Abstract
In this study, the effect of solvent type and solvent concentration on the ultrasound assisted extraction (UAE) of polyphenols from popular tea samples (black, green, mate, blackberry and black mulberry) which have high antioxidant activities were investigated by chemometric approaches. For this purpose, green extraction method was preferred and hydroalcoholic solvents for applied this system in industry. Water, MeOH 100%, MeOH 75%, MeOH 50%, EtOH 100%, EtOH 75%, EtOH 50% were used as extraction solvent. Multivariate calibration analytical technique preferred for quantification of individual phenolic compounds in tea samples and the relationship between total phenolic content (TPC), total flavonoid content (TFC) and total antioxidant activity (TAA) and individual phenolics was determined by chemometric approaches. The results of the study suggest that only spectroscopic comparisons based on TPC, TFC and TAA correlations are insufficient or even incorrect, and this is due to the fact that in tea samples different molecules besides different flavonoid structures are sensitive to spectroscopic techniques. The determination of the appropriate type and the concentration of solvent would contribute the usage of the herbal plants as a source of natural antioxidants in foods and pharmacology in large-scale industrial applications.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In recent years, researches on many plants, especially medicinal plants, have been increased in order to obtain new natural antioxidant sources. Among these natural antioxidant compounds, phenolic compounds are the most extensively researched because they are associated with lower risks of degenerative diseases, particularly cardiovascular diseases and cancer [1]. Extraction is the initial and essential step for the recovery of bioactive compounds from plant materials [2]. Several techniques such as maceration, Soxhlet extraction, microwave assisted extraction, supercritical fluid extraction, accelerated solvent extraction and ultrasound assisted extraction (UAE) were reported in order to extract poyphenolic compounds from various plants [3,4,5]. Maceration and Soxhlet extraction methods as the conventional extraction methods consume large volumes of organic solvent and take long extraction time. Also, long time and high temperature increase the risk of oxidation of phenolic compounds which decrease the bioactive effects in the extracts [3]. UAE has been proposed as an alternative to the conventional solvent extraction methods recently. Sonication gives a better recovery of bioactive compounds by intensification of mass transfer and easier access of the solvent to the cell [6]. In addition to lower solvent consumption and faster extraction, it also prevents the risk of degradation of polyphenols due to high temperature. Therefore it provides the application for thermolabile compounds [7, 8]. The extraction of phenolic compounds in the plant is affected by the extraction method as well as by the solvent used. The selection of solvent for extraction of polyphenolic compounds is important because it determines the amount and type of phenolic compounds extracted [1, 8, 9]. However, plants have different phenolic compounds of varied polarities and chemical characteristics that affect their solubility in a spesific solvent [9]. Water or organic solvents (ethanol-EtOH, methanol-MeOH, acetone, diethyl ether) and their aqueous mixtures have been commonly used for the extraction of polyphenols from plants [10]. Increase in researches on the most efficient solvent in the extraction of polyphenols from plants may help increase the usage of the potential natural antioxidants in pharmaceutical and food industry.
Tea is a widely consumed beverage throughout the world. In addition to the taste and aroma behind tea's growing popularity, there are also health benefits supported by numerous studies in recent years. Tea has proven to be beneficial by preventing the risk of some diseases such as cancer and cardiovascular problems [2, 11, 12]. Additionally, the biological functions of tea such as antiinflammatory, antioxidant, antiallergic, antiobesity, antimutagenic, antibacterial activities have also been reported [13,14,15,16,17]. Hundreds of different teas are now produced and consumed in the World. People's general attitude increased due to the relationship between the tea consumption and the risk of degenerative diseases [18]. The health benefits of teas have been correlated with the presence of high amount of phenolic compounds mainly flavonoids which have antioxidant activity [19, 20].
Teas prepared from the dried leaves of Camellia sinensis, are especially rich sources of phenolic compounds [21]. Green tea (unfermented) and black tea (fermented) have been consumed for centuries for their medicinal properties. Black tea is usually consumed in the West, while green tea consumption is especially popular in Asia mainly for its health benefits [22, 23]. Green tea is lesser processed than black tea and it has high amount of polyphenols such as catechins, epicatechins, epigallocatechins, epicatechingallate, epigallocatechin gallate, and gallic acid. Among them, the catechin’s family has been reported that as the most beneficial healts effects [8]. Black tea leaves are subjected to a complete crushing and fermentation process in which oxidation of catechin derivatives result in oxidized dimers (e.g., theaflavins) and complex condensed tannins (e.g., thearubigins) in additon to flavanols, flavonol glycosides, and phenolics [24, 25].
Mate is a traditional tea-like beverage obtained from the leaves of Ilex paraguariensis A. St.-Hil. a native plant of South America. It is widely consumed in Brazil, Argentina, Paraguay, and Uruguay. Also, it is exported to different parts of the world, mainly to the Middle East, USA and Europe [26]. Recently, interest in mate has been grown mostly due to its antioxidant, anticancer, antiobesity, antiinflammatory, antimutagenic, antirheumatic pharmacological effects. Health benefits may be related to possessing phytochemicals mainly phenolic compounds such as phenolic acids (chlorogenic acid) and flavonoids (quercetin, kaempferol, and rutin) besides triterpenoid saponins, minerals and purine alkaloids [27, 28].
Blackberry (Rubus fruticosus) leaf which is generally used as a tea substitute has been used as a traditional herbal medicine for treating digestive disorders especially acute diarrhea, nervous disorders, atherosclerosis, hypertension and radiation diseases [29,30,31,32]. It has been demonstrated that blackberry leaf has high level of antioxidants mainly phenolic compounds which are responsible for beneficial health effects [29, 33].
Black mulberry leaf (Morus nigra L.) which has been used mainly for sericulture also has been used traditionally for curing diabetes mellitus, cough, hypertension and cancer [34,35,36]. The leaf of black mulberry has been commonly consumed in Asian countries as a herbal infusion/tea beverage [37]. The health benefits due to high phenolic composition of black mulberry leaves have been investigated in recent studies [37, 38].
This research aimed to investigate the effect of solvent type and solvent concentration (water, MeOH 100%, MeOH 75%, MeOH 50%, EtOH 100%, EtOH 75%, EtOH 50%) with UAE of phenolic compounds from black tea (Camellia sinensis), green tea (Camellia sinensis), mate (Ilex paraguariensis A. St.-Hil.) tea, blackberry leaf (Rubus fruticosus) tea, black mulberry leaf (Morus nigra L.) tea which have been consumed commonly due to their health benefits.
The concentration of solvents—absolute (100%) and aqueous mixtures (75% and 50%)—were chosen according to literature [1, 10, 39, 40]. The extraction method of UAE was especially preferred to prevent the degradation of polyphenols due to high temperature in conventional methods and to protect their radical scavenging potential. The effect of extraction solvent and solvent mixtures on TPC, TFC, TAA and phenolic composition of obtained extracts were investigated and it was determined whether there was any correlation between the extracts of TPC, TFC, phenolic composition and TAA.
Materials and methods
Chemicals
All the reagents and chemicals used were of analytical grade. Folin-Ciocalteu’s reagent, sodium carbonate anhydrous, 1,1-Diphenyl-2-picrylhydrazyl radical (DPPH˙), aluminium chloride, sodium nitrite, sodium hydroxide, acetic acid and MeOH were obtained from Sigma (St. Louis, MO, USA). Phenolic standards for LPLC such as gallic acid monohydrate, (−)-gallocatechin, caffeic acid, vanillic acid, ellagic acid, p-coumaric acid, trans-ferulic acid, resveratrol, rutin trihydrate, hydroxycinnamic acid, chlorogenic acid, kaempferol, (+)-catechin, quercetin hydrate, syringic acid, (−)-epicatechin were purchased from Sigma-Aldrich (St. Louis, MO, USA). Ultra pure deionized water (18.2 MΩ) filtered through a 0.45 µm NC (nitrocellulose) membrane was used throughout.
Materials
Leafy herbal teas, including green tea (gt) (Camellia sinensis), black tea (bt) (Camellia sinensis), mate tea (m) (Ilex paraguariensis), blackberry leaf tea (bb) (Rubus fruticosus) and black mulberry leaf tea (bm) (Morus nigra) were provided from the market in İstanbul, Turkey. Products with a production date between July and September 2018 were preferred.The samples contained no additives which was mentioned on the label. The dried samples were ground to a fine powder and passed through a 1 mm sieve. The particle size was identified according to study which found the optimum particle size for the extraction of catechins from green tea [41]. The ground powder was kept in sealed containers and stored at 4 °C until used.
Preparation of extracts
Absolute (M1 and E1) and aqueous (M2, M3, E2, E3) hydroalcoholic solvent systems of MeOH and EtOH were used in green extraction. Water (W), 100% MeOH (M1), 75% MeOH (M2), 50% MeOH (M3) and 100% EtOH (E1), 75% EtOH (E2), 50% EtOH (E3) were used as solvents in the UAE to extract phenolic compounds from five different teas. UAE was performed in an ultrasonic bath (CleanEX 911, Everest Ultrasonic, 28 kHz) and the extraction parameters were 15 min extraction time, 85 mL/g leaf solvent:solid ratio, 55 °C extraction temperature. The parameters were determined according to the optimum results obtained from laboratory experiments on ultrasonic extraction of polyphenols from black mulberry tea in a previous study [42]. After the extraction, the extracts were cooled to room temperature, then filtered (Whatman No. 1 Paper) and used for the determination of TPC, TFC, TAA by spectroptometry and quantification of some phenolic compounds by LPLC. All measurements were carried out in triplicate and the data were expressed as the mean ± SD (standart deviation).
Total phenolic content (TPC)
TPC in the extracts was analyzed using the Folin-Ciocalteu method with some modifications [43]. 100 µL of extract was mixed with 2 mL of 2% (w/v) Na2CO3 solution. The mixture was incubated for 3 min, then 100 µL of Folin Ciocalteu reagent was added. The final mixture was incubated for 30 min at room temperature for colour development. Absorbance was then measured at 750 nm using UV–VIS spectrophotometer (PG T80-UV–VIS, PG Instruments, UK). Results are expressed as gallic acid equivalents per g of dried weight (mg GAE/g dw).
Total flavonoid content (TFC)
TFC in the extracts was analyzed using the modified method of Iqbal et al. [44]. 4 mL deionized water, 1 mL extract and 0.3 mL 5% NaNO2 solution were mixed. After 5 min. 0.3 mL of 10% AlCl3 was added. At 6th minute, 2 mL of 1 M NaOH and 2.4 mL deionized water was added and mixed. Absorbance of the mixture was measured at 510 nm (PG T80-UV–VIS, PG Instruments, UK). TFC in the extracts was calculated as catechin equivalents per g of dried weight (mg CAT/g dw).
Total antioxidant activity (TAA)
TAA of the extracts was measured using a DPPH free radical scavenging assay according to the method described by Lee et al. [45]. 0.5 mL extract was diluted tenfold with water and mixed with 2.5 mL 0.12 mM DPPH methanolic solution. After standing 30 min at room temperature, the absorbance of the final solution was measured at 517 nm using UV–VIS spectrophotometer (PG T80-UV–VIS, PG Instruments, UK). The scavenging activity of DPPH free radicals as TAA was calculated according to the following equation:
Multivariate calibration of phenolic compounds analyzed by low pressure liquid chromatography (LPLC)
There are number of problems in analytical chemistry where multivariate calibration is appropriate. One of these problems, as in our study, is a multi-component mixture in which all pure standards are present, such as a mixture of 16 polyphenolic substances (gallic acid monohydrate, (−)-gallocatechin, caffeic acid, vanillic acid, ellagic acid, p-coumaric acid, trans-ferulic acid, resveratrol, rutin trihydrate, hydroxycinnamic acid, chlorogenic acid, kaempferol, (+)-catechin, quercetin hydrate, syringic acid, (−)-epicatechin), representing a more complex situation (Table 1).
A 5-level (− 2, − 1, 0, + 1, + 2), 4-factor design was performed using five concentration levels (10–500 mg/L) for the phenolic compounds to be analyzed. The cyclic generator (− 2 → 1 → 2 → 1 → − 2), where the factors are related to each other, was used. Levels from − 2 (lowest) to + 2 (highest) were numbered, corresponding to coded concentrations; 10–500 mg/L; followed by a repeater level recommended to be the middle level, 0 (100 mg/L) was selected. The first experiment given in Table 2 was taken at this level for all factors. In this study the model was optimized with the aid of the 5-level factor design resulting in 25 sample mixture [46]. The [0 2 3 1] difference vector was chosen, which ensured that six successive factors were mutually orthogonal and also had a value for each level of each factor. The samples given in Table 2 were divided into two groups. For building the models, 13 training mixtures were selected whereas for measuring predictive power of the models, 12 validation mixtures were selected.
The wavelengths used were in the range of 220–360 nm. Due to the noisy content, wavelengths of less than 220 nm were not used. At the same time, wavelengths higher than 360 nm were not used because they were uninformative. The LPLC-DAD data from 220 to 360 nm at 2 nm intervals (71 wavelengths) for retention time points from 1.7 to 68 min (12,000 sampling points) were exported from LC Solution software (Shimadzu, Japan) in CSV format and imported to MatlabR2017a (Mathworks, Natick, MA, USA) for further data processing.
The purpose of partial least squares discriminant analysis (PLS-DA) method was to build a calibration model between the concentration of the components under study and the latent variables of the data matrix [47]. Partial Least Squares can be expressed in two different views, PLS-1 and PLS-2. PLS-2 uses total information related to the all concentration [47]. 25 calibration spectra was performed for PLS-2 calibration and, using this calibration, the concentration of the sample left out during the calibration process was predicted. The predicted concentrations of the components in each sample were compared with the actual concentrations in this calibration samples. The root mean squares error of cross validation (RMSECV) was calculated to use as a diagnostic test for examining the error in the predicted concentrations (Table 1).
Low pressure liquid chromatography (LPLC)
Shimadzu LC10A liquid chromatograph (Shimadzu, Kyoto, Japan) equipped with diode array dedector was used. The system included an LPLC; Shimadzu LC-10 AD pump, SPD-M10AVP Diode Array detector (200–550 nm), CTO-10 A Column Oven. 50 μL of sample loop with Reodayn Walve model 7725i Manual Sample Injection and CBM-10A Communications Bus Module.
Chromatographic conditions
The determination of chromatographic conditions was based on Algan Cavuldak et al. [42]. Quantitative analysis of sixteen phenolic compounds [gallic acid monohydrate (GA), (−)-gallocatechin (GC), caffeic acid (CA), vanillic acid (VA), ellagic acid (EA), p-coumaric acid (p-CA), trans-ferulic acid (t-FA), resveratrol (Res), rutin trihydrate (Rut), hydroxycinnamic acid (Hy-CinA), chlorogenic acid (CGA), kaempferol (Kae), (+)-catechin (Cat), quercetin hydrate (Quar), syringic acid (SA), (−)-epicatechin (Epi-C)] in tea extracts was performed using a reverse phase LPLC. The seperation was achieved on an Intersil ODS-3 reversed phase column (25 cm × 4.6 mm, 5 μm particle size). Column oven temperature was 30 °C. The flow rate of mobil phase was 1 mL/min and the injection volumes were 20 μL of the standards and extracts. All the solutions were filtered through 0.45 μm syringe filter before LPLC analysis. The mobile phase was MeOH (solvent A) and acetic acid solution (%2) (v/v) (solvent B). The gradient conditions were; 0 min, 100% B; 3 min, 95% B; 18 min, 80% B; 25 min 80% B; 30 min, 75% B; 35 min, 70% B; 40 min, 60% B; 55 min, 50% B; 65 min, 40% B; 68 min, 100% B. Chromatograms were recorded at 280, 320, and 360 nm. The retention time, absorbance, calibration curve, linearity (R2), limit of detection (LOD) and limit of quantification (LOQ) of phenolic compounds were shown in Table 1.
Statistical analysis
The results were presented in average values and standard deviations of the replicates. The results were submitted to variance analysis (ANOVA) and Kruskal Wallis test (p < 0.05) Kruskal Wallis test (p < 0.05) was used for comparison of all solvents and solvent mixtures. Spearmen correlation analysis was used to determine the relationship between all parameters, because the variables were obtained by intermitant scale but didn’t show normal distribution. Spearman’s correlation coefficient is a statistical measure of the strength of a monotonic relationship between two variables and it is denoted by rs and varies from − 1 to + 1. Cluster analysis was applied to the purpose of grouping a set of objects in such a way that objects in the same group (called a cluster) in a more similar to each other than to those in other groups (clusters). ANOVA, Kruskal Wallis test, Spearman correlation analysis, Cluster analysis were performed using the Minitab 17.1.0,UK.
Results and discussion
Effect of solvent system on total phenolic content (TPC)
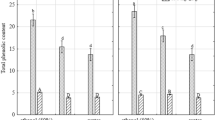
Phenolic compounds which have aromatic ring and hydroxyl group are commonly found in plants [3]. Extraction efficiency of polyphenols is dependent on the extraction solvent and its polarity [48]. TPC content of different tea leaf extracts using different solvent systems (W, M1, M2, M3, E1, E2, E3) obtained by UAE were shown in Table 3. The data showed that extraction solvents have significant effect on the extraction yield of TPC from different tea leaves according to the Kruskal–Wallis test (p < 0.05). TPC content in the five different tea leaf varies within wide range in water, MeOH and EtOH. Generally higher TPC was obtained in 50% aqueous organic solvents compared to their absolute solvents. This result was similar to the results of other studies in the literature in which high TPC was obtained in aqueous solvents rather than absolute solvents [9, 39, 40].
TPC results obtained from extracts of black tea (C. sinensis) were shown in Table 3. EtOH (50%) had the highest TPC. A high TPC content in EtOH (50%) than other solvents was reported in other plant extracts also [39]. TPC was ranged from 8.56 ± 0.34 to 64.95 ± 0.29 mg GAE/g dw and the decreasing order for the extracts of black tea: 50% EtOH > 75% EtOH > 75% MeOH > 50% MeOH > 100% (absolute) MeOH > water > 100% (absolute) EtOH. The lowest solvent system was shown as absolute EtOH, however water extracts and absolute MeOH extracts were also lower than aqueous-alcoholic extracts. Higher TPC was in 50% aqueous-organic solvents compared to their absolute solvents. Similarly, Turkmen et al. [49] reported that water extracted polyphenols were lower than those in alcohol solutions in the studying of the effect of solvent systems on the extraction of phenolics from black tea. Also, Üstündağ et al. [50] found that total phenolic yield of water extracts was significantly lower than that of aqueous-EtOH solvents for black tea samples in which 50% EtOH had significantly higher total phenolic yield than 80% EtOH, followed by water.
TPC results of extracts from green tea (C. sinensis) was shown in Table 3. TPC values of green tea extracts range from 20.53 ± 0.80 mg GAE/g dw for 100% EtOH extract to 90.87 ± 1.52 mg GAE/g dw for 50% MeOH extract. TPC in 50% MeOH extract was significantly higher than the other solvents (p < 0.05). TPC results of 100% EtOH extracts were the lowest in green tea extracts similar to the results of black tea extracts. It was seen that MeOH had enough potential for maximum extraction of green tea polyphenols in agreement with Pasrija and Anandharamakrishnan [8].
TPC results from mate tea extracts (I. paraguariensis) was shown in Table 3 TPC was as follows; 50% EtOH > 50% MeOH > 75% MeOH > 75% EtOH > 100% MeOH > water > 100% EtOH. TPC content was ranged from 17.88 ± 1.04 to 81.12 ± 2.20 mg GAE/g dw. Mate tea extracts had the highest TPC (81.12 ± 2.20) when the leaves were significantly extracted with 50% EtOH. Similar trends have been reported in mate tea extracts by other investigators. The result of the study of Turkmen et al. [49] was in agreement with our results in which the extracts of black and mate tea prepared with aqueous (50%) solvents had highest level of TPC than absolute solvents and followed by those with 80% and 100% solvents. It was determined that the higher content of polyphenols was obtained with an increase in the polarity of the solvent used [49]. It was reported that EtOH 50% is one of the most commonly used solvents for extracting polyphenolic compounds from plant materials [51]. EtOH is more preferable in the extraction studies since it is cheap, reusable and non toxic which can be used directly in human consumption (e.g. beverages, foods and cosmetics) [39, 52].
TPC results of extracts from blackberry leaf tea (R. fruticosus) showed a wide range of concentrations, from 16.25 ± 0.21 to 79.48 ± 2.94 mg GAE/g dw depending on the solvent used which was shown as follows; 50% MeOH > 50% EtOH > 75% EtOH > 75% MeOH > water > 100% MeOH > 100% EtOH (Table 3). Sultana et al. [9] found that the aqueous MeOH and aqueous EtOH extracts had the highest TPC among all the solvent extracts for the extraction of polyphenols from various medicinal plants. It may related with that phenolics are often extracted in higher amounts in aqueous solvents compared with absolute solvents [9].
TPC results of extracts from black mulberry leaf tea (M. nigra) was shown in Table 3 TPC of black mulberry leaf tea was generally lower compared to the other tea samples studied. The highest phenolic compounds content (19.86 mg GAE/g dw) was obtained with 75% EtOH and followed by 75% MeOH, 50% EtOH, 50% MeOH, water, 100% MeOH and 100% EtOH. The lowest TPC (5.68 mg GAE/g dw) was obtained in 100% EtOH extracts. Similar to our results, the highest TPC for M. nigra was obtained in EtOH (75%) in the study of Bhebhe et al. [39]. In another study, EtOH and MeOH were more effective than water for phenolic extraction from peanut skin than water, especially 80% EtOH was the most efficient [53]. Water in hydroalcoholic solvents can more easily penetrate the plant material, making it easier to extract polyphenols and bioactive substances in the solid matrix. Therefore, it was thought that higher amount of TPC can be reached in hydroalcoholic solutions [39]. Overall, TPC results indicate that extraction solvent mixtures play an important role in the extraction of phenolic compounds from different tea samples and aqueous EtOH or MeOH extracts contain more phenolic compounds than water extracts for all samples.
Generally, 50% MeOH enabled highest value of TPC for green, mate and blackberry leaf tea however 75% EtOH and 50% EtOH provided the highest value for black mulberry tea and black tea respectively. The lowest TPC was shown in 100% EtOH extracts of all tea samples studied (Table 3).
Effect of solvent system on total flavonoid content (TFC)
Phenolic compounds found in plant materials are mainly classified into two groups as flavonoids and phenolic acids [3]. In general, flavonoids that contain multiple hydroxyl groups show higher TAA than phenolic acids [54]. TFC of different leaf tea extracts studied in our study generally ranged from 2.27 ± 0.05 to 62.03 ± 0.93 mg CAT/g dw (Table 3). The highest flavonoid content was found in the 50% EtOH extract of mate tea. This result was in agreement with Oh et al. [43] who also evaluated the flavonoid levels of various leafy herbal tea extracts. The high level of TFC in mate tea EtOH extract may be explained owing to having high caffeoyl derivatives including caffeic acid, chlorogenic acid and dicaffeoylquinic acid which these also contribute antioxidant capacity of mate tea [43, 55]. The lowest flavonoid value was obtained in 100% EtOH extract of black mulberry leaf tea.
Table 3 shows the highest TFC for black tea (19.45 ± 0.64 mg CAT/g dw) that was obtained in the 50% EtOH extract, followed by the 75% EtOH, 75% MeOH, 100% MeOH, 50% MeOH, water and 100% EtOH extract. 50% EtOH extract of black tea was found significantly different from the other tea extracts. This following trend was similar with the variation in TPC of black tea extracts. Also, TFC in the extracts decreased when the water concentration in EtOH decreased.
For green tea extracts, TFC depended significantly (p < 0.05) on the solvent mixture (Table 3). The 75% MeOH extract had highest flavonoid content (30.36 ± 0.93 mg CAT/g dw), followed by 100% MeOH, 75% EtOH, 50% EtOH, 50% MeOH, water and 100%EtOH. The lowest content (7.44 ± 0.08 mg CAT/g dw) was found in absolute EtOH extract.
In the case of mate tea extracts, statistically significant differences were found on the flavonoid content of all solvents used for mate tea extract (Table 3). 50% EtOH extract was obtained as the highest flavonoid content whereas the lowest one was the 100% EtOH extract which was the most statistically different from the other solvent systems. Trend of TFC of extracts was found similar to TPC results.
Aqueous alcoholic solutions gave better results on the extraction of flavonoids from blackberry tea. The solvents showed significant differences in flavonoid content of extracts as shown in Table 3.The mixture of 50% water with MEOH had the highest extraction yield of flavonoids (37.94 ± 0.66 mg CAT/g dw) and it was followed by 50% water with EtOH extract (37.88 ± 0.95 mg CAT/g dw). Absolute EtOH (100%) had the lowest level of flavonoids (7.14 ± 0.03 mg CAT/g dw) in blackberry tea extracts. The decreasing order of TFC in the extracts was similar to TPC.
For black mulberry tea extracts, the highest level of TFC belonged to 75% EtOH extracts with the value of 11.23 ± 0.02 mg CAT/g dw (Table 3). It was followed by the aqueous mixtures of EtOH and MeOH and the lowest level of TFC was obtained in absolute EtOH (100%) extracts with the value of 2.27 ± 0.05 mg CAT/g dw. The all solvents used had significantly different from each other whereas especially 75% EtOH was found significant effect compared to the other solvents.
It was shown that the lowest values of TFC was obtained in 100% EtOH extracts for all tea samples. 50% EtOH gave the maximum values of TFC for black and mate tea extracts, however 75% MeOH, 75% EtOH and 50% MeOH gave the maximum values for green, black mulberry and blackberry tea respectively. The extraction solvents had significant effect on the extraction of TFC (p < 0.05).
Effect of solvent system on total antioxidant activity (TAA)
Analysis of TAA can be performed with different methods however each method has its advantages and disadvantages. TAA was analyzed by DPPH method since it has similar mechanisms with TPC and TFC analysis. It was thought that using similar methodologies in TPC, TFC and TAA analysis in order to establish the correct correlation between these parameters was an correct approach. In other words the effect of solvent type on tea varieties should be mainly evaluated eliminating the errors from the method differences. Phenolic compounds can show their TAA with their radical scavenging effects. Additionally the method of DPPH radical scavenging activity has been widely used to determine the TAA of extracts obtained from various plants [39, 56, 57]. The results of antioxidant activity using different extraction solvent systems are shown in Table 3. The results showed that the values of DPPH radical scavenging activity of all tea extracts vary significantly with them. Among all the samples analyzed, 50% MeOH extract of green tea (94.18 ± 0.49%) exhibited the highest TAA than the other tea extracts. For green tea extracts, the significant difference (p < 0.05) was observed between all solvent systems used. The highest DPPH activity was found in 50% MeOH extract with an inhibition % value of 94.18 ± 0.49%, whereas the lowest ability to scavenge DPPH with a value of 68.15 ± 2.20% was found in water extracts. The results were consistent with findings by Do et al. [1] who reported that using a absolute and aqueous organic solvents gave stronger radical scavenging capacity than that of the water extract when the extraction solvent effect was investigated [1]. Higher free radical scavenging activity in 50% MeOH extracts were reported in the studies from different plants [39, 58].
For black tea extracts, significant differences (p < 0.05) were seen in DPPH scavenging activities of extracts due to solvents used (Table 3). The best DPPH scavenging capacity (86.27 ± 1.69%) was obtained with 50% MeOH, followed by absolute MeOH (100%), 50% EtOH, 75% MeOH, 100% EtOH, 75% EtOH than water which was the lowest one (46.57 ± 1.17%) (Table 3). The similar trend in the result of green tea extracts was obtained in black tea extracts. Similar result for DPPH radical scavenging activity of black tea extracts was found in the study of Turkmen et al. [49] in which the concentrations of 50% and 80% of aqueous solvents used exhibited considerably higher DPPH activity than those with their respective absolute ones [49]. Additionally Bhebhe et al. [39] obtained with absolute organic solvents lower free radical scavenging activity of than their aqueous (50% MeOH, 50% EtOH and 50% acetone) organic preparations in the research of the effect of solvent type in the extracts of black tea and herbal infusions.
Table 3 shows that all solvent systems significantly affected TAA of mate tea extracts. The antioxidant activity was in decreasing order with the corresponding solvents used: 50% EtOH > 50% MeOH > 75% MeOH > 75% EtOH > absolute MeOH > water > absolute EtOH. Highest antioxidant activity value of 50% EtOH extract of mate tea was also found in another study that the high polarity solvents were more effective radical scavengers than less polarity solvents [49]. Additionally, EtOH (50%) extracts of different plants ensured high radical savenging activity in a study [39] which indicates that high TPC may also show high antioxidant activity in some plant materials.
Blackberry tea extract obtained by 75% EtOH yielded the significantly highest DPPH activity (89.10 ± 1.57%) whereas the extract obtained by water yielded the lowest DPPH activity (74.52 ± 0.84%). The results are significantly different from those of the other extracts. Water extracts of blackberry tea were found lower compared to alcoholic solvents similar to most of the tea extracts studied in our study.
For black mulberry tea extracts, the significance difference was observed in DPPH results between the extracts (Table 3). It was found that 75% MeOH extract possesses the strongest DPPH activity (88.85 ± 1.23%), followed by 75% EtOH, 50% EtOH, 50% MeOH, water, 100% MeOH and 100% EtOH (45.7 ± 0.14%) respectively. The lowest antioxidant activity was found in the absolute EtOH (100%) extracts of black mulberry tea samples. It has resulted that for black mulberry tea extracts, aqueous solvents may be better solvents. Low percentages (25%) water addition to the alcohol yielded antioxidant activity, total polyphenols and total flavonoids.
Generally minimum TAA values were obtained with water extracts of black, green and blackberry tea, however in mate and black mulberry tea extracts it was determined in 100% EtOH. The highest values of TAA were in 50% MeOH extracts of black and green tea whereas 50% EtOH, 75% MeOH and 75% EtOH provided maximum TAA for mate, black mulberry and blackberry tea extracts respectively. As can be seen from TAA results, the extraction efficiency was low with absolute solvents used. Variations in the level of antioxidant activities of the extracts may attribute to the change in polarity of solvents. Generally, the addition of water enhances the extraction efficiency in antioxidant activity as well as polyphenols and flavonoids. However, opposite results were observed in the correlations between TPC/TFC and TAA in the literature. The high correlation was observed in some studies [1, 52, 59] whereas there was no or weak correlation was found in others [60,61,62]. It was thought that comparison of TAA, TPC, TFC results with only spectroscopic methods was insufficient also it should be compared with the amount of individual phenolics determined by LPLC which was more important. As in literature giving data with correlations in the comparison between TPC and TAA was insufficient especially in complex structures such as plant extracts.
Effect of solvent system on phenolic composition (LPLC)
Sixteen phenolic compounds were identified in five tea extracts according to the retention time and UV spectra of their peaks in comparison with their standards. The individual phenolic compounds identified were as follows: (1) flavonoids (gallocatechin, (+)-catechin, (−)-epicatechin, resveratrol, rutin, kaempferol, quercetin), (2) phenolic acids (gallic acid, caffeic acid, vanillic acid, ellagic acid, p-coumaric acid, t-ferulic acid, hydroxycinnamic acid, chlorogenic acid, syringic acid) (Table 4).
Phenolics in green tea are generally formed by catechin derivatives, however flavonols and phenolic acids are also present in lower quantity [63]. Catechins which are also referred to flavan-3-ols are important phenolic compounds that were contributed to high antioxidant activity of green tea. The main catechins in green tea are epigallocatechin gallate, epigallocatechin, epicatechin gallate, and epicatechin [2, 18, 43, 64]. Among catechins; epigallocatechin gallate, epigallocatechin, epicatechin levels were found higher than other catechins [18]. In our study, the levels of total catechins ((+)-catechin, (−)-gallocatechin, (−)-epicatechin] in the green tea extracts varied from 0.246 ± 0.003 to 9.735 ± 0.212 mg/g (Table 4). Epicatechin level was found higher than catechin and gallocatechin which was similar to literature [65]. Water was shown as the best solvent in the extraction of total catechins in green tea whereas the lowest extraction being observed with 50% MeOH (Table 4). Similarly Khokhar and Magnusdottır [18] found the highest extraction of all catechins in various teas with water compared with 80% MeOH and 70% EtOH.
The highest antioxidant activity of green tea extracts among the other teas was highlighted come from mainly catechins such as epigallocatechin gallate, epigallocatechin, epicatechin gallate, and epicatechin [43, 65]. These major catechins were highly contributed TAA of green tea. Phenolic compounds having antagonistic or synergistic effect with themselves or with other constituents of the extracts have different antioxidant activity [61, 66]. Also, there can be other compounds that acted as antioxidants in plant material which may contributed to free radical-scavenging activity.
While green tea is from fresh leaves of C. sinensis, black tea is manufactured from green tea by oxidation followed by polymerisation. During this process, the concentration of flavan-3-ols was decreased whereas complex components such as theaflavins and thearubigins were increased [59, 65]. Therefore the catechin’s concentration was lower than those in green tea. Üstündağ et al. [50] found total catechin content (18.3–22.5 mg total catechins/g tea) of different grades of Turkish black tea. Similar results were obtained for catechin content in literature in which epigallocatechingallate and epigallocatechin were observed as the predominant catechins whereas catechin, epicatechin, gallocatechin and epigallocatechin also present [18, 67]. Also, it was pointed out that the presence of the four catechins (epigallocatechin gallate, epigallocatechin, epicatechin, epicatechin gallate) especially epigallocatechingallate in black tea is important to play a role in the formation of the main teaflavins responsible for the quality of black tea [68]. However, Üstündağ et al. [50] found higher total theaflavin and epicatechin content in which specified that high epicatechin content can be indicative of the extent of oxidation [50, 69]. Our results showed that the levels of catechins were lower (0.069 ± 0.002–0.638 ± 0.018 mg/g dw) compared to green tea extracts (Table 4) which supported that the reason for the lesser amount of catechins in black teas was the oxidation and polymerization of catechins during fermentation [65, 70]. 50% EtOH gave the maximum results for total catechins and phenolic acids (Table 4) which was similar the results of Üstündağ et al. [50].
The main phenolic compounds found in mate tea extracts were chlorogenic acid, caffeic acid, rutin (Table 4) which their levels were similar with the literature [2, 71, 72]. LPLC results of total phenolic acids (3.549 ± 0.110- 15.51 ± 0.268 mg/g dw) and total flavonoids (1.226 ± 0.025–10.668 ± 0.238 mg/g dw) were higher in mate tea extracs among all teas. 50% MeOH extracts gave the highest values for phenolic acids and flavonoids in mate tea extracts (Table 4).
The phenolic composition of blackberry tea extracts was given in Table 4. The compounds mainly rutin, kaempferol, quercetin, (−)-epicatechin were highly level determined compared to other compounds in the extracts. 50% EtOH was the best solvent for the extraction of total catechins whereas phenolic acids and other flavonoids extracted best by 75% MeOH (Table 4). The amount of chlorogenic acid which was at the range of 1.210 ± 0.041–5.591 ± 0.152 mg/g dw was the highest among phenolic acids.
In the black mulberry tea extracts, the contents of phenolic acids and other flavonoids determined by LPLC were found higher than total catechins (Table 4). 75% EtOH gave the maximum results for phenolic acids and other flavonoids by LPLC. Chlorogenic acid which was the major phenolic acid in black mulberry tea extracts was at the range of 0.471 ± 0.012—5.395 ± 0.221 mg/g dw. Additional to chlorogenic acid; kaempferol, rutin, quercetin, resveratrol, (−)-epicatechin were mainly exist in black mulberry leaf and their amounts were generally related with previous studies [35, 38, 73].
When the effect of extraction solvent on the individual phenolics of various tea extracts was studied, it was shown that solvents of different polarities showed significant difference (p < 0.05) in the amount of phenolic compounds (Table 4). The TAA of tea extracts depends on the individual phenolic contents, also their structure and interaction. An extract shows a higher antioxidant property if the phenolic compound contained in it contains more hydroxyl groups [74]. Since synergy between different chemicals is important for biological activity, other minor phenolics should also be considered [75]. LPLC results of individual phenolics were correlated with TPC and TFC whereas they were not all correlated with high TAA results. Although TAA was very high, it can be thought that high TAA may come from other compounds not from all phenolics.
Spearman correlation analysis
There are some results observed in Spearman analysis on all data considering the effect of all solvent systems for each tea extracts. For green tea, the correlation between TFC and phenolic acids of the extracts was found high (rs = 0.964) (Table 5a). For black tea, the correlation coefficient (rs) between TPC and TFC of tea extracts was found 0.964 (Table 5). Additionally TPC correlated with TCAT + PACIDS (rs = 0.857), GA (rs = 0.964) and Rut (rs = 0.929). Similar to TPC results, TFC highly correlated with GA (rs = 0.893) and Rut (rs = 0.857), SA (rs = 0.857) and Epi-C (rs = 0.847). The correlation between the TAA and TPC/TFC for tea extracts were not observed (Table 5b).
The correlation between TPC and TFC results of mate tea extracts was found highly correlated (rs = 0.964) (Table 5c) similar to black tea extracts. It was shown that flavonoids of mate tea has important role in TPC. At the same time, phenolic acids has significant place according to their correlation with TPC (rs = 0.893). Also, the correlation between TFC and phenolic acids of mate tea extracts was found high (rs = 0.857) (Table 5c) similar to green tea extracts. Among phenolic acids, the correlation coefficients of CGA (rs = 0.857) and GA (rs = 0.857) with TFC were highly correlated. A high and positive correlations between TPC and DPPH activity (rs = 1.000) and between TFC and DPPH radical scavenging activity (rs = 0.964) were observed, respectively. It was shown that TAA was strongly associated with the phenolics and flavonoids which the phenolic compounds and flavonoids contribute significantly to the TAA of mate tea extracts. The result was agreement with Turkmen et al. [49] and Mello et al. [59] who found high correlations between polyphenol content and TAA for mate tea extracts (R2 = 0.98 and R2 = 0.986, respectively). Additionally, the higher correlations between phenolic acids (rs = 0.893) and especially CGA (rs = 0.929) and TAA were obtained (Table 5c). This results were related with previous studies which indicated that simple phenols mainly phenolic acids especially chlorogenic acid was major compound and potential antioxidant compound that correlated antioxidant activity of mate tea [72, 76, 77].
For blackberry tea, a high Spearman correlation coefficient (rs = 0.964) (Table 5d) was found between TFC and TPC of extracts. It was observed that the effect of solvent systems on TFC was similar to that on TPC. Flavonoids of blackberry tea have been shown to be significant amount in the total phenolic content. As opposed to high correlation of TPC and TFC (rs = 0.964) there was no obvious relationship between TPC and TAA or TFC and TAA. Total catechins were more correlated with TPC (rs = 0.857) whereas CGA were more correlated with PACIDS (rs = 0.929). This results were conformed with the previous studies in which similar phenolic compounds were determined [29, 32].
TFC was found as highly correlated (rs = 0.964) (Table 5) with TPC of black mulberry extracts which was demonstrated that TFC of the extracts had similar trend in the change of TPC. This result was similar to blackberry, mate and black tea extracts. Additionally, Res (rs = 0.929) and CGA (rs = 0.929) were highly correlated with TPC. On the other hand, Table 5 indicates a significant relationship between TPC and TAA (rs = 0.963) and TFC and TAA (rs = 0.893) with significant correlation coefficients. The similar results of high correlation was shown in mate tea extracts which demonstrate that high TAA was attributed to high phenolics also high flavonoids in the extract (Table 5). When the correlation was determined between individual phenolic compounds and TAA of black mulberry tea extracts, the correlation coefficients of total CGA (rs = 0.964) and resveratrol (rs = 0.964) were found significantly high. Antioxidant activity of black mulberry tea can be explained especially by two major compounds (chlorogenic acid and resveratrol) in this study similar to the literature [37, 38].
TFC of all tea extracts had high correlated with TPC which high correlation were found in the extraction of polyphenols from various plants [1, 52]. In our study, the similar results were found in all tea samples except green tea. Although the results of TPC, TFC and TAA of tea samples were relatively high, there are especially significant correlation between TAA and TPC or TFC in mate tea and black mulberry tea extracts.
Cluster analysis
A fully linked clustering method, one of the hierarchical clustering analysis methods, was used in the study for the Cluster analysis. The distance between the clusters was calculated from the distance measurements using the Euclidean distance and divided into clusters according to the Euclidean distance. Cluster analysis was performed on all data considering the effect of all solvent systems for each tea extract. Clusters were formed to contain 19 components. At this stage, the defined clustering analysis method was chosen as the non-hierarchical k-means method. This process was continued until each variable center was collected in the cluster closest to it. In the study, the successive stages of clustering are shown using a dendogram (Fig. 1a-f).
Cluster analysis to variables of tea extracts obtained by different solvents. GA gallic acid, GC gallocatechin, CA caffeic acid, VA vanillic acid, EA ellagic acid, p-CA p-coumaric acid, t-FA trans-ferulic acid, Res resveratrol, Rut rutin, Hy-CinA hydroxycinnamic acid, CGA chlorogenic acid, Kae kaempferol, Cat catechin, Quar quercetin, SA syringic acid, Epi-C epicatechin, TCAT total catechins (GC + Cat + Epi-C), PACIDS phenolic acids (GA + CA + VA + EA + p-CA + t-FA + Hy-CinA + CGA + SA), AFLAV other flavonoids (Res + Rut + Kae + Quar), TFLAV total flavonoids (TCAT + AFLAV). Similarity is expressed in %
For green tea, the nearest variables (99.95%) were found to be EA-Rut and the least similar variables were found to be GA-EA with a similarity of 11.63%. When Fig. 1a was examined, it was determined that the 19 variables were included in different clusters associated with each other and represented by two basic clusters. Of these clusters; cluster 1 was consisted of the components of GA, GC, CA, VA, p-CA, t-FA, Res, Hy-CinA and TAA whereas cluster 2 was consisted of the components of EA, Rut, CGA, Kae, Cat, Quar, SA, Epi-C, TPC and TFC. When the dendogram was examined (Fig. 1a), the highest similarities were observed between binary variables as CA-pCA (98.63%), GA-CA (97.53%), tFA-Res (97.37%), tFA-HyCinA (96.49%), TPC-TFC (93.80%), EA-Kae (92.56%), CGA- Cat (90.85%), VA-tFA (86.69), Quar-SA (81.69), GA-TAA (79.09%), GC-VA (74.08%), Quar-TPC (73.08%). Among these clusters, tFA-Res was found to be significant (p = 0.004) in terms of spearman correlation coefficiant (rs = 0.918) and was identified by positive correlation (Table 5).
In the study for black tea (Fig. 1b), the closest variables were TPC-TFC (97.96%) and the least similar variables were GA-CA (9.02%). The variables are represented in Fig. 1b by three basic clusters. Of these clusters; cluster 1 was composed of the components of GA, VA, p-CA, t-FA, Res, Rut, Hy-CinA, CGA, Kae, Cat, SA, Epi-C, TPC and TFC; cluster 2 was composed of the component of GC and cluster 3 was composed of the components of EA, Rut, CGA, Kae, Cat, Quar, SA, Epi-C, TPC and TFC. When the dendogram was examined (Fig. 1b), the highest similarities were observed between VA-Cat (96.99%), GA-Rut (96.92%), CGA-EpiC (86.22%), GA-SA (81.41%), VA-Res (79.71%), VA-tFA (77.80%) binary variables. Among these clusters, the GA-Rut cluster (rs = 0.964) was identified by significant (p = 0.000) and positive correlation, and the GA-SA cluster (rs = 0.857) was identified by significant (p = 0.013) and positive correlation (Table 5).
For mate tea (Fig. 1c) the closest variables were found as pCA-EpiC (98.87%) and the least similar variables were found as GA-CA with a similarity of 19.67% which was similar to black tea. The variables are represented in Fig. 1c by five basic clusters. Of these clusters; cluster 1 contained the components of GA, CGA, TPC, TFC and TAA; cluster 2 contained the components of GC and Kae; cluster 3 contained the components of CA and VA; cluster 4 contained the components of EA, p-CA, Rut, Quar, SA and Epi-C; cluster 5 contained the components of Res, Hy-CinA and Cat. When the dendogram was examined (Fig. 1c), the highest similarities were observed between binary variables as TFC-TAA (96.95%), Rut-SA (96.88%), TPC-TFC (96.25%), pCA-Quar (95.64%), VA-CA (93.56%), GA-TPC (90.17%), pCA-Rut (90.07%), HyCinA-Cat (82.13%), GA-CGA (79.16%) and Res-HyCinA (69.60%). Among these clusters, TFC-TAA (rs = 0.964) was identified with significant (p = 0.000) and positive correlation and TPC-TFC (rs = 0.964) with significant (p = 0.000) and positive correlation (Table 5).
For blackberry tea (Fig. 1d), VA-EpiC (99.56%) was observed as the closest variables and GA-CA (16.56%) were observed as the least similar variables. They are represented by five basic clusters in Fig. 1d. Of these clusters; cluster 1 was composed of the components of GA, GC, TPC and TFC; cluster 2 was composed of the components of CA, Res and SA; cluster 3 was composed of the components of VA, Quar and Epi-C; cluster 4 was composed of the components of EA, p-CA, t-FA, Rut, Hy-CinA and Cat; cluster 5 was composed of the components of CGA, Kae and TAA. When the dendogram was examined (Fig. 1d), the highest similarities were; TPC-TFC (98.31%), tFA-HyCinA (97.78%), EA-tFA (95.44%), Rut-Cat (94.78%), CA-SA (93.49%), GA-GC (92.68%), CGA- Kae (91.57%), EA-Rut (85.69%), CA-Res (81.56%), VA-Quar (81.56%) and CGA-TAA (75.78%). Among these clusters, TPC-TFC (rs = 0.964) was identified by significant (p = 0.000) and positive correlation (Table 5).
Cluster analysis for black mulberry tea (Fig. 1e) revealed the nearest variables as CGA-TAA (98.54%) and the lowest variables as GA-Res (24.41%). The variables were represented by three basic clusters in Fig. 1e. Of these clusters; cluster 1 was consisted of the components of GA, CA, EA, Hy-CinA and SA; cluster 2 was consisted of the components of GC, VA, p-CA, t-FA and Cat; cluster 3 was consisted of the components of Res, Rut, CGA, Kae, Quar, Epi-C, TPC, TFC and TAA. When the dendogram was examined (Fig. 1d), the highest similarities were observed between binary variables as CGA-TPC (96.82%), HyCinA-SA (96.73%), GC-VA (96.52%), pCA-tFA (96.26%), CGA-TFC (95.54%), CA-HyCinA (94.40%), Quar- EpiC (93.38%), GA-EA (90.66%), GC-pCA (86.70%), Rut-Kae (85.95), Res-CGA (84.43%) and GA-CA (77.91%). Among these clusters, the correlation between CGA-TPC (rs = 0.929) was significantly (p = 0.003) positive, pCA-tFA (rs = 0.955) was significantly (p = 0.001) positive, CGA-TFC (rs = 0.857) was significantly (p = 0.012) positive, Quar-EpiC (rs = 0.857) was positive (p = 0.013), GA-EA (rs = 0.882) was positive (p = 0.009), and Res-CGA (rs = 0.893) was significant (p = 0.007) (Table 5).
In the cluster study of the extracts of all tea varieties in different solvents, clustering was made in 23 steps consisting of variables with full connection clustering method according to the Euclidean distance when the dendogram in Fig. 1f was examined. In the first branch, 10 basic clusters were formed under the effect of different tea types and in the second branch 13 basic clusters were formed under different solvent effects. In the dendogram, the nearest variables were determined as EpiC-TCAT (97.71%) and the least similar variables were determined as GA-GC (27.44%). It was determined that the variables were represented by five basic clusters in the whole dendogram. Of these clusters; cluster 1 was composed of the components of GA, CGA, Cat, Quar, Epi-C, TPC, TFC, TAA, TCA and TCAT + PACIDS; cluster 2 was composed of the components of GC, EA, p-CA and t-FA; cluster 3 was composed of the components of VA and CA; cluster 4 was composed of the components of Res, Rut, Hy-CinA, Kae and AFLAV; cluster 5 was composed of the components of SA and PACIDS. When the dendogram was examined (Fig. 1e), the highest similarities were observed between binary variables as Kae-AFLAV (97.48%), GA-TFC (89.59%), EpiC-TCAT + PACIDS (87.31%), Rut-Kae (83.58%), CA-VA (82.02%), pCA-tFA (78.54%), SA-PACIDS (77.57%), GA-TPC (75.45%) and Res-HyCinA (73.42%).
Conclusions
In our study, the correlation of individual phenolics based on LPLC measurements with TPC, TFC and TAA were also considered together with spectroscopic method comparisons. When the correlation studies of similar plant origin samples in the literature were examined, it was seen that the correlation of TPC, TFC and TAA values measured by spectroscopic methods was considered. The results of this correlation studies have led us to conclude that only correlations on spectroscopic TPC, TFC and TAA analysis are insufficient, especially in complex plant materials.
Thus, the importance of chemometric approaches in the comparison of multi-component structures of complex plant resources such as tea, which was the material of our study, was emphasized in terms of providing a broad statistical perspective. The results of the research showed that solvent type and concentration significantly affected the variables analyzed. Different from the studies which based on the effect of the solvents on TPC, TFC and TAA results, the LPLC analysis also revealed the individual phenolics especially major phenolics in different tea samples. This was used to suggest the appropriate solvent to extract the phenolic compounds in every tea sample. Although different hydroalcoholic solvents were efficient in different tea samples, generally aqueous organic solvents especially EtOH 50% and MeOH 50% extracted the highest phenolics in most of the teas compared to absolute solvents and water. Because EtOH has less toxic nature and has no need of further processing, aqueous EtOH mixture may be recommended in the extraction of valuable phenolic compounds as a solvent compared to MeOH.
References
Q.D. Do, A.E. Angkawijaya, P.L. Tran-Nguyen, L.H. Huynh, F.E. Soetaredjo, S. Ismadji, Y. Ju, Effect of extraction solvent on total phenol content, total flavonoid content, and antioxidant activity of Limnophila aromatica. J. Food Drug Anal. 22, 296–302 (2014)
I.K. Bae, H.M. Ham, M.H. Jeong, D.H. Kim, H.J. Kim, Simultaneous determination of 15 phenolic compounds and caffeine in teas and mate using RP-HPLC/UV detection: method development and optimization of extraction process. Food Chem. 172, 469–475 (2015)
J. Dai, R.J. Mumper, Plant phenolics: extraction, analysis and their antioxidant and anticancer properties. Molecules 15, 7313–7352 (2010)
H. Wijngaard, M.B. Hossaın, D.K. Rai, N. Brunton, Techniques to extract bioactive compounds from food by-products of plant origin. Food Res Int 46, 505–513 (2012)
F. Chemat, M. Abert-Vian, A.S. Fabiano-Tixier, J. Strube, L. Uhlenbrock, V. Gunjevic, G. Cravotto, Green extraction of natural products. Origins, current status, and future challenges. Trends Anal. Chem. 118, 248–263 (2019)
R.A. Jacques, L.S. Freitas, V.F. Perez, C. Dariva, A.P. Oliveira, J.V. Oliveira, E.B. Caramao, The use of ultrasound in the extraction of Ilex paraguariensis leaves: a comparison with maceration. Ultrason. Sonochem. 14, 6–12 (2007)
G.J. Gil-Chavez, J.A. Villa, J.F. Ayala-Zavala, J.B. Heredia, D. Sepulveda, E.M. Yahia, G.A. Gonzalez-Aguilar, Technologies for extraction and production of bioactive compounds to be used as nutraceuticals and food ıngredients: an overview. Compr. Rev. Food Sci. F. 12, 5–23 (2013)
D. Pasrija, C. Anandharamakrishnan, Techniques for extraction of green tea polyphenols: a review. Food Bioprocess. Technol. 8, 935–950 (2015)
B. Sultana, F. Anwar, M. Ashraf, Effect of extraction solvent/technique on the antioxidant activity of selected medicinal plant extracts. Molecules 14, 2167–2180 (2009)
K.H. Musa, A. Abdullah, K. Jusoh, V. Subramaniam, Antioxidant activity of pink-flesh guava (Psidium guajava L.): effect of extraction techniques and solvents. Food Anal. Methods 4, 100–107 (2011)
T. Kuzuhara, M. Suganuma, H. Fujiki, Green tea catechin as a chemical chaperone in cancer prevention. Cancer Lett. 261, 12–20 (2008)
Q.M. Wang, Q.Y. Gong, J.J. Yan, J. Zhu, J.J. Tang, M.W. Wang, Z.J. Yang, L.S. Wang, Association between green tea intake and coronary artery disease in a Chinese population. Circ. J. 74, 294–300 (2010)
K. Yoshino, K. Yamazaki, M. Sano, Preventive effects of black tea theaflavins against mouse type IV allergy. J Sci Food Agric 90, 1983–1987 (2010)
B.N. Singh, Prateeksha, A.K.S. Rawat, R.M. Bhagat, B.R. Singh, Black tea: phytochemicals, cancer chemoprevention, and clinical studies. Crit. Rev. Food Sci. Nutr. 57(7), 1394–1410 (2017)
Y. Xu, M. Zhang, T. Wu, S.D. Dai, J. Xu, Z. Zhou, The anti-obesity effect of green tea polysaccharides, polyphenols and caffeine in rats fed with a high-fat diet. Food Funct. 6, 297–304 (2015)
C.-S. Rha, H.W. Jeong, S. Park, S. Lee, Y.S. Jung, D.-O. Kim, Antioxidative, anti-ınflammatory, and anticancer effects of purified flavonol glycosides and aglycones in green tea. Antioxidants 8, 278 (2019). https://doi.org/10.3390/antiox8080278
H. Zhang, R. Qi, Y. Mine, The impact of oolong and black tea polyphenols on human health. Food Bioscience 29, 55–61 (2019)
S. Khokhar, S.G.M. Magnusdottır, Total phenol, catechin, and caffeine contents of teas commonly consumed in the United Kingdom. J. Agric. Food Chem. 50, 565–570 (2002)
A.K. Atoui, A. Mansouri, G. Boskou, P. Kefalas, Tea and herbal infusions: their antioxidant activity and phenolic profile. Food Chem. 89, 27–36 (2005)
B. Astill, M.R. Birch, C. Dacombe, P.G. Humphrey, P.T. Martin, Factors affecting the caffeine and polyphenol contents of black and green tea infusions. J. Agric. Food Chem. 49, 5340–5347 (2001)
B.K. Panda, G. Mishra, A.K. Datta, Orthodox tea (Camellia sinensis L.) oxidation under the influence of compressed air: process optimization. J. Food Process. Preserv. 42:e13573 (2018). https://doi.org/10.1111/jfpp.13573
N.P. Seeram, S.M. Henning, Y. Niu, R. Lee, H.S. Scheuller, D. Heber, Catechin and caffeine content of green tea dietary supplements and correlation with antioxidant capacity. J. Agric. Food Chem. 54, 1599–1603 (2006)
S. Lin, E. Liu, J. Mau, Effect of different brewing methods on antioxidant properties of steaming green tea. LWT Food Sci. Technol. 41, 1616–1623 (2008)
M. Bancirova, Comparison of the antioxidant capacity and the antimicrobial activity of black and green tea. Food Res. Int. 43, 1379–1382 (2010)
A. Serpen, E. Pelvan, C. Alasalvar, B.A. Mogol, H.T. Yavuz, V. Gökmen, N. Özcan, B. Özçelik, Nutritional and functional characteristics of seven grades of black tea produced in Turkey. J. Agric. Food Chem. 60, 7682–7689 (2012)
A.B. Pomilio, S. Trajtemberg, A.A. Vitale, High-performance capillary electrophoresis analysis of mate infusions prepared from stems and leaves of Ilex paraguariensis using automated micellar electrokinetic capillary chromatography. Phytochem. Anal. 13, 235–241 (2002)
C.I. Heck, E.G. Demejıa, Yerba Mate Tea (Ilex Paraguariensis): a comprehensive review on chemistry, health ımplications, and technological considerations. J. Food Sci. 72, 138–151 (2007)
N. Grujic, Z. Lepojevic, B. Srdjenovic, J. Vladic, J. Sudji, Effects of different extraction methods and conditions on the phenolic composition of mate tea extracts. Molecules 17, 2518–2528 (2012)
J. Oszmianski, A. Wojdyło, P. Nowicka, M. Teleszko, T. Cebulak, M. Wolanin, Determination of phenolic compounds and antioxidant activity in leaves from wild Rubus L. species. Molecules 20, 4951–4966 (2015)
A.V. Patel, J. Rojas-Vera, C.G. Dacke, Therapeutic constituents and actions of Rubus species. Curr. Med. Chem. 11, 1501–1512 (2004)
R.G. Melkadze, N.Sh. Chikovani, E.Z. Kakhniashvili, Characteristics of the composition of Caucasian blackberry (Rubus caucasicus L.) leaves as a raw material for tea production. Appl. Biochem. Microbiol. 44, 647–651 (2008)
Ö. Aybastıer, E. Işık, S. Şahin, C. Demir, Optimization of ultrasonic-assisted extraction of antioxidant compounds from blackberry leaves using response surface methodology. Ind. Crops Prod. 44, 558–565 (2013)
A.V. Pavlovic, A. Papetti, D.C.D. Zagorac, U.M. Gasic, D.M. Misic, Z. Lj Tesic, M.M. Natic, Phenolics composition of leaf extracts of raspberry and blackberry cultivars grown in Serbia. Ind. Crops Prod. 87, 304–314 (2016)
S. Srivastava, R. Kapoor, A. Thathola, R.P. Srivastava, Mulberry (Moms alba) leaves as human food: a new dimension of sericulture. Int. J. Food Sci. Nutr. 54, 411–416 (2003)
S.R. Cestic, M.M. Radojkovic, A.D. Cvetanovic, P.Z. Maskovic, S.D. Durovic, Influence of steeping time on biological activity of black mulberry leaves tea. APTEFF 47, 177–191 (2016)
A. El-Mawla, K.M. Mohamed, A.M. Mostafa, Induction of biologically active flavonoids in cell cultures of Morus nigra and testing their hypoglicemic efficacy. Sci. Pharm. 79, 951–961 (2011)
I. Thabti, W. Elfalleh, H. Hannachi, A. Ferchichi, M.D.G. Campos, Identification and quantification of phenolic acids and flavonol glycosides in Tunisian Morus species by HPLC-DAD and HPLC–MS. J. Funct. Foods 4, 367–374 (2012)
A.A. Memon, N. Memon, D.L. Luthria, M.I. Bhanger, A.A. Pitafi, Phenolic acids profiling and antioxidant potential of mulberry (Morus laevigata W., Morus nigra L., Morus alba L.) leaves and fruits grown in Pakistan. Pol. J. Food Nutr. Sci. 60, 25–32 (2010)
M. Bhebhe, T.N. Füller, B. Chipurura, M. Muchuweti, Effect of solvent type on total phenolic content and free radical scavenging activity of black tea and herbal infusions. Food Anal. Methods 9, 1060–1067 (2016)
H. Metrouh-Amir, C.M.M. Duarte, F. Maiza, Solvent effect on total phenolic contents, antioxidant, and antibacterial activities of Matricaria pubescens. Ind. Crop. Prod. 67, 249–256 (2015)
Q.V. Vuong, J.B. Golding, C.E. Stathopoulos, M.H. Nguyen, P.D. Roach, Optimizing conditions for the extraction of catechins from green tea using hot water. J. Sep. Sci. 34, 3099–3106 (2011)
Ö. Algan-Cavuldak, N. Vural, M.A. Akay, R.E. Anlı, Optimization of ultrasound-assisted water extraction conditions for the extraction of phenolic compounds from black mulberry leaves (Morus nigra L.). J Food Process Eng. 42(5), e13132 (2019). https://doi.org/10.1111/jfpe.13132
J. Oh, H. Jo, A.R. Cho, S. Kim, J. Han, Antioxidant and antimicrobial activities of various leafy herbal teas. Food Control 31, 403–409 (2013)
S. Iqbal, U. Younas, Sirajuddin, K.W. Chan, R.A. Sarfraz, M.K. Uddin, Proximate composition and antioxidant potential of leaves from three varieties of Mulberry (Morus sp.): a comparative study. Int. J. Mol. Sci. 13, 6651–6664 (2012)
L. Lee, N. Lee, Y.H. Kim, C. Lee, S.P. Hong, Y. Jeon, Y. Kim, Optimization of ultrasonic extraction of phenolic antioxidants from green tea using response surface methodology. Molecules 18, 13530–13545 (2013)
R.G. Brereton, Multilevel multifactor designs for multivariate calibration. Analyst 122, 1521–1529 (1997)
D.L. Massart, B.G.M. Vandeginste, L.M. Buydens, S. de Jong, P.J. Lewi, J. Smeyers-Verbeke, Handbook of Chemometrics and Qualimetrics, vol. A (Elsevier, London, 1987)
J.S. Boeing, E.O. Barizão, B.C. Silva, P.F. Montanher, V.C. Almeida, J.V. Visentainer, Evaluation of solvent effect on the extraction of phenolic compounds and antioxidant capacities from the berries: application of principal component analysis. Chem. Cent. J. 8, 48 (2014)
N. Turkmen, F. Sari, Y.S. Velioglu, Effects of extraction solvents on concentration and antioxidant activity of black and black mate tea polyphenols determined by ferrous tartrate and Folin-Ciocalteu methods. Food Chem. 99, 835–841 (2006)
Ö.G. Üstündağ, S. Erşan, E. Özcan, G. Özan, N. Kayra, F.Y. Ekinci, Black tea processing waste as a source of antioxidant and antimicrobial phenolic compounds. Eur. Food Res. Technol. 242, 1523–1532 (2016)
N.E. Durling, O.J. Catchpole, J.B. Grey, R.F. Webby, K.A. Mitchell, L.Y. Foo, N.B. Perry, Extraction of phenolics and essential oil from dried sage (Salvia officinalis) using ethanol–water mixtures. Food Chem. 101, 1417–1424 (2007)
M. Alothman, R. Bhat, A.A. Karim, Antioxidant capacity and phenolic content of selected tropical fruits from Malaysia, extracted with different solvents. Food Chem. 115, 785–788 (2009)
J. Yu, M. Ahmedna, I. Goktepe, Effects of processing methods and extraction solvents on concentration and antioxidant activity of peanut skin phenolics. Food Chem. 90, 199–206 (2005)
K. Robards, P.D. Prenzler, G. Tucker, P. Swatsitang, W. Glover, Phenolic compounds and their role in oxidative processes in fruits. Food Chem. 66, 401–436 (1999)
R. Filip, S.B. Lotito, G. Ferraro, C.G. Fraga, Antioxidant activity of Ilex paraguariensis and related species. Nutr. Res. 20, 1437–1446 (2000)
N. Turkmen, Y.S. Velioglu, F. Sari, G. Polat, Effect of extraction conditions on measured total polyphenol contents and antioxidant and antibacterial activities of black tea. Molecules 12, 484–496 (2007)
Y.V. Yuan, D.E. Bone, M.F. Carrington, antioxidant activity of dulse (Palmira palmata) extract evaluated in vitro. Food Chem. 91, 485–494 (2005)
G.A. Akowuah, Z. Ismail, I. Norhayati, A. Sadikun, The effects of different extraction solvents of varying polarities on polyphenols of Orthosiphon stamineus and evaluation of the free radicalscavenging activity. Food Chem. 93, 311–317 (2005)
L.D. Mello, A.A. Alves, D.V. Macedo, L.T. Kubota, Peroxidase-based biosensor as a tool for a fast evaluation of antioxidant capacity of tea. Food Chem. 92, 515–519 (2004)
L. Yu, S. Haley, J. Perret, M. Harris, J. Wilson, M. Qian, free radical scavenging properties of wheat extracts. J. Agric. Food Chem. 50, 1619–1624 (2002)
H. Zielinski, H. Kozłowska, Antioxidant activity and total phenolics in selected cereal grains and their different morphological fractions. J. Agric. Food Chem. 48, 2008–2016 (2000)
M. Bajpai, A. Pande, S.K. Tewari, D. Prakash, Phenolic contents and antioxidant activity of some food and medicinal plants. Int. J. Food Sci. Nutr. 56, 287–291 (2005)
J.M. Lorenzo, P.E.S. Munekata, Phenolic compounds of green tea: health benefits and technological application in food. Asian Pac. J. Trop. Biomed. 6(8), 709–719 (2016)
R. Apak, K. Güçlü, B. Demirata, M. Özyürek, S.E. Çelik, B. Bektaşoğlu, K.I. Berker, D. Özyurt, Comparative evaluation of various total antioxidant capacity assays applied to phenolic compounds with CUPRAC assay. Molecules 12, 1496–1547 (2007)
A.J. Stewart, W. Mullen, A. Crozler, On-line high-performance liquid chromatography analysis of the antioxidant activity of phenolic compounds in green and black tea. Mol. Nutr. Food Res. 49(1), 52–60 (2005)
C.A. Rice-Evans, N.M. Miller, G. Paganda, Structure-antioxidant activity relationships of flavonoids and phenolic acids. Free Radic Biol. Med. 20, 933–956 (1996)
I.C.W. Arts, B. van De Putte, P.C. Hollman, Catechin contents of foods commonly consumed in The Netherlands. 2 Tea, wine, fruit juices, and chocolate milk. J Agric Food Chem 48, 1752–1757 (2000)
L.P. Wright, N.I.K. Mphangwe, H.E. Nyirenda, Z. Apostolides, Analysis of caffeine and flavan-3-ol composition in the fresh leaf of Camellia sinensis for predicting the quality of the black tea produced in Central and Southern Africa. J. Sci. Food Agric. 80, 1823–1830 (2000)
K.L. Bajaj, T. Anan, T. Tsushida, K. Ikegaya, Effects of (−)-picatechin on oxidation of theaflavins by polyphenol oxidase from tea leaves. Agric. Biol. Chem. 51, 1767–1772 (1987)
E. Nishitani, Y.M. Sagesaka, Simultaneous determination of catechins, caffeine and other phenolic compounds in tea using new HPLC method. J. Food Compos. Anal. 17, 675–685 (2004)
T.F.F. Da Silveira, A.D. Meinhart, C.A. Ballus, H.T. Godoy, The effect of the duration of infusion, temperature, and water volume on the rutin content in the preparation of mate tea beverages: an optimization study. Food Res. Int. 60, 241–245 (2014)
T.F.F. Da Silveira, A.D. Meinhart, T.C.L. De Souza, E.C.E. Cunha, M.R. de Moraes, H.T. Godoy, Chlorogenic acids and flavonoid extraction during the preparation of yerba mate based beverages. Food Res. Int. 102, 348–354 (2017)
E.M. Sánchez-Salcedo, P. Mena, C. García-Viguera, F. Hernández, J.J. Martínez, (Poly)phenolic compounds and antioxidant activity of white (Morus alba) and black (Morus nigra) mulberry leaves: their potential for new products rich in phytochemicals. J. Funct. Foods 18, 1039–1046 (2015)
S. Arabshahi-Delouee, D.V. Devi, A. Urooj, Evaluation of antioxidant activity of some plant extracts and their heat, pH and storage stability. Food Chem. 100, 1100–1105 (2007)
A. Djeridane, M. Yousfi, B. Nadjemi, D. Boutassouna, P. Stocker, N. Vidal, Antioxidant activity of some Algerian medicinal plants extracts containing phenolic compounds. Food Chem. 97, 654–660 (2006)
B.S. Bizzoto, A.D. Meinhart, A.C.P. Rybka, M.R. Sobrinho, S.B. Junior, C.A. Ballus, H.T. Godoy, Quantification of phenolic compounds by capillary zone electrophoresis in extracts of four commercial types of mate herb before and after acid hydrolysis. Food Res. Int. 48, 763–768 (2012)
M. A. Rostagno, N. Manchón, M. D’Aarrigo, E. Guillamón, A. Villares, A. García Lafuente, Fast and simultaneous determination of phenolic compounds and caffeine in teas, mate, instant coffee, soft drink and energetic drink by high-performance liquid chromatography using a fused-core column. Anal. Chim. Acta 685, 204–211 (2011)
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
No potential conflict of interest was reported by the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Vural, N., Algan Cavuldak, Ö., Akay, M.A. et al. Determination of the various extraction solvent effects on polyphenolic profile and antioxidant activities of selected tea samples by chemometric approach. Food Measure 14, 1286–1305 (2020). https://doi.org/10.1007/s11694-020-00376-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11694-020-00376-6