Abstract
With the aim of choosing an appropriate standard for determining total phenol content (TPC) in food extracts, a theoretical study was done to demonstrate the electronic properties of nine phenolic compounds. Besides, TPC of three different tea extracts was determined by the Folin–Ciocalteu (F–C) assay with nine phenolic compounds as the standards. The frontier molecular orbitals (FMO), molecular electrostatic potential (MEP) and ionization potential (IP) of these standards were calculated with density functional theory. Results indicated the active sites of the nine standards by FMO and MEP. Moreover, the IP value of epigallocatechin gallate was about 15% lower than that of epigallocatechin, indicating that the 3-galloy group at C ring rendered a higher reactivity in the F–C assay. TPC of green tea measured by epicatechin was about 19% lower than that of gallic acid, suggesting that epicatechin was not an appropriate standard for tea extracts. It is deduced that gallic acid is a comparably good standard among commercial standards (relative standards). However, it is recommended that researchers should not choose a universal standard for all food extracts considering the heterogeneity and diversity of polyphenols in food extracts.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Polyphenols, ubiquitous in various kinds of food, have many potential health benefits, such as antioxidant, anti-proliferative, anti-inflammatory, anti-allergic, anti-hypertensive and antithrombotic activities [1,2,3,4]. Therefore, the study of polyphenol has attracted many researchers’ attention. In the research of polyphenol, the determination of total phenol content (TPC) in food extracts plays an important role due to the dose–effect relationship. The determination procedure is the basic and foundation for further research and is of great importance to the whole study [5, 6].
At present, colorimetric methods are employed as the mainstream analytical tool to determine TPC in food extracts, which is simple and fast compared with other analytical methods, such as high-performance liquid chromatography, and fluorescent detection [7]. The Folin–Ciocalteu (F–C) assay is the most commonly used colorimetric method in the determination of TPC in food extracts. The color density in the reaction medium is linearly correlated to the level of reducing substances, such as polyphenols. The F–C assay is not specific to particular groups of phenolic compounds, but serves to quantify the total concentrations of phenolic hydroxyl groups [8, 9]. Moreover, the F–C assay relies on the electron transfer in alkaline medium from phenolic compounds to phosphomolybdic or phosphotungstic acid complexes [8,9,10]. Therefore, the ionization potential (IP) value, which is defined as the energy needed to remove an electron from the parent molecule in ground state to form a cation in the gas phase [11], was introduced to evaluate the electron-transfer potential of phenolic compounds by density functional theory in present study.
TPC is expressed as the form of “mg standard equivalent/g sample” in colorimetric methods [12], showing the importance of standard on the determination of TPC. Therefore, in order to improve the accuracy, researchers have dedicated to investigating appropriate standards over the past decades. Standards are divided as absolute standards and relative standards [12]. It is reported by Wisdom and co authors [13] that the most appropriate standard for determining the absolute TPC may only be obtained by isolating and purifying from plant of interest. However, it is very difficult to be widely applied and may exist inter-laboratory difference, indicating the significance of choosing an appropriate commercial standard (such as gallic acid, epicatechin, epigallocatechin gallate etc.).
How to choose the suitable standard according to the properties of samples is the key point to the determination of TPC. In present study, nine commercial standards, varying in number and position of hydroxyl, and differing in type of subunit and molecular mass, and ranging from simple phenolic compounds (such as gallic acid) to oligomers (such as proanthocyanidin), were chosen to investigate this issue. We theoretically demonstrated the electronic properties of phenolic compounds with density functional theory and experimentally determined TPC in different tea extracts using the F–C assay with the nine phenolic compounds as standards. This study gave insights to the structure–activity relationship of chosen standards, and demonstrated the difference of TPC brought by different standards, thus providing information on choosing an appropriate standard in the determination of TPC in food extracts.
Materials and methods
Computational methods
The geometries of neutral molecules and their radicals were optimized at the B3LYP [14, 15] exchange–correlation functional level without constraints, employing the 6-311++G (d, p) basis set [16, 17]. The computation was carried out under vacuum condition. The IP value was computed at 298.15 K. The IP value was calculated as the difference in energy between cation and neutral molecule (IP = Ecation − Eneutral) [18]. All calculations referred in this work were performed with the Gaussian09 software (version 09 for Windows, GAUSSIAN Inc., USA) [19].
Materials and reagents
Phenol, catechol, resorcinol, pyrogallic acid, gallic acid, proanthocyanidin, epicatechin (EC), epigallocatechin (EGC) and epigallocatechin gallate (EGCG) were purchased from Sigma-Aldrich (St. Louis, MO, USA). F–C reagent, dimethyl sulfoxide (DMSO), sodium carbonate was purchased from Aladdin (Shanghai, China). Tea was purchased from a local supermarket in Chengdu, China. All other reagents used were of analytical grade, and the water was purified by a Milli-Q water purification system (Millipore, Bedford, MA, USA).
Preparation of standard solutions and tea extracts
Nine phenolic compounds were chosen as standards, and their structure was shown in Fig. 1. First, the standards were dissolved in distilled water or DMSO according to their solubility. Then, different standard solutions were diluted with distilled water and/or DMSO to different concentration ranges (Table 1).
Green tea, oolong tea and black tea were selected as the testing samples to determine TPC. During the preparation of tea extracts, all samples were crushed into powders with a mixer (JYL-350, Jiuyang Co., Ltd., China) and passed through a 60-mesh screen (sieve size, < 0.25 mm). Then, tea powders (100 g) were suspended in ethanol (1000 mL, 75%, v/v) with stirring at room temperature for 24 h. Thereafter, the mixture was filtered and the extraction procedure was repeated twice. All supernatants were condensed at 45 °C by rotary evaporator. Then, the dried tea extracts were obtained by freeze–drying with a yield (g extracts /100 g dried tea powders) of 8.36% (green tea), 6.21% (black tea) and 7.67% (oolong tea), respectively. All the tea extracts were stored at 4 °C for the determination of TPC using the F–C assay [20].
Determination of the TPC by the F–C assay
The F–C assay was carried out according to Singleton and co authors method [21] with some modifications. Briefly, 1 mL of testing solution (tea extracts: 4 mg/mL, standards: the concentration was shown in Table 1) was mixed with 10 mL of F–C reagent (10%, v/v), and 8 mL of sodium carbonate solution (7.5%, w/v) was added later. The mixture was mixed well and incubated at 45 °C for 30 min. Then, a Lambda 25 UV–Vis spectrophotometer (PerkinElmer Co., Ltd., MA., USA) was used to scan and record the maximum wavelength of the reaction mixture between 600 and 900 nm, and its absorbance was measured at the maximum wavelength.
Nine phenolic compounds were used as standards, and the calibration curves correlating the concentration of standard solution to absorbance were obtained, respectively. Then, all calibration curves were used to calculate TPC (mg/g) in tea extracts, and TPC was expressed as mg standard equivalent/g extract, respectively.
Statistical analysis
Data was presented as means ± standard deviations of three replicates. Statistics of the data was analyzed with SPSS (version 12.0 for Windows, SPSS Inc., CO, USA). Differences were considered significant at P < 0.05.
Results and discussion
Frontier molecular orbital
The highest occupied molecular orbital (HOMO) and the lowest unoccupied molecular orbital (LUMO) compositions for the nine phenolic compounds calculated with the DFT/6-311g++ (d, p) were depicted in Fig. 2, respectively. The energy of HOMO is the highest, so the electron of HOMO is very active and is most likely to donate. The charge density distribution of HOMO, indicating the electron-donating behavior, could give us insights to the antioxidant reactivity of phenolic compounds in the F–C assay. It is suggested that the site of the biggest electron charge density is the most active site [22, 23].
As shown in Fig. 2, the charge density covered the benzene ring and the phenolic hydroxyl(s) of phenol, catechol and resorcinol. However, the charge density of pyrogallic acid distributed less on the third phenolic hydroxyl compared with the ortho di-hydroxyl, providing theoretical evidence for the experimental finding that the activity of ortho di-hydroxyl was higher than tri-hydroxyl [21]. The charge density of gallic acid was mainly distributed on the benzene ring, the ortho di-hydroxyl and the oxyl of carboxyl, while the charge density on the third hydroxyl was comparatively less dense.
The charge density of EC was mainly distributed on the A ring, which contains a meta di-hydroxyl. Therefore, the A ring was the primary active site for electron-transfer reaction, and this is consistent with former report [24], which demonstrated that the A ring of EC was the preferential site for electrophilic attack. The distribution of EGC spread over the basic skeleton, and the electron on A, B and C ring were all active. The charge density of EGCG spread over A, B and C ring but not over the galloyl group, which corroborated Valcic’s observation [25] that the galloyl D-ring was not the primary site for antioxidant reactions. The charge distribution of proanthocyanidin was concentrated on the (epi) catechin subunit, and this is consistent with Mendoza-Wilson and Glossman-Mitnik’s report [24], indicating the difference between monomer and dimer.
Molecular electrostatic potential
Molecular electrostatic potential (MEP) is a very useful and important approach to explore the antioxidant reactivity of various compounds [23]. In most MEP surfaces, negative site is the preferred site for the electrophilic attack and positive site is preferred for nucleophilic attack. The nucleophilic and electrophilic sites of tested compounds were depicted in different colors. The red color indicates an electron-rich site, and a blue color indicates an electron-deficient site. The electron density indicated by colors follows this order: red > orange > yellow > green > blue [26]. MEP for compounds a–i was shown in Fig. 3.
For all phenolic compounds, the color code ranged from − 0.08 a.u (deepest red) to 0.08 a.u (deepest blue). It is clear that the electron was concentrated on the hydroxyl(s) of phenol, catechol and resorcinol. The ortho-hydroxyl of gallic acid was the electron-rich site, while the third hydroxyl was less electron-rich. Besides, the oxyl of carboxyl was another electron-rich site. The possible site for electrophilic attack of EC was on A ring at 5–OH, 7–OH and B ring at 3′–OH, and C ring at 3–OH. The electron density of EGC was mainly concentrated on A ring and B ring.The negative electrostatic potential of EGCG was predominant over A ring, B ring and C ring, while D ring was electron-deficient site. The distribution of proanthocyanidin dimer was rather dispersed and the electron was mainly concentrated on the (epi) catechin subunit.
IP evaluation
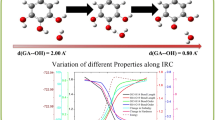
The IP, computed by the different value of enthalpy between the parent molecule and the cation, has correlation with electron-transfer pathway and is widely applied in electron-transfer reaction to calculate the electron-donating potential of phenolic compounds. The lowerthe IP value, the easier the electron-transfer process [27]. Therefore, the reactivity of phenolic compounds in the F–C assay can be evaluated by IP value because the F–C assay is based on the electron-transfer mechanism [8,9,10, 21].
The IP value of the tested phenolic compounds was shown in Table 2. The IP value of resorcinol was the highest, and the IP value of phenol was about 0.1% lower than that of resorcinol, which indicated that removing electron of the two compounds was the most difficult, thus showing the lowest reactivity in the F–C assay. Besides, it could be deduced that resorcinol with a meta-hydroxyl reacted like a mono-phenol, which is consistent with Singleton and co authors report [21]. The IP value of phenol was about 4% higher than that of catechol, since the antioxidant activity of phenolic compounds containing ortho-dihydroxyl was higher than that of mono-phenol [21, 27]. The IP value of pyrogallic acid was about 3% lower than that of catechol, which could be deduced that the trihydroxyl had a small influence on the electron-donating behavior. The IP value of gallic acid was about 10% lower than that of pyrogallic acid. Considering the noticeable difference between them, it could be concluded that the carboxyl on gallic acid took important participation in the electron-transfer reaction. The carboxyl group was considerably electron-rich, making the electron-donating behavior easier.
The IP value of EC was similar to that of EGC (about 1% lower). It could be speculated that the 5′–OH at the B ring of EGC was not the primary active position, making very little contribution to electron-donating. However, the IP value of EGCG was about 15% lower than that of EGC, which indicated that the 3-galloy group at C ring had a big influence on the electron-transfer reaction. Though the galloyl group at C ring was not the electron-rich site, its existence could facilitate the electron-transfer process. It is reported that the ester linkage to gallic acid via the 3–OH at C ring of EGCG further increased the electron-transfer reactivity [28].
Proanthocyanidin is the dimer of (epi) catechin, and its IP value was about 7% lower than EC, which showed that proanthocyanidin had a higher reactivitythan EC in the electron-transfer F–C assay. It is reported that proanthocyanidin dimer had a greater ability to donate electron than monomer [29]. What’s more, proanthocyanidin contains a number of hydroxyl groups, and they can form intramolecular and intermolecular hydrogen bonding to enhance the reactivity [29, 30]. In the electron-transfer reaction, IP value is mainly influenced by the extended delocalization and conjugation of the π-electrons [31]. Proanthocyanidin is not completely planar due to the existence of twisted B ring. However, the extended delocalization and conjugation of π electrons do occur in proanthocyanidin, involving the aromatic ring(s) and the substituents [31]. Therefore, proanthocyanidin had a rather high reactivity to donate electron in the electron-transfer F–C assay, showing the significance of polymerization. It is surprising that the IP value of proanthocyanidin was similar to that of gallic acid (about 1% lower). The structure of the two compounds is quite different. However, they are both good candidates to donate electron in the F–C assay and have almost equal potential to react with the F–C reagent.
Choosing an appropriate standard for determining TPC in the F–C assay
In present study, different phenolic compounds (phenol, catechol, resorcinol, pyrogallic acid, gallic acid, proanthocyanidin, EC, EGC and EGCG) were employed as standards to determine TPC in three different kinds of tea extracts. As shown in Table 2, TPC measured by different standards varied since the reactivity of different phenolic compounds was different in the F–C assay. TPC measured by EC was about 19% lower than that of GA in green tea. It is reported that TPC measured by GA was underestimated when compared with TPC measured by the standard isolated and purified from pomegranate [32]. Therefore, EC is not a good standard. It is not recommended to choose EC as the standard in the determination of TPC in tea extracts. TPC measured by EGC was 20% higher than that of GA, suggesting that EGC was a more appropriate standard than GA in tea extracts.
Food extracts can be considered as a mixture of different phenolic compounds, so the determination of TPC in food extracts is challenging. Considering the expression of TPC (mg standard equivalent/g sample), choosing a suitable standard for determining TPC in food extracts is of great importance and difficulty. Standards are divided as absolute standards and relative standards [12]. It is recommended by Wisdom and co authors [13] that appropriate standards for determining the absolute TPC may only be obtained by isolating and purifying from plant of interest. It is reported that the use of highly purified persimmon tannin as the standard to measure TPC in persimmon might be more accurate [32]. Analogous studies were carried out by Martin and co authors [33] and Feliciano [34], and TPC measured by these highly purified standards from plants of interest were more accurate and convincing since the standards had much similar structure to the extracts. Thus, these standards could be more representative. However, unfortunately, this work is time-consuming, complicated and heavy, which is quite difficult to be applied widely. What’s more, there may exist inter-laboratory difference. Therefore, obtaining an absolute standard for determining the absolute phenol content of food extract is rather arduous and may be impossible [12], indicating the significance of choosing an appropriate commercial standard (relative standard) for determining the relative phenol content. An appropriate commercial standard can not only eliminate inter-laboratory difference, but also make the experiment procedure simple to perform and make the data reliable.
Commercial standards, usually some simple phenolic compounds (such as gallic acid, EC, EGCG), can relatively represent the structure of polyphenols in various food extracts [34]. Gallic acid is the most widely used standard in the determination of TPC in food extracts. Compared with absolute standards, TPC measured by GA was lower. However, among commercial standards, GA is considerably an excellent candidate. It is not only cheap and stable, but also makes the analyzing procedure convenient and simple. Besides, the IP value of proanthocyanidin was similar to that of gallic acid (about 1% lower), and TPC measured by GA was similar to that of EGCG and proanthocyaidin (about 5% different), which could support the conclusion that GA is considerably a good standard.
However, considering the fact that the structure of polyphenols varies greatly, we do suggest that researchers should choose standards according to the properties of food extracts, and should bear in mind that there does not exist a perfect standard which can be applied to every food extract. It is recommended that the main component of food extract is a good choice to be employed as the standard. For example, proanthocyanidin or gallic acid is appropriate when determining TPC in grape extract, wine and other food extracts containing rich proanthocyanidin; as for those food extracts containing highly polymerized polyphenol, the dimer or trimer, tetramer standard should be taken into consideration; as for those food extracts containing highly esterified tannin with glucose, tannic acid is a good choice [32,33,34].
Conclusion
As for phenolic compounds containing only a benzene ring, the reactivity of meta-hydroxyl was similar to that of mono-hydroxyl. Besides, the carboxyl on gallic acid rendered a higher electron-donating activity. As for phenolic compounds of flavan–3–ol structure, the 3-galloy group at C ring rendered a higher antioxidant activity in the electron-transfer reaction since EGCG had a lower IP value than EGC. As for non-monomer phenolic compounds, the degree of polymerization was an important factor that could influence the phenolic compounds’ reactivity since proanthocyanidin had a higher antioxidant activity than EC. It is not recommended to choose EC as the standard in the determination of TPC in tea extracts since it underestimated TPC. Besides, we corroborated that GA was a comparably good standard among commercial standards by theoretically predicting the IP value and experimentally determining TPC in different tea extracts with nine phenolic compounds as standards. Moreover, considering the heterogeneity and diversity of polyphenol, it is suggested that we should choose the most abundant component of food extracts as the standard if possible.
References
C. Fernando, A.L. Cristina, T. Sara, J.T. Francisco, I.Q.O. María, J. Nutr. Biochem. 24, 1415–1422 (2013)
R.D. Del, L.G. Costa, M.E. Lean, A. Crozier, Nutr. Metab. Cardiovasc. Dis. 20, 1–6 (2010)
Y. Zhou, J. Zheng, Y. Li, D.P. Xu, S. Li, Y.M. Chen, H.B. Li, Nutrients 8, E515 (2016)
J. Dai, R.J. Mumper, Molecules 15, 7313–7352 (2010)
H. Lee, M.J. Ha, H.M. Shahbaz, J.U. Kim, H. Jang, J. Park, J. Food Eng. 238, 141–147 (2018)
P. Pajak, R. Socha, J. Broniek, K. Krolikowska, T. Fortuna, Food Chem. 275, 69–76 (2019)
I. Ramirez-Sanchez, L. Maya, G. Ceballos, F. Villarreal, J. Food Compos. Anal. 23, 790–793 (2010)
G. Daniel, S.S. Janio, G.M. Laercio, S.N. Domingos, TrAC Trends Anal. Chem. 80, 266–279 (2016)
B. Andressa, C.L. Gisely, C.P.M. Joao, Molecules 18, 6825–6856 (2013)
J.C. Sánchezrangel, J. Benavides, J.B. Heredia, L. Cisneroszevallos, D.A. Jacobovelázquez, Anal. Methods 5, 5990–5999 (2013)
V. Deepha, R. Praveena, R. Sivakumar, K. Sadasivam, Spectrochim. Acta 121, 737–745 (2014)
A.E. Hagerman, L.G. Butler, J. Chem. Ecol. 15, 1795–1810 (1989)
C.S. Wisdom, A. Gonzalez-Coloma, P.W. Rundel, Oecologia 72, 395–401 (1987)
A.D. Becke, J. Chem. Phys. 98, 5648 (1993)
C. Lee, W. Yang, R.G. Parr, Phys. Rev. B 37, 785–789 (1988)
R. Ditchfield, W.J. Hehre, J.A. Pople, J. Chem. Phys. 54, 724–728 (1971)
W.J. Hehre, R. Ditchfield, J.A. Pople, J. Chem. Phys. 56, 2257–2261 (1972)
Y.Z. Rong, Z.W. Wang, B. Zhao, Food Biophys. 8, 90–94 (2013)
M.J. Frisch, G.W. Trucks, H.B. Schlegel, G.E. Scuseria, M.A. Robb, J.R. Cheeseman, J.A. Montgomery, T. Vreven, K.N. Kudin, J.C. Burant, J.M. Millam, S.S. Iyengar, J. Tomasi, V. Barone, B. Mennucci, M. Cossi, G. Scalmani, N. Rega, G.A. Petersson, H. Nakatsuji, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, M. Klene, X. Li, J.E. Knox, H.P. Hratchian, J.B. Cross, C. Adamo, J. Jaramillo, R. Gomperts, R.E. Stratmann, O. Yazyev, A.J. Austin, R. Cammi, C. Pomelli, J.W. Ochterski, P.Y. Ayala, K. Morokuma, G.A. Voth, P. Salvador, J.J. Dannenberg, V.G. Zakrzewski, S. Dapprich, A.D. Daniels, M.C. Strain, O. Farkas, D.K. Malick, A.D. Rabuck, K. Raghavachari, J.B. Foresman, J.V. Ortiz, Q. Cui, A.G. Baboul, S. Clifford, J. Cioslowski, B.B. Stefanov, G. Liu, A. Liashenko, P. Piskorz, I. Komaromi, R.L. Martin, D.J. Fox, T. Keith, M.A. Al-Laham, C.Y. Peng, A. Nanayakkara, M. Challacombe, P. M. W. Gill, B. Johnson, W. Chen, M. W. Wong, C. Gonzalez, Gaussian 09. Revision A.1 (Gaussian, Inc, Wallingford, CT, 2009)
W.C. Zeng, W.C. Zhang, W.H. Zhang, Q. He, B. Shi, Food Chem. Toxicol. 58, 311–317 (2013)
R.M. Lamuela-Raventós, V.L. Singleton, R. Orthofer, Methods Enzymol. 299, 152–178 (1999)
M. Paczkowska, K. Lewandowska, W. Bednarski, M. Mizera, A. Podborska, A. Krause, J.C. Piontek, Spectrochim. Acta 140, 132–139 (2015)
D. Jeevitha, K. Sadasivam, R. Praveena, R. Jayaprakasam, J. Mol. Struct. 1120, 15–24 (2016)
A.M. Mendoza-Wilson, D. Glossman-Mitnik, J. Mol. Struct. THEOCHEM 761, 97–106 (2006)
S. Valcic, J.A. Burr, B.N. Timmermann, D.C. Liebler, Chem. Res. Toxicol. 13, 801–810 (2000)
N. Russo, Food Chem. 125, 288–306 (2011)
M. Leopoldini, N. Russo, M. Toscano, Food Chem. 125, 288–306 (2010)
Q. Guo, B. Zhao, S. Shen, J. Hou, J. Hu, W. Xin, Biochim. Biophys. Acta 1427, 13 (1999)
A.M. Mendoza-Wilson, S.I. Castro-Arredondo, R. R. Balandrán-Quintana. Food Chem. 161, 155–161 (2014)
A.M. Mendoza-Wilson, M.E. Armenta-Vázquez, S.I. Castro-Arredondo, A. Espinosa-Plascencia, H. González-Ríos, A. González-León, R.R. Balandrán-Quintana, J. Mol. Struct. 1035, 61–68 (2013)
M. Leopoldini, F. Rondinelli, N. Russo, M. Toscano, J. Agric. Food Chem. 58, 8862 (2010)
B. Zou, X. Dong, Z. Ge, Z. Xu, J. Du, C. Li, Eur. Food Res. Technol. 239, 385–391 (2014)
K.R. Martin, C.G. Krueger, G. Rodriquez, M. Dreher, J.D. Reed, J. Sci. Food Agric. 89, 157–162 (2009)
R.P. Feliciano, M.P. Shea, D. Shanmuganayagam, C.G. Krueger, A.B. Howell, J.D. Reed, J. Agric. Food Chem. 60, 4578 (2012)
Acknowledgements
We are grateful to Prof. Bi Shi, College of Light Industry, Textile and Food Engineering, for his helpful comments and suggestions. This work was financially supported by the National Natural Science Foundation of China (Grant No. 31801548) and Sichuan Science and Technology Program (Grant Nos. 2018HH0134, 2018GZ0003).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Gao, MR., Xu, QD., He, Q. et al. A theoretical and experimental study: the influence of different standards on the determination of total phenol content in the Folin–Ciocalteu assay. Food Measure 13, 1349–1356 (2019). https://doi.org/10.1007/s11694-019-00050-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11694-019-00050-6