Abstract
Citrullus lanatus seed is a potential source of edible protein, hence effective extraction of protein components plays a vital role in food application. Therefore, this study aims for optimization and hydrolysis of protein using single factor and Box Behnken design (BBD). Various concentrations of the protein hydrolysates were studied for antioxidant activity using different assays. The one factor test revealed that important parameters such as alkali concentration (0.8%), temperature (40 °C), time (30 min) and solid to alkali ratio (1:30) enhance the protein concentration from C. lanatus (903.1 mg/g). The maximum degree of hydrolysis 39.30% was achieved at pH 2.4, an enzyme to substrate ratio 3% (w/w), and hydrolysis time 180 min by using BBD design. C. lanatus protein hydrolysate showed 15.71 and 61.67% of DPPH and ABTS radical scavenging activity at 5 mg/ml. It exhibited reducing power optical density 0.18593 nm and metal chelating activity of 42.69% at the concentration of 25 mg/ml. This implied that C. lanatus could be used as a natural antioxidant agent.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The global statistical analysis has revealed that around 50% of Indian children suffer from under nourishment [1]. The last accessible national data specifies that 48% under five children are stunted, 42.5% are underweight [2]. According to World Bank [3], India has a higher stunting rate compared to other countries in South Asia facing similar or worse economic crisis. The major nutritional obstacle in the developing world is a protein deficiency (Marasmus and kwashiorkor). Enlargement of present agricultural practices into marginal lands is projected to solve this chronic protein scarcity. Hence search for natural edible protein derivatives from plants is the need of the hour. Significant pharmacology and medicinal values were found in seed dietary proteins which are reported as bioactive protein [4, 5].
Citrullus lanatus (watermelon) seeds are highly nutritive and used in the treatment of diseases such as cancer, cardiovascular, blood pressure and have also been used as a home therapy for edema and urinary tract infections [6]. According to the study of Sonawane and Arya, the defatted watermelon seeds are rich in protein, and has considerable amount of minerals viz., Na, Mn, Mg, K, Cu, Fe, and Zn and essential amino acids (isoleucine, leucine, lysine, histidine, methionine, phenylalanine, threonine, tryptophan, and valine) [7].
To the best of our knowledge, very limited literature is available on preparation of protein from C. lanatus seed. The target of the present study was to enhance the extraction factors for C. lanatus seed protein and hydrolysis conditions using response surface methodology (RSM)- BBD in order to obtain a high degree of protein hydrolysate as well as to evaluate its potential as an antioxidant agent to be used in food formulation.
Materials and methods
Chemicals
1,1-Diphenyl-2-picrylhydrazyl (DPPH), 2,2-azino-bis (3-ethylbenzothiazoline-6-sulphonic acid) diammonium salt (ABTS), methanol, ethanol, potassium persulphate,ferrous chloride, 3-(2-pyridyl)-5,6-diphenyl-1,2,4-triazine-4,4-disulphonic acid sodium salt (ferrozine), glutathione(GSH), EDTA, trolox, were purchased from Sigma Chemicals Co. (St. Louis, MO, USA). All other chemicals and reagents used in the present study were of analytical grade. Pepsin, Papain and protease were gift samples from Advanced Enzymes India Pvt Ltd. (Mumbai, India). Trypsin and Chymotrypsin were procured from Hi media (Mumbai, India).
Sample and preparation of defatted C. lanatus seed flours
Decorticated C. lanatus seeds were purchased from a local market in one lot (APMC market, Navi Mumbai, India) in March 2013. These dried seeds were stored at −20 °C until their final usage to prevent spoilage and to maintain uniformity in the quality throughout the entire research study.
The fat was extracted by using petroleum ether from seed flour at room temperature with stirring for a period of 6 h. The solvent was separated and process was repeated thrice to remove the fat. The procedure was repeated thrice for separating the fat from the solvent. The solvent was decanted after each extraction and the flour was air-dried at room temperature. Further, it was ground and sieved using through 40 mesh sieves (0.425 mm according to Indian standard). All resulting flours were packed into clean airtight polyethylene bags and kept at 4 °C until utilization.
Optimization of extraction parameter for defatted C. lanatus seed protein
The defatted C. lanatus seed powder was suspended in extraction solution (alkali) and subsequently incubated at designated time, temperature and buffer-to-sample ratio. The resulting slurry was then centrifuged at 12,000 rpm for 30 min at 4 °C after incubation. The supernatant was collected and the extracted protein content of the sample was determined using Bradford assay [8]. The extraction yield was expressed as mg protein per gram of flour sample. The effect of extraction parameters (i.e. extraction time, temperature and buffer-to-sample ratio) was optimized by single factor experiment and is detailed in Table 1. The obtained supernatant was further precipitated at pH 4.5 and washed thrice with distilled water and dried at 40 °C in vacuum oven.
Enzymatic hydrolysis of C. lanatus protein using one factor
Six proteases (papain, pepsin, protease, pancreatin, trypsin, and chymotrypsin) were screened for enzymatic hydrolysis, in which pepsin showed the highest degree of hydrolysis (DH) (data not shown) was selected to optimize the optimal condition for hydrolysis. C. lanatus proteins were suspended in distilled water at a concentration of 1% (w/v) and the conditions for enzymatic hydrolysis with pepsin were optimized. The pH for hydrolysis ranged between 2 and 3, an enzyme to substrate ratio (ES) was 0.5–3% (w/w) and hydrolysis time varied from 30 to 180 min. During hydrolysis, pH was maintained and the reaction was carried out at 37 °C. Enzymes were inactivated by keeping the hydrolysate in a water bath at 100 °C for 10 min. Further the hydrolysates were centrifuged at 10,000 rpm for 20 min, and this supernatant was used for measuring DH as mentioned below.
Degree of hydrolysis
The DH was determined by the Hoyle and Merritt’s [9] TCA method with the slight modifications. In the first part, 500 μl of hydrolyzed protein samples were mixed with equal amount i.e. 500 μl of 20% TCA solution. The mixture was allowed to rest for 30 min. After 30 min, samples were centrifuged at 10,000 rpm for 20 min.. The soluble protein content of the supernatant was determined by the method of Lawry et al. [10]. Results were expressed as the mg of protein. Finally, DH was determined using following equation:
Experimental design
RSM was useful to predict the optimal hydrolysis conditions of C. lanatus by using pepsin, which could produce the hydrolysate with the highest DH. A three-level-three-factor Box-Behnken design using Design-Expert1 6 software (Stat-Ease Inc., Minneapolis, USA) provides 17 experiments [11] as shown in Table 2. Based on the commercially labeled suitable enzymatic hydrolysis conditions of pepsin, the three levels of the selected variables were: pH (2.0, 2.2 and 2.4), ES (2, 2.5 and 3%) and time (120, 150 and 180 min). Each enzymatic hydrolysis experiment was measured according to the DH and the experimental results are shown in Table 2.
The experimental data obtained were fitted by the following regression equation:
where Y is the predicted response of DH, β0, β1, β2, β3…β33 are the regression coefficients, X1, X2 and X3 represent pH, the ratio of ES and time respectively.
This is a square regression model in terms of real values. Analysis of parameters of the equation (Eq. 2) as well as the experimental design and calculation of projected data were approved by using Design-Expert1 6 software to assessment the response of the independent variables.
Determination of molecular weight of peptides
The molecular weight of peptides formed during hydrolysis was determined by the SDS-PAGE. For electrophoresis, 30% acrylamide-bis-acrylamide solution mixture was used for the preparation of 4% stacking gel and 15% resolving gel. 10–20 μl of sample mixed with the sample buffer was loaded into the well. Gel was run at 60 V for approximately 90–120 min. Silver staining was performed for visualizing the protein bands.
Antioxidant studies of C. lanatus seed protein hydrolysate
The optimal conditions provided by the BBD were used to produce protein hydrolysate from wood apple. The hydrolysate was centrifuged at 10,000 rpm for 20 min at 4 °C and the supernatant was dried in vacuum oven at 40 °C.
ABTS free radical scavenging assay
Antioxidant activity was measured using Hitachi Spectrophotometer with the improved ABTS method [12, 13]. The ABTS reagent was prepared freshly and used within 3 days. The reagent was made by mixing 7 mM ABTS and 2.45 mM potassium persulfate. This reagent was incubated for 16–17 h at 37 °C. The ABTS cations were diluted with 0.01 M Phosphate buffer saline to set optical density at 0.7 (± 0.02) at 734 nm. 260 µl (absorbance of 0.700 ± 0.02) of ABTS was added to the 40 µl of the hydrolyzed sample and mixed thoroughly and absorbance was measured at 734 nm immediately after 6 min. Results were expressed in terms of percentage inhibition.
\(\% {\text{ Inhibition}}\; = 1 - \frac{{A{}_{s}}}{{A_{c} }} \times 100\)where Ac = absorbance of control, As = Absorbance of sample.
DPPH free radical scavenging assay
The ability to scavenge DPPH free radicals was determined based on the method [13, 14] with little modification in the mixture of test sample concentration and DPPH concentration. 40 µl of sample mixed with 120 µl of methanol which further mixed with 40 µl of DPPH (0.15 mM) prepared in methanol and kept for 15 min incubation in dark room and absorbance was measured at 517 nm. Results were expressed in terms of percentage inhibition.
\({\text{DPPH Scavenging activity}}\; = \frac{{\left( {Absorbance} \right)_{{t = 0}} - (Absorbance)_{{~t = 15~}} }}{{(Absorbance)_{{~t = 0}} }} \times 100\).
Radical formation by ferric reducing antioxidant power (FRAP)
Ferric reducing antioxidant power (FRAP) assay was developed by Benzie and Strain [15], that is in the mixture of test sample concentration and FRAP reagent concentration. Firstly, FRAP reagent was prepared by mixing the following solutions: tenfold 300 mM Acetate buffer + onefold TPTZ (10 mM in 40 mM HCl) + onefold FeCl3 (20 mM). This 200 µl of FRAP reagent was added to the 20 µl of the sample which shaken vigorously and absorbance was read at 593 nm after incubation of 15 min at 37 °C. Results were expressed in terms of absorbance.
Metal chelation activity
In the chelation test, 50 µl of peptide and entrapped peptide solution were mixed with 10 µl of FeCl2 (2 mM) and 250 µl of double distilled water. Subsequently, 20 µl of ferrozine solution (5 mM) were added to the mixture, followed by vigorous mixing for 2 min. The mixture was then kept for 10 min at room temperature. Further, the color reduction, due to the chelation of Fe2+ was recorded by measuring the absorbance at 562 nm. The control sample contained 300 µl of double distilled water, 10 µl of FeCl2 and 20 µl of ferrozine solution (5 mM) [16]. The chelating activity was calculated in percentage using
Statistical analysis
All determinations obtained from triplicate measurements and expressed as a mean ± standard deviation. The Statistical Package for Social Sciences (SPSS) for Windows version (16.0) was used to analyze the data (SPSS Inc., Chicago, IL). Statistical significance was declared at p < 0.05.
Results and discussion
Optimization of process parameter for extraction of protein from defatted C. lanatus seed
The effect of four independent variables such as alkali concentration, a buffer to sample ratio, pH, temperature, and extraction time on the protein yield was studied. Generally, each factor has an obvious effect on the protein content. During protein extraction, these parameters significantly affect the protein extractability [17].
Figure 1a explains the role of alkali in the extraction of protein. In case of C. lanatus, the high content of protein was observed at 0.8% of alkali. No significant improvement in the extraction of protein was observed in presence of 0.8% alkali.
The role of temperature on the concentration of protein was shown in Fig. 1b. Generally, the protein is sensitive to temperature. The increase in temperature significantly helps in enhancement of the protein in alkali extractions. This was due to higher temperature raises the solubility of the protein. As the temperature ascended to 45 °C, the protein content decreased due to the lower solubility of protein due to protein denaturation. The effect of temperature on peanut protein yield and the optimal temperature was 36.35 °C [18]. Hence, in this study the 40 °C was peak temperature for extraction of protein.
Figure 1c demonstrates the effect of extraction time on the protein content of defatted C. lanatus seed flours. In the case of alkali C. lanatus seed flour; the protein content increased upto 30 min and had no significant extraction after 30 min, this result indicated that a longer extraction time did not improve the protein extraction efficiency. Hence optimal extraction time for alkali C. lanatus seed was 30 min.
Influence of the solid-to-liquid ratio on the protein content is showed in Fig. 1d. The protein content was significantly high at 1:30 ratio for C. lanatus seed. There was no significant difference between the protein concentration of C. lanatus seed as 1/30, 1/40 and 1/50 ratios increased in the solid to alkali ratio.
From the above single factor test, it was observed that maximum total protein content achieved from C. lanatus was 903.1 mg/g of flour, which was obtained by optimizing extraction condition such as alkali concentration (0.8%), temperature (40 °C), time (30 min) and solid to alkali ratio (1:30). This may be due to the high alkali concentration, which is responsible to inhibit the common activity of protease and causes ionization of phenolic compounds and also prevent the forming hydrogen bonding with protein [19].
Optimization of process parameters for hydrolysis of protein from defatted C. lanatus seed
In protein hydrolysates, average peptide chain length (PCL) is produced with desired functional properties which can be calculated from the inverse of the DH value, which means that higher the DH value; the smaller will be PCL [20]. Figure 2a demonstrates effect of pH on hydrolysis of C. lanatus protein. As pH increased from 2 to 2.4, there was no significant difference observed, but after pH 2.4 it decreased significantly as compared to pH 2, 2.2 and 2.4. Hence, this range (2–2.4) of pH was designated for BBD.
The effect of enzyme concentration on hydrolysis of C. lanatus proteins was shown in Fig. 2b. No signficant increase in hydrolysis upto 1.5% concentration was observed. Further it increased upto 2.5% and no significant difference between 2.5 and 3% was observed. Hence, 2–3% (w/w) enzyme concentration was selected for further optimization. There was no significant difference in hydrolysis time upto 90 min (Fig. 2c), further DH increased as time increased. Therefore, we used the steepest range i.e. 120–150 min in the optimization study. This could be due to the fact that polypeptide chain will release to the surface and begin to hydrolyze the protein particles slowly due to the interaction of proteolytic enzyme rapidly with insoluble protein particles. Thus, the more squeezed core of proteins would cut more slowly [21]. Our results confirmed that hydrolysis was affected by hydrolysis time and enzyme concentration.
Response surface methodology
The effects of three hydrolysis parameters: pH (X1), ES (X2) and time (X3), on the DH of C. lanatus protein, were studied using a BBD design. The range of each factor was selected on the basis of single factor experiment. The DH (which was expressed as %) was selected as the response of interest. Table 2 shows the experimental conditions design in a total of 17 runs, along with the experimental outcomes obtained. The experimental DH was ranged from 25.03 to 42.14% under different tested conditions. The DH varied depending on the given hydrolysis conditions.
Model fitting
We examined the effect of parameters such pH, E/S ratio and time on the DH value by using RSM. The experimental design and results are shown in Table 2. According to the model regression analysis by eliminating model factors with p > 0.05, the best explanatory model equation fitted into Eq. (1) is given in Eq. (4) which corresponds to DH (%).
Analysis of variance (ANOVA) was achieved to evaluate the significance of the coefficient of the models. The ANOVA presented in Table 3 which shows that quadratic model was significant (p < 0.05) and Eq. 4 represent for DH which fitted quadratic model. The significance of each coefficient was determined using the F value and p value. The corresponding variables would be more significant if the absolute F value becomes greater and the p value becomes smaller.
Results revealed that the largest effect on the DH was a linear term of the ES ratio (X2) followed by a quadratic term of the ES ratio (X2 2), the linear term of hydrolysis time (X3) and quadratic term of hydrolysis time (X3 2). The linear terms of pH (X1 and X1 2) and other interaction terms of X1X2, X1X3 and X2X3 were insignificant (p > 0.05).The insignificant p value of 0.8619 (p > 0.05) was shown in Table 3, indicating that the selected model is fitted well and was in agreement with the observed data. The coefficient of determination value (R2 = 0.9096) indicated that the fitted model could explain 90.96% of the variation in the data. A high degree of correlation was observed between Adj R2 (of 0.79) and Pred R2 (0.66). The coefficient of variation (CV) represents the ratio of standard deviation to the mean which shows the extent of variability in the data. This model had a CV of 5.95% suggesting a good precision and high reliability of the experiment.
Interpretation of response surface model
Three-dimensional response surfaces and contour plots of the models are generally used for graphical representation of the regression equation to determine the optimum values of the variables within the given range. Response surfaces with a contour (at the base) plot showed the interaction of two independent variables when another variable was fixed at zero. The maximum predicted value was indicated by the surface confined in the smallest ellipse in the contour diagram. The elliptical contours are obtained when there is a perfect interaction between the independent variables. Figure 3 present the response surface curves established for the DH in this study.
Figure 3a shows the effect of the interaction between E/S and pH on the DH of the hydrolysates of C. lanatus seed protein at any constant hydrolysis time. Increased in DH as the concentration of enzyme was an increase, whereas pH increased DH increased upto pH 2.2 beyond that slightly decreased in DH. Figure 3b depicts the effect of the interaction between time and pH on the DH of the hydrolysates of C. lanatus seed protein at any constant ES. Increased in DH as the hydrolysis time was an increase, whereas pH increased DH increased upto pH 2.2 beyond that slightly decreased in DH. Whereas Fig. 3c noted that DH increased with increase in ES and hydrolysis time.
Verification of predictive models
Confirmation of the model was accomplished in order to validate its suitability in the experiment. A combination of hydrolysis parameters was suggested to optimize the DH taking into account of the efficiency and the feasibility of the experiment. The optimal conditions for the predicted target DH 42.14% corresponded to pH 2.4, an ES ratio 3% (w/w), and hydrolysis time 180 min. The experimental value of 39.30 ± 2.32 (%) was found close to the predicted value. Hence, the model is valid to be used in optimizing the process.
Molecular weight distribution of C. lanatus protein hydrolysates
Polypeptide band pattern was observed in C. lanatus protein hydrolysates shown in Fig. 4. C. lanatus protein hydrolysates composed of protein bands with a molecular weight between 22 and 95 kDa, where five peptide bands of them are characterized with low molecular weight ranging from 22 to 25 kDa. Previous reports also have found that pepsin is capable of producing peptides from other food proteins [22].
Antioxidant activity of C. lanatus protein hydrolysates
Due to the possible irregularity of different radical systems used for antioxidant assessment, it is widely suggested that two or more methods should be applied to study the radical-scavenging activities. In the present study, therefore, the primary antioxidant potential was measured by DPPH, FRAP and ABTS assays. The secondary antioxidant potential was measured by the metal chelating assay, which demonstrates the inhibitory effect on the generation of radicals. In this study, the antioxidant activities of the hydrolysates were investigated and compared with that of Trolox, a widely used synthetic antioxidant.
ABTS radical-scavenging activity
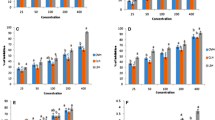
Antioxidant activity of both lipophilic and hydrophilic molecules was analyzed by using ABTS de-colorization assay that is based on the reaction of hydrogen donating antioxidants with the ABTS radical, which is intensely colored and is determined by measuring absorbance at 734 nm. ABTS scavenging activity by protein hydrolysate was increased as the concentration of peptides increased (shown in Table 4). 3 mg/ml of protein hydrolysate shows 45.79% of scavenging activity which was closed to 20 µg/ml of Trolox that is 46.2%. Antioxidant capacity of ber peptides reported by different enzyme such as alcalase (8.09 µM of TE/g), papain (9.14 µM of TE/g), protease (8.92 µM of TE/g) which correspond to DH of alcalase (45.52%), papain (40.95%), and protease (47.26%) respectively [23]. There was difference in the inhibition of DPPH and ABTS radical by C. lanatus protein hydrolysates. This may be due to the DPPH assay that was carried out in organic media, while the ABTS assay was performed in aqueous media. Thus, the contradictory phenomenon between the radical scavenging activity for DPPH and ABTS radicals could be due to the difference of solubility and diffusivity of radicals and peptides in the reaction system [24].
DPPH radical-scavenging activity
The maximum absorbance of DPPH (stable free radical) shows at 517 nm. When DPPH radicals come across a proton-donating substrate such as an antioxidant, the radicals are scavenged with decrease in the absorbance [25].
Radical scavenging activity is measured by reduction in the absorbance. Thus, the DPPH radicals were widely used to examine the scavenging activity of natural compounds. Table 4 represents the DPPH radical-scavenging activity of the C. lanatus protein hydrolysates at various concentrations. The results clearly indicated that the C. lanatus protein hydrolysates have DPPH radical scavenging activity at high concentration that was 1–5 mg/ml as compared to standard Trolox which were 2–10 µg/ml (shown in Table 4). Generally, the accumulation of shorter peptides and amino acids could make the hydrolysate more hydrophilic. The increased polarity of the low-MW protein hydrolysate makes them more difficult to react with the hydrophobic [26].
FRAP
In the FRAP assay, the presence of reducing agents in the tested samples results in the development of the complex tripiridiltriazine (TPTZ)–Fe(II), which is monitored at 595 nm. Table 5 shows that as the concentration of protein hydrolysate increases the absorbance increases at 593 which were comparably low to Trolox. 25 mg/ml of protein hydrolysate shows the absorbance 0.18 which corresponds to 0.22 absorbance at 10 µM/ml of Trolox. Absorbance increases as the concentration of protein hydrolysates and Trolox increases which are due to the peptide cleavages that make the availability of hydrogen ions [27].
Metal chelating ability
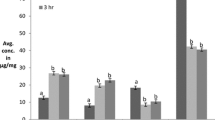
The ferrous ion chelating ability of hydrolysates is shown in Table 5. The high chelating effect was observed at high concentration of hydrolysates that is 5–25 mg/ml. 24.67% of chelating activity shown by 5 mg/ml of hydrolysates which corresponds to 0.4 mg/ml of Trolox. It is well-known that transition metal ions such as iron or copper may catalyze the formation of reactive oxygen species (ROS) that accelerates lipid oxidation. Carboxyl and amino groups in the side chains of the acidic (Glx, Asx) and basic (Lys, His, Arg) amino acids are thought to play an important role in chelating metal ions [28].
The difference in the antioxidant activity obtained with different assay could be due to the different reaction mechanisms involved. The FRAP assay detects compounds that act only by the single electron transfer mechanism, whereas ABTS assay detects compounds that act either by direct reduction via electron transfer or by radical quenching via the hydrogen atom transfer mechanism [29]. Proteins and their hydrolysates generally possess significant antioxidant capacity [30]. It is postulated that the capacity comes from their ability to inactivate ROS, quench free radicals, chelate pro-oxidative transition metals, and donate electron/hydrogen. The amino acid composition and sequence, the size and the configuration are reported to be the main factors influencing on the antioxidant properties of proteins and their hydrolysates [31]. Antioxidant abilities of the peptides are thought to be due to their amino acid composition and hydrophobicity [32]. Some amino acids, such as Tyr, Lys, Arg, Gly, Leu, and His had been reported to exhibit antioxidant ability [33]. Especially, histidine residue showed high radical scavenging ability due to the decomposition of its imidazole ring [34].
Conclusion
In summary, the study of single factors (i.e. alkali concentration, temperature, time and solid to alkali ratio) had an impact on the protein extraction from C. lanatus. BBD design was used to optimize the hydrolysis process from C. lanatus protein on the basis of the single factor test. The predicted profile and the regression equation showed that the optimum conditions for protein hydrolysis were a DH 39.30% corresponded to pH 2.4, an ES ratio 3% (w/w), and hydrolysis time 180 min. Through the test data analysis and confirmative test, the experimental result is consistent with the predicted value. Therefore, the response model can be widely used for hydrolysis of protein. This investigation confirms that C. lanatus protein hydrolysate may be a valuable source of antioxidative peptides. This may forecast their future application into functional foods or dietary supplements.
References
UNICEF, The state of the world‘s children 2012: children in an urban world. (United Nations Children‘s Fund. UNICEF, New York, 2012)
National Family Health Survey (NFHS), NHFS 3, 2005–06. (Indian Institute of Population Sciences, Mumbai, 2007)
World Bank, Nutrition country profiles: Nutrition at a Glance (India), http://siteresources.worldbank.org/NUTRITION/Resources/2818461271963823772/India.pdf (2010)
R.J. Henry, P. Kettlewell, Cereal Grain Quality. (Chapman and Hall, London, 1996)
M. Duranti, A. Consonni, C. Magni, F. Sessa, A. Scarafoni, The major proteins of lupin seed: characterisation and molecular properties for use as functional and nutraceutical ingredients. Trends Food Sci. Technol. 19, 624–633 (2008)
M.S. Teotia, P. Ramakrishna, Chemistry and technology of melon seeds. J. Food Sci. Technol. 21, 332–340 (1984)
S.K. Sonawane, S.S. Arya, Nutritional, functional, thermal and structural characteristics of Citrullus lanatus and Limonia acidissima seed flours. Food Meas. 10(1), 72–79 (2016)
M.M. Bradford, A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72, 248–254 (1976)
N.T. Hoyle, J.O.H.N. Merritt, Quality of fish protein hydrolysates from herring (Clupea harengus). J Food Sci 59(1), 76–79 (1994)
O.H. Lowry, N.J. Rosebrough, A.L. Farr, R.J. Randal, Protein analysis with Folin-phenol reagent. J. Biol. Chem. 193, 265–275 (1951)
G.E.P. Box, N.R. Draper, Empirical Model-Building and Response Surfaces (John Wily and Sons, New York, 1987), p. 249
R. Re, N. Pellegrini, A. Proteggente, A. Pannala, M. Yang, C. Rice-Evans, Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 26, 1231–1237 (1999)
S. Sonawane, S.S. Arya, Antioxidant activity of jambhul, L acidissima, ambadi and ambat chukka: an indigenous lesser known fruits and vegetables of India. Adv. J. Food Sci. Technol. 5(3), 270–275 (2013)
S. Sahreen, M. Khan, R. Khan, Evaluation of antioxidant activities of various solvent extracts of Carissa opaca fruits. Food Chem. 122, 1205–1211 (2010)
I.F.F. Benzie, J.J. Strain, The ferric reducing ability of plasma as a measure of “antioxidant power”: the FRAP assay. Anal. Biochem. 239, 70–76 (1996)
E.A. Decker, B. Welch, Role of ferritin as a lipid oxidation catalyst in muscle food. J. Agric. Food Chem. 38, 674–677 (1990)
A.A. Wani, D.S. Sogi, L. Grover, D.C. Saxena, Effect of temperature, alkali concentration, mixing time and meal/ solvent ratio on the extraction of watermelon seed proteins—a response surface approach. Biosyst. Eng. 94, 67–73 (2006)
T.Z. Ma, Q. Wang, H.W. Wu, Optimization of extraction conditions for improving solubility of peanut protein concentrates by response surface methodology. LWT–Food Sci. Technol. 43, 1450–1455 (2010)
D.F. Hochstrasser, M.G. Harrington, A.C. Hochstrasser, M.J. Miller, C.R. Merril, Methods for increasing the resolution of two-dimensional protein electrophoresis. Anal. Biochem. 173(2), 424–435 (1988)
J. Adler-Nissen, Enzymic Hydrolysis of Food Proteins (Elsevier Applied Science, London, 1986)
S. Benjakul, M.T. Morrissey, Protein hydrolysates from Pacific whiting solid wastes. J. Agric. Food Chem. 45(9), 3423–3430 (1997)
K. Suetsuna, T. Nakano, Identification of an antihypertensive peptide from peptic digest of wakame. J. Nutr. Biochem. 11, 450–454 (2000)
K.D. Kanbargi, S.K. Sonawane, S.S. Arya, Functional and antioxidant activity of Ziziphus jujube seed protein hydrolysates. Food Meas. 10, 226 (2016)
M. B. Arnao, Some methodological problems in the determination of antioxidant activity using chromogen radicals: a practical case. Trends Food Sci. Technol. 11, 419–421 (2000)
K. Shimada, K. Fujikawa, K. Yahara, T. Nakamura, Antioxidative properties of xanthan on the autioxidation of soybean oil in cyclodextrin emulsion. J. Agric. Food Chem. 40, 945–948 (1992)
L.J. Zhu, J. Chen, X.Y. Tang, Y.L. Xiong, Reducing, radical scavenging, and chelation properties of in vitro digests of alcalase treated zein hydrolysate. J. Agric. Food Chem. 56, 2714–2721 (2008)
B.H. Kong, Y.L. Xiong, Antioxidant activity of zein hydrolysate in liposome system and thepossible mode of action. J. Agric. Food Chem. 54, 6059–6068 (2006)
A. Saiga, S. Tanabe, T. Nishimura, Antioxidant activity of peptides obtained from porcine myofibrillar proteins by protease treatment. J. Agric. Food Chem. 51, 3661–3667 (2003)
R.L. Prior, X. Wu, K. Schaich, Standardized method for the determination of antioxidant capacities and phenolics in foods and dietary supplements. J. Agric. Food Chem. 53, 4290–4302 (2005)
R.J. Elias, S.S. Kellerby, E.A. Decker, Antioxidant activity of proteins and peptides. Crit Rev. Food Sci. Nutr. 48(5), 430–441 (2008)
H.M. Chen, K. Muramoto, F. Yamauchi, K. Nokihara, Antioxidant activity of designed peptides based on the antioxidative peptide isolated from digests of a soybean protein. J. Agric. Food Chem. 44(9), 2619–2623 (1996)
B.H. Sarmadi, A. Ismail, Antioxidative peptides from food proteins. Peptides 31, 1949–1956 (2010)
Z. Xie, J. Huang, X. Xu, Z. Jin, Antioxidant activity of peptides isolated from alfalfa leaf protein hydrolysate. Food Chem. 111, 370–376 (2008)
J.S. Wang, M.M. Zhao, Q.Z. Zhao, M. Y., Jiang,. Antioxidant properties of papain hydrolysates of wheat gluten in different oxidation systems. Food Chem. 101, 1658–1663 (2007)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sonawane, S.K., Arya, S.S. Citrullus lanatus protein hydrolysate optimization for antioxidant potential. Food Measure 11, 1834–1843 (2017). https://doi.org/10.1007/s11694-017-9565-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11694-017-9565-7