40.8 and 28.3%, respectively. To the best of our knowledge, no data existed in the literature on the solubility of pomegranate seed proteins. In general, a clear trend between extraction technique and % solubility did not seem to exist when all 3 samples were considered.
Statistical analysis was carried out to determine whether significant differences occurred in solubility values of each sample between different preparation methods (p < 0.05). The corresponding findings are also indicated in Table 2. In all cases, solubility was found to be significantly affected by the type of protein concentrate preparation methods.
Water and oil holding capacity
Water holding capacity (WHC) is related to the ability of protein molecules to retain water against gravity. This property clearly relates to the amino acid composition and spatial distribution of amino acids in the protein molecules. Especially the increased number of charged amino acids tends to influence the WHC values. In addition, factors such as molecular conformation, hydrophobicity, pH, temperature, ionic strength, and protein concentration affect WHC [60]. Fat absorption capacity is the binding of fat by nonpolar side chains of proteins. Fat absorption is usually measured by adding an excess liquid fat to protein dispersions and determining the amount of bound oil.
Water and oil holding capacities of the current protein concentrates were investigated (Table 3). AE-IP, SE, and MP pumpkin seed protein concentrates were found to have WHC values of approx. 175.8, 90.6, and 81.2 (100 g water/g protein), respectively. AE-IP and SE pomegranate seed protein concentrates were found to be 178.3 and 12.2 (100 g water/g sample), respectively. Finally, AE-IP and SE grape seed protein concentrates were found to be 258 and 157.7 (100 g water/g sample), respectively. In all cases, AE-IP samples had higher WHC values than the SE samples. The primary interactions related to WHC include the protein–water interactions [61]. Consequently, the amount of protein within the protein concentrates has a bearing on the WHC values observed. Since the protein contents of AE-IP samples were higher than the SE samples, WHC and protein content values were found to be coherent. WHC values obtained here were mostly lower compared with the WHC value of a commercial soy protein isolate (i.e., 202.68 (100 g water/g protein)) (Table 3).
The oil holding capacity (OHC) values for all samples are also presented in Table 3. AE-IP pumpkin, pomegranate, and grape seed protein concentrates demonstrated OHC values of approx. 337.3, 182.2, and 414.5 (100 g oil/g protein), respectively. In the same order, OHC values for the SE samples were 364.5, 240.4, and 1131.3 (100 g oil/g protein), respectively. Finally, MP sample for pumpkin represented an OHC value of 267.6 (100 g oil/g protein). As a reference, OHC value of commercial soy protein isolate was 119 (100 g oil/g protein). Consequently, OHC values of the current concentrates were higher than the soy protein isolate in all cases (Table 3), while WHC values were generally lower. This finding could be attributed to the relatively low abundance of polar amino acids in the protein concentrates and the presence of considerable extents of nonpolar side chains, which may bind the hydrocarbon units of oils, thereby resulting in higher oil absorption [62]. Our earlier findings indicated that in the presence of an organic extraction step, water and oil holding capacities of seed proteins could be further enhanced [40].
Statistical analysis was carried out to determine whether significant differences occurred in WHC or OHC values of each sample between different preparation methods (p < 0.05) (Table 3). In most cases, WHC or OHC values were found to be significantly affected by the protein concentrate preparation methods.
Emulsification activity
Oil-in-water emulsions (o/w) were prepared using soybean oil and protein concentrate dispersions. The results are summarized in Table 4 in terms of emulsifying activity index (EAI), which describes the ability of a protein to form an emulsion. This value presents an estimate for the maximum extent of interfacial area that can be stabilized by a certain amount of protein [47].
For AE-IP pumpkin, pomegranate, and grape seed protein concentrates, EAI values were approx. 6.1, 15.2, and 22.3 (m2·g−1), respectively. In the case of SE samples, EAI values were found as approx. 21, 31.3, and 20.2 (m2·g−1) for pumpkin, pomegranate, and grape seed protein concentrates, respectively. Finally, EAI value was approx. 1.6 (m2·g−1) for MP pumpkin seed protein concentrates prepared by micellar precipitation (Table 4). Protein concentrates produced by salt extraction had significantly higher solubilities, which contributed to their higher EAI when compared with alkali extracted concentrates (Table 2). Solubility plays an important role as highly insoluble proteins tend to perform as poor emulsifiers and lead to coalescence, since the emulsifying properties are correlated to the presence of hydrophobic residues on the protein surface. In most cases, the EAI values of the current samples were mostly comparable to that of a commercial soy protein isolate (17.6 m2·g−1) (Table 4).
Statistical analysis was carried out to determine whether significant differences occurred in EAI values of each sample between different preparation methods (p < 0.05) (Table 4). In all cases, EAI values were found to be significantly affected by the protein concentrate preparation methods.
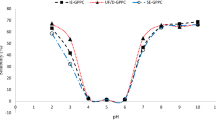
Drop shape tensiometry
The capabilities of proteins to lower surface and/or interfacial tension indicate their potential activity in the formation of foams and emulsions, respectively. Both processes are of both commercial and fundamental importance. Generation of plant protein concentrates with significant surface activity generates a potential to partly or wholly replace synthetic surfactants in industrial applications. In this regard, the surface tension at the air-protein solution surface was determined using drop tensiometry (Fig. 2). After 10,000 s, equilibrium surface tension values were approximately 45.5, 47.5, and 56.1 mN·m−1 for AE-IP pumpkin, pomegranate, and grape seed samples, respectively, at a protein concentration of 0.1%, which implied that grape seed proteins were comparatively less surface active compared with pumpkin and pomegranate samples. The relatively high WHC and OHC values of the grape seed protein concentrates implied that non-proteinaceous large molecules were also present in the system (i.e., complex carbohydrates) which could have limited the surface activity of grape seed proteins. The fairly high crude fiber content of grape seeds (i.e., 38.6% on dry weight basis) [35] might have affected the adsorption characteristics.
Also, as the long-term kinetics of the protein concentrates were investigated (i.e., ΔΠ vs t−0.5) [38], maximum surface pressure values of 30 (γ = 42.3 mN·m−1), 27.8 (γ = 44.5 mN·m−1), and 21 (γ = 51 mN·m−1) mN·m−1 were predicted as t approached infinity for pumpkin, pomegranate, and grape seed protein concentrates. Long-term prediction essentially yielded similar results. Bucko et al. [50] reported the surface pressure for pumpkin protein concentrates as 20–30 mN·m−1 as a function of pH. This finding in average corresponds to a surface tension of 47.3 mN·m−1, which was comparable to the current findings. Once again, these findings can be attributed to the presence of non-proteinaceous impurities in the samples. Although the impurities delayed adsorption in short-term adsorption, as increasingly more molecular rearrangements took place in long term, eventual surface activity performances were comparable. However, the industrial performance could vary based on the necessity to lower surface activity abruptly or over time. For example, in chaotic environment of high-pressure homogenization, rapidly adsorbing small molecules might lower surface tension and stabilize the system, whereas long-term stability of the emulsions formed might depend on the molecular identity of the adsorbing moieties. Large biomolecules or particulate emulsifier systems (i.e., Pickering emulsifiers) might further extend the stability of the system [63]. Similar adsorption principles are also applicable for stabilization of foams. Commercial soy protein isolate was analyzed under similar conditions, and a surface tension value of 47.9 mN·m−1 was attained. Based on these findings, surface activity of the current protein concentrates was found to be comparable to the commercial soy protein isolate sample and literature data.
Rheological characteristics
In order to determine the thermal gelation characteristics of the protein concentrates (AE-IP), a temperature controlled rheometer system with a parallel plate geometry was utilized. The protein dispersions (10%) were heated from 25 to 80 °C, held at 80 °C, and cooled back to 25 °C at a constant heating and cooling rate of 5 °C·s−1 in all cases. G′ values for pumpkin and pomegranate seed protein concentrates were found to become higher than G″ after approx. 10 and 5 min of heating, respectively (Fig. 3). In addition, during the heating and cooling experiments, loss moduli values were larger than or roughly equal to the storage moduli which indicated the formation of weaker gels, since G″ measures the viscous contribution to the system and represents the molecular interactions that do not lead to the formation of 3D gel networks [64]. In the case of grape seed protein concentrates, however, G″ values were found to be higher than G′ in all cases indicating the absence of gel formation. As also observed by the changes in drop tensiometry, the components present in the system deeply influenced the gelation capabilities of grape seed proteins. Since the oil content in the system is relatively low, the results are most likely due to the presence and influence of complex carbohydrates. As already mentioned above, the crude fiber content is fairly high in the grape seeds, the grape seed protein concentrates held relatively high amounts of water as determined by the WHC value, and the water holding capabilities did not lead to thermally induced gel formation. The findings on tensiometry and rheology are coherent with each other on the potential influence of impurities on protein functionality. The crude fiber content being lower in pumpkin seeds (approx. 16.4%) [65] and pomegranate seeds (approx. 12.1%) [66] further supports this hypothesis.
Conclusion
In this study, 3 different aqueous extraction methods were used to obtain protein concentrates from cold press deoiled pumpkin, pomegranate, and grape seed meals. The physicochemical characteristics, molecular weight distributions, and functional properties of these seed protein concentrates were determined. Although the water holding capacities of these proteins were relatively weak, oil holding and emulsion formation characteristics were significant which could be instrumental especially in the production of foods and other industrial products. Emulsification activity generally increased with the solubility of protein concentrates. Dynamic surface tension analyses indicated that that all of the current protein samples lowered surface tension significantly at air-protein dispersion interface, while pumpkin seed proteins were the most effective in that sense. The thermal gelling characteristics of pumpkin and pomegranate protein concentrates were relatively weak, but it must be noted that we have worked at a limited range of protein concentrations, pH values, temperatures, and ionic strengths. The fiber content in the samples might have a bearing on thermal gelation, especially since all of the seeds investigated here originally contained > 10% crude fiber. Under the current circumstances, grape seed proteins were not found to form 3D gels. The extractability of pumpkin seed proteins was superior to the other two samples, whereas potentially due to their glycoprotein or fiber content, pomegranate and grape seed proteins demonstrated superior WHC and OHC performance. While further processing could potentially enhance the functionality of current samples [40], all of the current concentrates demonstrated considerable functionality after utilization of simple aqueous extraction techniques that are conveniently adaptable to industrial settings.
Abbreviations
- AE-IP:
-
Alkali extraction-isoelectric precipitation
- SE:
-
Salt extraction
- MP:
-
Micellar precipitation
- WHC:
-
Water holding capacity
- OHC:
-
Oil holding capacity
- SDS:
-
Sodium dodecyl sulfate
- CCD:
-
Charge coupled device
- ANOVA:
-
Analysis of variance
- EAI:
-
Emulsifying activity index
References
Oreopoulou V, Tzia C. Utilization of plant by-products for the recovery of proteins, dietary fibers, antioxidants, and colorants. In: Utilization of by-products and treatment of waste in the food industry, Vol.3, Eds: V. Oreopoulou and W. Russ (Springer Science-Business Media, LLC, USA, 2007), pp. 216–223.
Friedman M. Nutritional value of proteins from different food sources. A Review. J Agric Food Chem. 1996;44:6–29.
Zeng HY, Cai LH, Cai XL, Wang YJ, Li YQ. Amino acid profiles and quality from lotus seed proteins. J Sci Food Agric. 2013;93(5):1070–5.
Wolf RB, Cavins JB, Kleiman R, Black LT. Effect of temperature on soybean seed constituents: oil, protein, moisture, fatty acids, amino acids and sugars. JAOCS. 1982;59:230–2.
Liadakis GN, Floridis A, Tzia C, Oreopoulou V. Protein concentrates with reduced gossypol content from screw-pressed cottonseed meal. J Agric Food Chem. 1993;41:918–22.
Yadav M, Jain S, Tomar R, Prasad GBKS, Yadav H. Medicinal and biological potential of pumpkin: an updated review. Nutr Res Rev. 2010;23:184–90.
Tomar PPS, Nikhil K, Singh A, Selvakumar P, Roy P, Sharma AK. Characterization of anticancer, DNase and antifungal activity of pumpkin 2S albumin. Biochem Biophys Res Commun. 2014;448:349–54.
Bucko SD, Katona JM, Popoviç LM, Vastag ZG, Petroviç LB. Functional properties of pumpkin (Cucurbita pepo) seed protein isolate and hydrolysate. J Serb Chem Soc. 2016;81(1):35–46.
Quanhong L, Ze T, Tongyi C. Study on the hypoglycemic action of pumpkin extract in diabetic rats. Acta Nutr Sin. 2003;25(1):34–6.
Wang HX, Ng TB. Isolation of cucurmoschin, a novel antifungal peptide abundant in arginine, glutamate and glycine residues from black pumpkin seeds. Peptides. 2003;24:969–72.
Caili FU, Huan SH, Quanhong LI. A review on pharmacological activities and utilization technologies of pumpkin. Plant Food Hum Nutr. 2006;61(2):70–7.
Nkosi CZ, Opoku AR, Terblanche SE. Antioxidative effects of pumpkin seed (Cucurbita pepo) protein isolate in CCl4-induced liver injury in low-protein fed rats. Phytother Res. 2006;20(11):935–40.
Quanhong L, Caili F. Application of response surface methodology for extraction optimization of germinant pumpkin seeds protein. Food Chem. 2005;92:701–6.
Pericin D, Popovic L, Vastag Z, Popoviç SM, Trivic S. Enzymatic hydrolysis of protein isolate from hull-less pumpkin oil cake: application of response surface methodology. Food Chem. 2009;115:753–7.
Anon. Global pumpkin seeds market analysis & trends – industry forecast to 2027. Accuray Research LLP 2017.
Promprom W, Kupittayanant P, Indrapichate K, Wray S, Kupittayanant S. The effects of pomegranate seed extract and beta-sitosterol on rat uterine contractions. Reprod Sci. 2010;17(3):288–96.
de Nigris F, Williams-Ignarro S, Sica V, Lerman LO, D’Armiento FP, Byrns RE, et al. Effects of a pomegranate fruit extract rich in punicalagin on oxidation sensitive genes and eNOS activity at sites of perturbed shear stress and atherogenesis. Cardiovasc Res. 2007;73(2):414–23.
Mirmiran P, Fazeli MR, Asghari G, Shafiee A, Azizi F. Effect of pomegranate seed oil on hyperlipidaemic subjects: a double-blind placebo-controlled clinical trial. Br J Nutr. 2010;104(3):402–6.
Schubert SY, Lansky EP, Neeman I. Antioxidant and eicosanoid enzyme inhibition properties of pomegranate seed oil and fermented juice flavonoids. J Ethnopharmacol. 1999;66(1):11–7.
Yang H, Li M, Qi X, Lv C, Deng J, Zhao G. Identification of seven water-soluble non-storage proteins from pomegranate (Punica granatum Linn.) seeds. Food Sci. Technol. Int. 2011;18(4):329–38.
FAOSTAT: Agricultural production domain online. (2011). Rome, Italy: Food and Agricultural Organization of the United Nations. Available from: http://faostat.fao.org/.
Fantozzi P. Grape seed: a potential source of protein. JAOCS. 1981;58(12):1027–31.
Maier T, Schieber A, Kammerer DR, Reinhold C. Residues of grape (Vitis vinifera L.) seed oil production as a valuable source of phenolic antioxidants. Food Chem. 2009;112:551–9.
Schieber A, Stintzing FC, Carle R. By-products of plant food processing as a source of functional compounds- recent developments. Trends Food Sci Technol. 2001;12:401–13.
Igartuburu JM, Del Río RM, Massanet GM, Montiel JA, Pando E, Luis FR. Study of agricultural by-products. Extractability and amino acid composition of grapeseed (Vitis vinifera) proteins. J Sci Food Agric. 1991;54:489–93.
Zhou T, Zhang T, Liu W, Zhao G. Physicochemical characteristics and functional properties of grape (Vitis vinifera L.) seeds protein. Int. J. Food Sci. Technol. 2011;46:635–41.
Fazio G, Gattuso A, Cilluffo V, Arcoleo G. Preparation and characterization of protein materials from grapeseed meals. Riv Soc Ital Sci Aliment. 1983;6:469–78.
Fahim AT, Abd-el Fattah AA, Agha AM, et al. Effect of pumpkin-seed oil on the level of free radical scavengers induced during adjuvant-arthritis in rats. Pharmacol Res. 1995;31:73–9.
Hammer KA, Carson CF, Riley TV. Antimicrobial activity of essential oils and other plant extracts. J Appl Microbiol. 1999;86:985–90.
Jian L, Du CJ, Lee AH, Binns CW. Do dietary lycopene and other carotenoids protect against prostate cancer? Int J Cancer. 2005;113(6):1010–4.
Kohno H, Suzuki R, Yasui Y, Hosokawa M, Miyashita K, Tanaka T. Pomegranate seed oil rich in conjugated linolenic acid suppresses chemically induced colon carcinogenesis in rats. Cancer Sci. 2004;95(6):481–6.
Maheswari MU, Rao PGM. Antihepatotoxic effect of grape seed oil in rat. Indian J. Pharmacol. 2005;37(3):179.
Passos CP, Silva RM, Da Silva FA, Coimbra MA, Silva CM. Supercritical fluid extraction of grape seed (Vitis vinifera L.) oil. Effect of the operating conditions upon oil composition and antioxidant capacity. Chem Eng J. 2010;160(2):634–40.
Yamasaki M, Kitagawa T, Koyanagi N, Chujo H, Maeda H, Kohno-Murase J, et al. Dietary effect of pomegranate seed oil on immune function and lipid metabolism in mice. Nutrition. 2006;22(1):54–9.
Kamel BS, Dawson H, Kakuda Y. Characteristics and composition of melon and grape seed oils and cakes. JAOCS. 1985;62(5):881–3.
Rezig L, Chouaibi M, Msaada K, Hamdi S. Chemical composition and profile characterisation of pumpkin (Cucurbita maxima) seed oil. Ind Crop Prod. 2012;37(1):82–7.
Melgarejo P, Salazar DM, Amorós A, Artés F. Total lipids content and fatty acid composition of seed oils from six pomegranate cultivars. J Sci Food Agric. 1995;69(2):253–6.
Day L. Proteins from land plants–potential resources for human nutrition and food security. Trends Food Sci Technol. 2013;32(1):25–42.
Boye JI, Aksay S, Roufik S, Ribereau S, Mondor M, Farnworth E, et al. Comparison of the functional properties of pea, chickpea and lentil protein concentrates processed using ultrafiltration and isoelectric precipitation techniques. Food Res Int. 2010;43:537–46.
Coşkun Ö, Çakır B, Vahapoğlu B, Gülseren İ. Influence of extraction conditions on structural and functional characteristics of black cumin protein concentrates and ACE-inhibition in their hydrolyzates. J Food Meas Charact. 2019;13(3):2328–38. https://doi.org/10.1007/s11694-019-00152-1.
Liu SH, Low NH, Nickerson MT. Effect of pH, salt, and biopolymer ratio on the formation of pea protein isolate-gum Arabic complexes. J Agric Food Chem. 2009;57:1521–6.
Lampart-Szczapa E. Preparation of protein from lupin seeds. Nahrung. 1996;40:71–4.
AOAC, Official methods of analysis of AOAC International (17th ed.). Gaithersburg, MD. USA: Association of Official Analytical Chemists Inc. (Revision 2) (2003).
Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;5259:680–5.
Tsaliki E, Pegiadou S, Doxastakis G. Evaluation of the emulsifying properties of cottonseed protein concentrates. Food Hydrocoll. 2004;18:631–7.
Beuschel BC, Culbertson JD, Partridge JA, Smith DM. Gelation and emulsification properties of partially insolubilized whey protein concentrates. J Food Sci. 1992;57:604–609,634.
Karaca AC, Low N, Nickerson M. Emulsifying properties of chickpea, faba bean, lentil and pea proteins produced by isoelectric precipitation and salt extraction. Food Res Int. 2011;44:2742–50.
Gülseren İ, Güzey D, Bruce BD, Weiss J. Structural and functional changes in ultrasonicated bovine serum albumin solutions. Ultrason Sonochem. 2007;14(2):173–83.
Gölükçü M, Tokgöz H, Kıralan M. Ülkemizde yetiştirilen önemli nar (punica granatum) çeşitlerine ait çekirdeklerin özellikleri. GIDA - J Food. 2008;33(6):281–90 (in Turkish).
Bucko S, Katona J, Popovic L, Vastag Z, Petrovic L, Vucinic-Vasic M. Investigation on solubility, interfacial and emulsifying properties of pumpkin (Cucurbita pepo) seed protein isolate, LWT - Food Sci. Technol. 2015;64:609–15.
Rezig L, Chibani F, Chouaibi M, Dalgalarrondo M, Hessini K, Gueguen J, et al. Pumpkin (Cucurbita maxima) seed proteins: sequential extraction processing and fraction characterization. J Agric Food Chem. 2013;61:7715–21.
Nowshehri JA, Bhat ZA, Shah MY. Blessings in disguise: bio-functional benefits of grape seed extracts. Food Res Int. 2015;77:333–48.
Pesavento IC, Bertazzo A, Flamini R, Vedova AD. Differentiation of Vitis vinifera varieties by MALDI-MS analysis of the grape seed proteins. J Mass Spectrom. 2008;43:234–41.
Sosulski FW, McCurdy AR. Functionality of flours, protein fractions and isolates from field peas and faba bean. J Food Sci. 1987;52(4):1010–4.
Marcone FM, Kakuda Y, Yada RY. Salt-soluble seed globulins of various dicotyledonous and monocotyledonous plants – I. Isolation - purification and characterization. Food Chem. 1997;62:27–47.
Fang EF, Wong JH, Lin P, Ng TB. Biochemical characterization of the RNA-hydrolytic activity of a pumpkin 2S albumin. FEBS Lett. 2010;584:4089–96.
Zhou T, Li Q, Zhang J, Bai Y, Zhao G. Purification and characterization of a new 11S globulin-like protein from grape (Vitis vinifera L.) seeds. Eur. Food Res. Technol. 2010;230:693–9.
Gazzola D, Vincenzi S, Gastaldon L, Tolin S, Pasini G, Curioni A. The proteins of the grape (Vitis vinifera L.) seed endosperm: fractionation and identification of the major components. Food Chem. 2014;155:132–9.
Radha C, Kumar PR, Prakash V. Preparation and characterization of a protein hydrolysate from an oilseed flour mixture. Food Chem. 2007;106:1166–74.
Damodaran S. Food proteins: An overview. In: Damodaran S, Paraf A, editors. Food proteins and their applications. New York: Marcel Dekker; 1997. p. 1–21.
Stone AK, Karalash A, Tyler RT, Warkentin TD, Nickerson MT. Functional attributes of pea protein concentrate prepared using different extraction methods and cultivars. Food Res Int. 2015;76:31–8.
Lazos ES. Composition and oil characteristics of apricot, peach and cherry kernel. Grasas Aceites. 1991;42(2):127–31.
Gülseren İ, Corredig M. Interactions of chitin nanocrystals with β-lactoglobulin at the oil–water interface, studied by drop shape tensiometry. Colloids Surf B Biointerfaces. 2013;111:672–9.
Sun XD, Arntfield SD. Gelation properties of salt-extracted pea protein induced by heat treatment. Food Res Int. 2010;43(2):509–15.
Alfawaz MA. Chemical composition and oil characteristics of pumpkin (Cucurbita maxima) seed kernels. Food Sci Agric. 2004;2(1):5–18.
Robledo A, Aguilera-Carbó A, Rodriguez R, Martinez JL, Garza Y, Aguilar CN. Ellagic acid production by Aspergillus niger in solid state fermentation of pomegranate residues. J Ind Microbiol Biotechnol. 2008;35(6):507–13.
Acknowledgements
The authors would like to also express their gratitude to Neva Foods Ltd. (İstanbul, Turkey) for the donation of all of the deoiled plant meals. Anton Paar, Turkey is also acknowledged for their support in rheological analysis.
Funding
This study was funded by a grant from TÜBİTAK 3501 Programme (Grant No. 115O569; The Scientific and Technological Research Council of Turkey).
Author information
Authors and Affiliations
Contributions
İG acquired the necessary funding, supervised the present research work and gave the required scientific instructions. ÖC did the experiments and obtained the experimental data. Both authors analyzed the data and contributed to the preparation of the manuscript. Both authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no competing interests.
Ethical approval
None needed.
Rights and permissions
About this article
Cite this article
Coşkun, Ö., Gülseren, İ. Aqueous extraction and functionality of protein concentrates manufactured from cold press meals of pumpkin, pomegranate, and grape seeds. Nutrire 45, 11 (2020). https://doi.org/10.1186/s41110-020-00114-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s41110-020-00114-4