Abstract
Arthrospira platensis (Spirulina) is a widely known microalga with nutritional and therapeutic applications due to its richness in nutrients and bioactive elements. The aim of this research was to characterize the phytochemical and nutritional compounds of five different Spirulina strains from the USA, Chad and Algeria. Concentrations of carbohydrates and fats, fatty acid profile, and the total proteins were determined through biochemical and chromatographic methods. The concentration of phytochemical compounds was determined by using UV–Vis spectrophotometry. The Spirulina from the USA had the highest amounts of lipids, proteins, phycocyanin and chlorophyll a content compared to the others strains tested. The strains from Algeria had the highest amount of carotenoids (5.50 mg/g) among the strains investigated. In all the strains, the main fatty acid compound was the palmitic acid (16:0) with the highest concentration of 71.15% in the Spirulina from Chad. All the Spirulina strains tested contained high quantities of ω-6-fatty acids (avg. 24%) except in the Spirulina strain from Chad, which had only 10.59%. All the Spirulina strains investigated were rich in polyphenols with the highest value of 67.52 mg GAE/g in the strains from Algeria. The Spirulina from the USA has more potential to be used in the nutraceutical and a functional food area since it is very rich in bioactive compounds.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The Arthrospira platensis (commercial name ‘Spirulina’) is a phototrophic, filamentous and multicellular blue-green microalga which belongs to the Phylum Cyanobacteria. It converts sunlight, water and carbon dioxide into algal biomass. It is an important source of nutrients such as proteins, minerals, carbohydrates, and many phytopigments that can be used as food supplements. In addition, this microalga cells have high digestibility due to the lack of cellulose which facilitates their use for human consumption [1–3]. The most important species used for consumption are Spirulina maxima and Arthrospira platensis (Spirulina) [4, 5]. The Spirulina is produced naturally in alkaline water of volcanic lakes and warm brackish waters. However, this alga could also be grown for higher biomass production, under controlled conditions in saline water (>30 g/L) at a pH in the range of 8.5–11.0 with high levels of solar radiation (2500 Lux) and at temperatures in the range of 35–39 °C [2, 6, 7]. The current production of Spirulina in the world is estimated at 3000 metric tons and more than 70% of it is marketed for human consumption, mainly as health food [7]. The Spirulina is mostly consumed as tablets, capsules and powder or added into various kinds of foods like pasta, cakes and health drinks which are used as nutritional supplements or as natural colorants [8].
Spirulina is well known for its high content of proteins (60–70%), vitamins such as vitamin B12 (8 ppm) and pro-vitamin A (0.2%), minerals such as iron (0.1%), and polyunsaturated fatty acids especially the ω-6-fatty acids (up to 29.4–31.5% of the total fatty acids) [5, 9–11]. In addition to its nutrients richness, it is also a source of phytopigments like chlorophylls, carotenoids and phycobiliproteins, which are the light harvesting pigments in Spirulina. The phycobiliproteins that can be obtained from Spirulina are phycoerythrin (PE), allophycocyanin (A-PC) and phycocyanin (C-PC). However, several studies showed that the C-PC is the main phycobiliprotein in this microalga and it contains up to 50% of the phycobiliproteins in it [12–14].
The phytopigments play an important role in the photosynthetic metabolism of Spirulina and have anti-obesity, anti- inflammatory, anti-carcinogenic, antioxidant and neuroprotective effects on human health [15–17]. These effects have been attributed to the presence of components such as phycobiliproteins, phenolic compounds, beta-carotene and polyunsaturated fatty acid (PUFA) in the Spirulina [7, 18–20]. Mühling et al. [11] reported the significant difference in the fatty acid composition especially in the PUFA fraction, among the Spirulina strains. This difference in fatty acid composition is due to the differences in the growing conditions that have an influence on the fatty acids content of the algal strains [11]. In addition, many authors have reported that Spirulina is one of the best whole food sources for ω-6-fatty acids [7].
Many strains of Arthrospira have been identified as having different shapes: spiral, straight and wavy, under different culture conditions [21]. However, only a few of these strains are commercially exploitable due to their different productivities and qualities. Also, the available data on the nutrient composition and bioactive compounds of Spirulina are highly varied, and the values are dependent on the growing, harvesting and drying conditions of the microalgae. Hence, information on the phytochemical and biochemical composition of Spirulina is important for their use as food supplements. Therefore, this study compared and characterized the biochemical compositions of different A. platensis strains from Algeria, the USA and Chad.
Materials and methods
Algal strains
Five Spirulina strains were obtained from Algeria, the USA and Chad. Three of them were purchased as powder (Spirulina 1–3), whereas, the two other Spirulina strains (Spirulina 4 and 5) were obtained as fresh starter cultures. The latter strains were cultivated, harvested and dried to preserve them for further use. Table 1 presents the characteristics of all the Spirulina strains studied.
The morphology of fresh Spirulina (4 and 5) was observed with 10 and 40 times magnifications using an optical microscope (Optech Linear Biostar B4, München, Germany).
Culture conditions
The fresh cells of Spirulina strain samples 4 and 5 were separately grown under specific conditions in bath cultures with Zarrouk’s medium [22] (Table 2). The pH of the medium was maintained to be in the range of 8.5–10. The culture temperature was 30 ± 2.0 °C, exposed under cool-white fluorescent tubes with 2500 Lux (35 μmol/m 2/s) light intensity with light/dark cycles of 12:12. A steady-state agitation was applied by bubbling air using an aquarium pump to aerate the cells, ensuring even exposure of the algae to the light for optimal growth and nutrition. The pH was measured using a pH-meter (WTW, 315i /SET, Germany). The culture purity assessment, identification of the cells and the calculation of the concentration were carried out using a Nageotte cell under an optical microscope (Optech Linear Biostar B4, München, Germany). After 3 weeks of culturing, the algal cells were harvested by filtration through silkscreen canvas (150–200 mesh), washed with distilled water and finally dried for 3 days in shadow at ambient temperature (~20 °C). All the chemicals used to prepare the culture medium and for analysis were of analytical grade obtained from the Biochem Chemopharma (France).
The growth rate of Spirulina strains was determined by following the dry weight method of Madkour et al. [23]. In this method, 10 mL of algal culture samples were filtered through dried and pre-weighed Whatman filter membrane (Ø 47 mm and nominal pore size 0.45 µm) and washed twice with distilled water. The algal cells on the filter membranes were then dried for 4 h at 80 °C and were cooled in desiccators before weighing them.
Biochemical analysis
The moisture content was determined by drying 2 g of alga sample at 105 °C in a hot air oven until constant weight was obtained. The dietary fiber contents were determined by the method described by Van Soest (1963) [24]. Total ashes were determined by incinerating 5 g sample at 550 °C for 3 h using an incinerator (Linn Electro Therm, model, Germany). This method consisted of measuring the weight of samples before and after the incineration.
The total carbohydrate content was determined according to the methodology described by Dubois et al. [25] using glucose as a standard to plot the calibration curve.
The total protein concentration was determined using Kjeldahl method [26]. The mineralization and distillation of the samples were carried out using an incineration and distillation apparatus (Behr Labor-Technik, Behrosog and Behr S2, Germany).
The lipid contents were extracted by following a modified method of Xu et al. [27], using chloroform:methanol (2:1, v/v) mixture solvent. After refluxing the sample in a Soxhlet apparatus, the solvent was then evaporated under pressure by rotary vacuum evaporator (Rotavapor, Heidolph G1; Germany) and the residue was dried and weighted.
The algae fatty acid profile was determined by using Gas Chromatography (GC, Chrompack CP 9002, Netherlands) according to the methodology described by Tokusoglu and Ünal [28]. The fatty acids from the algae were converted to their corresponding fatty acids methyl esters (FAME) by methylation through Wolff method [29]. The separation of FAME was conducted using a capillary GC-column (DB-23, 50% Cyanopropyl, 30 m × 0.32 mm ID; film thickness 0.25 µm). Methyl-esters were injected (Split 1/100 injector) and detected (FID detector) at a temperature of 250 °C. A standard mixture of FAME (purity 99% by GLC, sigma Co.) was used for the identification of the major fatty acids in the algal strains.
The microalgae total calorific strength was determined by multiplying the total lipid, protein and carbohydrate content values by 9, 4 and 4 Kcal respectively, and then summing the obtained results.
Phytochemical analysis
Determination of Chlorophyll a, b and carotenoids
Chlorophyll a, b and carotenoids were determined according to the modified method described by El-Sheekh and Fathy [30]. In this method, 1 g of each sample was suspended in 50 mL of acetone and stirred vigorously with magnetic stirrer (Stuart stir SB161, UK). The solutions were then placed in the dark at 4 °C and centrifuged at 4000×g for 10 min using centrifuge (Kseroa Monaco, No. 4222). The obtained supernatants were used to determine the concentration of chlorophyll a (Chl a), chlorophyll b (Chl b) and total carotenoids (Car). The concentration of Chl a, Chl b and Car were determined spectrophotometrically at 645, 662 and 470 nm respectively using a UV/Vis spectrophotometer (Jenway Genova plus, Staffrdshire, UK). The contents (mg/g) of each pigment were quantified using Eqs. (1–3) [31].
where, A662, A645 and A470 are absorbance wavelengths at 662, 645 and 470 nm respectively by the sample.
Determination of water soluble pigments (phycobiliproteins)
The extraction and estimation of phycobiliprotein components including phycocyanin (C-PC), allophycocyanin (APC) and phycoerythrin (PE) were determined by using spectrophotometer with wavelengths 620, 652 and 562 nm respectively according to the method described by Anamika et al. [32]. The contents of each component were measured using Eqs. (4–6).
where, A620, A652 and A562 are the absorbance of light at 620, 652 and 562 nm respectively by the sample.
The percentage of crude phycocyanin was estimated by using the method described by Jourdan [33] and calculated by using Eq. (7). The experiment consists of dissolving 4 g of Spirulina into 100 mL distilled water.
where, DIL is the dilution factor and C is the percentage of the dry powder.
Determination of total phenols
The dry algal samples were ground to fine powder. A known powder amount (0.5 g) was used for the extraction of phenols using 10 mL of methanol (80%) at 35 °C for 24 h according to method described by Cai et al. [34]. The methanol samples were then cooled at room temperature and centrifuged at 4000×g for 10 min using a centrifuge (Kseroa Monaco, No. 4222). The obtained supernatant was used for the estimation of total phenolic content.
The total phenolic content present in microalgae was determined using Folin-Ciocalteu reagent [35]. The results were expressed as gallic acid equivalent (GAE)/g dry weight of microalgae.
Statistical analysis
The analyses were conducted in triplicate and the mean values are presented and expressed as mean value ± standard derivation (Mean ± SD). Analysis of variance (ANOVA) was performed using the General Linear Models of the STATGRAPHICS Centurion XVI.II software (STATPOINT Technologies, Inc. http://www.STATGRAPHICS.com). The multiple comparisons of means of each analysis were determined using the least significant difference (LSD) test at the confidence level of 95% and the difference between mean values greater than the LSD (0.95) was determined as significant.
Results and discussion
Strain morphology
The fresh Spirulina strain cells (Fig. 1) were multi-cellular and filamentous with cylindrical blue-green trichomes, forming an open helix which is the characteristic of the Arthrospira genus [3]. However, the Spirulina cells had different shapes. For example, the Spirulina 4 was straight filament, whereas, the Spirulina 5 (B and B′, Fig. 1) had a wavy shape. One of the main characteristics of this genus is the variable morphology under different environmental conditions [36]. Also, the degree of spiralisation show great variation and the Spirulina cells sometimes appear as straight trichomes in the culture [21]. These straight filaments are the Spirulina which need to be harvested [33].
The Spirulina B′ had changed from wavy to straight form, which is probably due to the change of the culture medium condition (Hiri medium vs Zarrouk medium). The physical and chemical conditions of the growing medium could alter the filamentous shape of the Spirulina strains [6]. According to Dhiab et al. [37], the change in the morphology of Spirulina trichome (from helical to straight shape) is due to the change in NaCl concentrations of the growing medium.
Biomass productivity
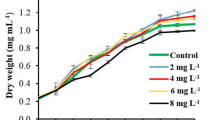
Different authors have reported that, in microalgae cultures, the nutritional condition is the main factor that can affect the growth and productivity [3, 6, 22, 23, 33, 38]. In this study, the Spirulina was cultivated and grown for a period of 23 days. The growth rate is expressed as dry weight (g/L) in ZM medium (Fig. 2). For the two fresh strains (Spirulina 4 and 5), the maximum biomass concentration and cell density were 0.60 g/L and 2.16 × 107 cell /mL respectively. These results are in accordance with those reported by Kumar et al. [6] and Aouir et al. [39].
The physicochemical composition
Table 3 presents the nutrient profiles and biochemical composition of the five Spirulina powders. The moisture contents of the samples were 4.70, 5.17, 8.40, 8.63 and 9.77% for Spirulina 1, 2, 3, 4 and 5 respectively. For all Spirulina samples, the moisture content was less than 10%, which is the recommended condition for long-term storage of Spirulina powders [40].
The total ash contents of the studied Spirulina varied significantly (p < 0.05) and were in the range of 8.23–18.11%. Except for Spirulina 3, for all strains, the ash contents were in the range of 7–10% and similar results were reported by Tokusoglu and Ünal [28]. The high ash content in Spirulina 3 cells can be attributed to the presence of sand and some impurities resulting from the traditional harvesting and drying process of Chad Spirulina which is called “Dihé” [41]. Recently, Bensehaila et al. [42] reported 6.88% of ash in A. platensis from Tamenrasset (South of Algeria). In our study, for the same strain (Spirulina 2), we found 9.3% of ash, which is probably due to the difference in growing conditions.
The total carbohydrate content of the Spirulina samples was almost the same (avg. 15%) except for Spirulina 3 or “Dihé” sample which had lower concentration (3.64%). The carbohydrate content of Spirulina 1, 2, 4 and 5 were almost the same (15–25%) and similar values were reported by Quillet [43].
The protein contents of the Spirulina strains studied were varied significantly. The samples had very high protein concentrations (avg. 60%) except in Spirulina 3, which had only 23.78% of protein. This could be attributed to the poor nutritive growing media condition (Lake Chad) of this species (Spirulina 3); while the other strains were grown in monitored conditions (pond cultures). According to Sorto and Gonnet [41], the presence of sand (over 30%), worms, plants debris, animals and insects in Lake Chad affect the nutritional and hygienic qualities of the Spirulina from there. Bensehaila et al. [42] reported 60.32% proteins content, which is higher than the 59.33% observed in our study, for the same strain (Spirulina 2). This slight difference could be due to the difference in analysis conditions. Nevertheless, the protein content of the Spirulina strains investigated still shows higher value than those of many other food protein sources (vegetables: 22%, meat: 25%, fish: 20%). A. platensis protein contents have shown very high digestibility (75–83%) due to the lack of cellulose cell walls in Spirulina. Therefore, it is a preferred source of food for major protein intake [10].
The highest total lipid content was observed for Spirulina 1 (8.08%). The concentrations for the other strains were 2.38, 1.5, 2.95 and 4.79% for Spirulina 2, 3, 4 and 5 respectively (Table 3). Bensehaila et al. [42] found 7.28% lipid content in Spirulina 2 which is very high compared to the result observed in this study for the same Spirulina strain (2.38%). This difference is closely related to the differences in pH, temperature and lighting in the culture conditions [6]. From the several microalgae species selected by Franke et al. [44], the Cyanophytes contained very low fat content (4.4–7.4%); which is in accordance with our results. According to Tokusoglu and Ünal [28] and Capelli and Cysewski [45], the lipid content in Spirulina varies between 5 and 8%. Tomabene et al. [46] found a higher content of lipids in S. platensis (16.6% of dry matter). However, Hudson and Karis [47] reported 11% of lipids in Spirulina maxima. Under different growth conditions, the lipid content in Spirulina was significantly different. For example, high light intensities and temperature of 38 °C don’t affect the fatty acid composition but had a drastic effect on the fatty acid content, reducing it by as much as 46% [48]. In addition, when there is a nutrient deficiency in term of nitrogen and phosphorus, the lipid accumulation in Spirulina was increased [49]. Hence, according to Orcutt and Patterson [50], light and pH parameters can also affect the lipid metabolism. The total lipid content increased at low pH and it decreased at high pH in Chlamydomonas sp [51]. However, Richmond [2] reported that microalgae lipid production depends on the species and their culture conditions such as nutrients, salinity, light intensity, temperature, pH and even the association with other microorganisms.
The fiber content in all the Spirulina was about 3%. These results are similar with those reported by Koru [9], who found 3% fiber content in Spirulina. These results confirm that the lower fiber content suggests an easily digestible biomass for human use [2, 10].
Finally, all the strains showed higher values of total energy contents in the range of 1327.34–1602.59 kJ, except for Spirulina 3, with lower energy (511.80 kJ), which is due to its poor carbohydrate, protein and lipid compositions (Table 3). This could be explained by the fact that the traditional “Dihé” production process is not much suitable to obtain a high Spirulina nutritional value, because algal growing conditions are not enriched and/or controlled. The harvesting and drying conditions are done traditionally, which could affect the nutrients and microbiological quality [41].
Fatty acids profile
The values obtained for fatty acids (FA) analyzed were expressed in percentages of total fat and are presented in Table 4. The major FA compound for all Spirulina strains is palmitic acid (16:0) with the mean of 43.60, 46.45, 71.15, 51.33 and 48.19% for Spirulina 1, 2, 3, 4 and 5 respectively. The highest amount was found for Spirulina 3 which is probably due to the lack of phosphorus in the growing medium [49]. Experiments carried out by Tokusoglu and Ünal [28] and Piorreck et al. [52] show that the palmitic acid (PA) content in Spirulina is between 26 and 39%. Falquet and Hurni [53] also reported a relatively high proportion of PA, in the range of 25–60% which probably reflects the variation in fatty acid content among the samples. Several other authors have also reported that the predominant fatty acid in Spirulina is a palmitic acid and are in the range of 35–54.1% [1, 53].
The statistical analysis shows significant differences (p < 0.05) in the stearic acid (SA) content of the samples. The highest and the lowest values were 3.33 and 0.69% for Spirulina 1 and 5, respectively. According to Falquet and Hurni [53], the SA content in Spirulina was between 0.5 and 2%. Oliveira et al. [54] found that the highest C18:0 content was 2.76% obtained in Spirulina grown in a medium with temperature of 30 °C.
The oleic acid (OA, 18:1n-9) content in our samples was in the range of 4.02–5.99% which is almost in the range (5–16%) reported by Hudson and Karis [47]. According to Habib et al. [1], oleic acid in Spirulina was varied from 1 to 15.5%.
Essential FAs means that the human body does not synthesize them in enough amounts and thus they need to be obtained through diet [55]. Therefore, the estimation of these FAs is very important for evaluating the potential of microalgae for human health. Linoleic acid (LA, 18:2n-6) levels of Spirulina were high, with a mean of 19–20% except for Spirulina 3 which had only 7.86%. These results are in accordance with the 17.6 ± 2.3% value reported by Xue et al. [56]. Several authors have reported that the linoleic acid content of Spirulina were in the range of 27% [45] to 30.7% [1].
Spirulina samples investigated in this study contained high amounts of γ-linolenic acid (GLA, 18:3n-6), with a mean concentration of 4.32%, except in Spirulina 3 that has only 2.73%. According to Kent et al. [57], Spirulina contain high quantities of γ-linolenic acid (9.42%) than all others microalgae strains (Nannochloropsis sp., Scenedesmus sp., Chlorella sp. and Dunaliella sp.). Tokusoglu and Ünal [28] have compared the GLA content of three kinds of algae (Spirulina platensis, Isochrisis galbana and Chlorella vulgaris) and they reported that Spirulina platensis species has a much higher γ–linolenic acid content (3.64–5.52%) compared to Isochrisis galbana (0.54%) and Chlorella vulgaris (traces %). In addition, Franke et al. [44] selected several species of microalgae and defined their fatty acid composition; they found that the GLA in Spirulina was in the range of 16–23%. Whereas, Habib et al. [1], reported that GLA in Spirulina was in the range of 8–31.7%. Tomaselli et al. [58] and Quoc and Dubacq [59] have reported that one of the reasons of these variations in FA content could be attributed to the difference in temperature used for cultivation of the Spirulina. Therefore, when the temperature of the medium increases, the lipid cells content increases considerably and the FA composition changed towards a higher degree of saturation (GLA biosynthesis was progressively hampered and LA was accumulated).
According to Mühling et al. [11] there are considerable differences in the FA composition, especially in the PUFA fraction, among the Spirulina strains. Cohen et al. [48], studied the FA distribution in 19 strains of Spirulina grown under various environmental conditions. They found that the fatty acid content increased with the cultivation temperature and the amount of PUFA was decreased. Also, the highest content of GLA was found in the strains grown at 30–35 °C.
Regarding the alpha-linolenic acid (ALA, 18:3n-3) or (ω-3), it was found that only Spirulina 1 and 5 contain a trace amount of it. It is reported in the literature that Spirulina in general do not have ω-3 fatty acids compared to other microalgae [57].
The levels of arachidic acid (AA, 20:0) in the strains investigated were high and the contents were varying significantly (p < 0.05) from 10.24 up to 15.71%. However, the Spirulina 3 had a lower nutrient content with 3.75% of AA. Nevertheless, this value of AA in Spirulina 3 is of reasonably high quantity, since the Spirulina platensis strains studied by Tokusoglu and Ünal [28] contained no amount of AA. In addition, the amount of AA of all the Spirulina investigated in this study is very higher than those found in Chlorella vulgaris and Isochrisis galbana strains (0.19 and 0.74%, respectively).
Mühling et al. [11] have noticed that the algal growing conditions could considerably affect the fatty acids content. For example, high light intensity and temperature favor the accumulation saturated FA [60, 61]. Meanwhile, low light intensities and low temperature promote the synthesis of PUFA [62].
Table 5 presents the sum of monounsaturated (MUFA), polyunsaturated (PUFA) and saturated (SFA) fatty acids in the Spirulina strains investigated. Spirulina algae contain greater quantities of ω-6 and traces of ω-3 FA. PUFA in the samples investigated were in the range of 23–24.5%, except in Spirulina 3 which had only 10.59%. The ω-6 quantities in Spirulina 1, 2, 4 and 5 were almost the same as that reported by Kent et al. [57] who found 20.04% of ω-6 in Spirulina.
According to the obtained result, the most important PUFA in Spirulina samples studied in this present work are linoleic acid and Gamma-linolenic acid (ω-6). Simopoulos [63] showed that the balance between ω-6 and ω-3 FA in foods is essential for human health benefits, and high ω-6/ω-3 ratios have been implicated in the higher chances of cancer, cardiovascular and inflammatory diseases. The same author recommended an optimal ratio of ω-6/ω-3 in the range of 1/1–4/1 as the most beneficial for human health, whereas the human diets have a ratio in the range of 15/1–16.7/1. However, in our case, we can’t define the ω-6/ω-3 ratio, since the ω-3 was almost undetectable in the Spirulina strains studied (Table 5). The same observations were reported by Kent et al. [57]. However, the deficiency of ω-3 in human diets has to be complemented by other foods rich with mostly PUFA such as nuts, seeds, and fish. Nevertheless, many researchers have reported that Spirulina is one of the best ω-6 whole food sources [7, 45].
Phytopigments composition
Chlorophyll a, b and carotenoids content
The amounts of chlorophylls and carotenoids observed in this study are shown in Fig. 3. The content of Chl a in Spirulina strains is significant (p < 0.05) with values of 6.39, 4.42, 1.79, 6.17 and 3.58 mg/g for Spirulina 1, 2, 3, 4 and 5, respectively. The Chl a is the major photosynthetic pigments in microalgae compared to Chl b [2].
According to Kumar et al. [6] the temperature condition affects the metabolic processes and biochemical composition of cells, and the optimal growth temperature is usually depending on the strain. In their study, the highest value of Chl a and carotenoids accumulation observed are 1.54 and 0.27%, respectively, obtained at a growth temperature of 35 °C.
El-sheikh and Al-fathy [30] reported that the autotrophic and heterotrophic growing conditions may considerably affect the phytopigments content. They found that, the Chl a in Chlorella vulgaris cells was higher in autotrophic and lower in heterotrophic growing conditions; whereas, the opposite trend was observed for Chl b.
In this study, the chlorophylls content was measured by summing Chl a and Chl b. The Spirulina 1 and 4 contained significantly higher mean values (p < 0.05) of chlorophylls and were 8.49 and 7.84 mg/g, respectively.
Carotenoids content were higher in Spirulina 5 (5.50 mg/g) than the other samples. This is in accordance with the values reported by M’baye et al. [64] that were in the range of 5.43–8.93 mg/g of carotenoids in 51 Mauritanian Spirulina samples.
Pierlovisi [65] and Ripley [3] suggested that the average phytopigments content in Spirulina are 11.5, 6.8 and 3.3 mg/g for chlorophylls, Chl a and carotenoids, respectively. In addition, Capelli and Cysewski [45] found that total chlorophylls and carotenoids in Spirulina are in the range of 10–5 mg/g, respectively. However, Kent et al. [57] found 12.33 and 1.45 mg/g of chlorophylls and carotenoids, respectively, in the same strain. As mentioned above, these differences may be due to the Spirulina growing conditions. According to the obtained results, compared to carotenoids, chlorophylls are the major pigment in the Spirulina microalgae.
Phycobiliprotein content
The amount of water soluble phycobiliprotein in Spirulina strains are shown in Table 6. The phycocyanin is the major phycobiliprotein in A. platensis. The phycoerythrin (PE) contents were very low in all the Spirulina strains investigated with a significant difference (p < 0.05) in the phycobiliprotein content among all the samples. The Spirulina 1 contains the highest C-PC and A-PC amounts with an average of 8.7 and 7.2% respectively, followed by Spirulina 5 (C-PC: 7.2%, APC: 5.1%), Spirulina 2 (C-PC: 6.4%, APC: 3.1%) and Spirulina 4 (C-PC: 5.9%, APC: 1.1%). These results are in accordance with those reported by Kumar et al. [6], who studied the effect of different temperature and light intensities on the pigments composition of S. platensis. They reported that the highest phycobiliproteins content (C-PC: 7.73%, APC: 3.46%, PE: 1.8%) was obtained when the Spirulina were grown at 35 °C temperature and with 2000 Lux light intensity. However, Anamika et al. [32] reported higher Spirulina phycobiliprotein content values (C-PC: 17.5%, APC: 3.8%, PE: 1.2%), confirming that growth conditions have significant effect on phytopigment content.
The values of crude Spirulina phycocyanin content are also shown in Table 6. The highest value was obtained for Spirulina 1 (29.03%). Except for Spirulina 3, the phycocyanin content values of all the Spirulina samples were more than 10% than the values reported by Jourdan [33]. According to Cuellar-Bermudez et al. [15], the phycocyanin is the major phycobiliprotein in A. platensis and can constitute up to 20% of its dry weight.
In this study, the average purity grade of the Spirulina strain, except for Spirulina 3, was more than 0.7 which is considered as food grade purity. According to Rito-Palomares [66], a purity grade of 0.7 is considered as food grade, 3.9 as reactive grade, and above 4 as analytical grade.
Total phenolic content
Figure 4 shows the total phenolic content of Spirulina strains expressed as a Gallic Acid Equivalent (GAE). The results showed that Spirulina 5 have the highest total phenolic content with 67.52 mg GAE/g of dry weight followed by Spirulina 1 (48.93 mg GAE/g), Spirulina 2 (45.22 mg GAE/g), Spirulina 3 (19.61 mg GAE/g) and Spirulina 4 (39.33 mg GAE/g). These results are in accordance with those reported by Benahmed et al. [67] who found 51.325 mg GAE/g in Spirulina. The work carried out by Harwati [68] on microalgae like Chlorococcum sp. and Nannochloropsis sp. showed that total phenolic content of these species were 2.41 and 2.05 mg GAE/g dry weight respectively. Whereas, Li et al. [69] reported that, Chlamydomonas rivialis and Chlorella vulgaris had relatively high total phenolic contents (>15 mg GAE/g). According to them, the medium composition had a significant influence on the total phenolic content of microalgae. According to our results Spirulina microalgae contain higher polyphenols content compared to other microalgae and fruits such as grapes, apples, pears, cherries and berries which contain only 2–3 mg/g of polyphenols [70]
Conclusion
This study has characterized different strains of Spirulina from Algeria, Chad and the USA towards their use in the nutraceutical and functional food area. The nutrient composition parameters and antioxidants compounds are more important in Spirulina samples 1 and 5 compared to the others. The strains growing in different culture media do not present the same nutrient composition. Hence, different strains with different shapes (straight vs wavy shape) cultivated in the same medium present difference in nutrient composition. Culture and nutrient conditions affect the cell composition and the biomass productivity. The majorities of Spirulina strains tested show an excellent nutritional profile and are rich in phytonutrients (phycocyanin, carotenoids and polyphenols) that have potential health benefits. Nevertheless, Spirulina 1 has higher nutritional contents, and a great potential for use in human nutrition. Spirulina 3 or “Dihé” has a lower nutritional composition. Therefore, it is highly recommended to use Spirulina strains grown under controlled growth conditions for production of nutrient and bioactive compounds for nutraceutical purposes.
References
M.A.B. Habib, M. Parvin, T.C. Huntington, M.R. Hasan, A review on culture, production and use of Spirulina as food for humans and feeds for domestic animals and fish. FAO Fisheries and Aquaculture Circular No. 1034, Rome, 2008), p. 33
A. Richmond, Handbook of Microalgal Culture: Biotechnology and Applied Phycology (Blackwell, Oxford, 2004), p. 566
R.D. Fox, Spiruline, Technique pratique et promesse (Edisud, Aix-en-Provence, 1999), p. 246
M. Abu Affan, D.W. Lee, S.M. Jeon, J.H. Noh, S.J. Heo, C. Oh, H.S. Park, H.S.A. Khomayis, D.H. Kang, Bituminous coal and sodium hydroxide-pretreated seawater stimulates Spirulina (Arthrospira) maxima growth with decreased production costs. Aquaculture 436, 121–126 (2015)
A. Vonshak, Spirulina platensis (Arthrospira) Physiology, Cell-Biology and Biotechnology (Taylor & Francis, London, 2002), p. 252
M. Kumar, J. Kulshreshtha, G.P. Singh, Growth and biopigment accumulation of Cyanobacterium Spirulina platensis at different light intensities and temperature. Braz. J. Microbiol. 42, 1128–1135 (2011)
T.S. Vo, D.H. Ngo, S.K. Kim, Nutritional and pharmaceutical properties of microalgal Spirulina, in Handbook of Marine Microalgae: Biotechnology Advanced (Elsevier, Amsterdam, 2015), p. 585
I. Priyadarshani, B. Rath, Commercial and industrial applications of microalgae. J. Algal Biomass Util. 3(4), 89–100 (2012)
E. Koru, Earth food Spirulina (Arthrospira): Production and quality standards, in Food Additive, ed. Y. El-Samragy (In Tech, Rijeka, 2012), p. 256
S. Sguera, Spirulina platensis et ses constituants intérêts nutritionnels et activités thérapeutiques. Thèse de Doctorat. Université Henri Poincaré - Nancy 1 Faculté De Pharmacie, 2008, p. 162
M. Mühling, A. Belay, A.B. Whitton, Variation in fatty acid composition of Arthrospira (Spirulina) strains. J. Appl. Phycol 17, 137–146 (2005)
R. Rodríguez-Sánchez, R. Ortiz-Butrón, V. Blas-Valdivia, A. Hernández-García, E. Cano-Europa, Phycobiliproteins or C-phycocyanin of Arthrospira (Spirulina) maxima protect against HgCl2-caused oxidative stress and renal damage. Food Chem. 135, 2359–2365 (2012)
K. Brejc, R. Ficner, R. Huber, S. Steinbacher, Isolation, crystallization, crystal structure analysis and refinement of allophycocyanin from the Cyanobacterium Spirulina platensis at 2.3 A resolution. J. Mol. Biol. 249, 424–440 (1995)
S. Boussiba, A. Richmond, Isolation and purification of phycocyanins from the blue-green alga Spirulina platensis. Arch. Microbiol. 120, 155–159 (1979)
S.P. Cuellar-Bermudez, I. Aguilar-Hernandez, D.L. Cardenas-Chavez, N.O. Soto, M. A. Romero-Ogawa, R. Parra-Saldivar, Extraction and purification of high-value metabolites from microalgae: essential lipids, astaxanthin and phycobiliproteins. Microb Biotechnol. 8(2), 190–209 (2015)
N.T. Eriksen, Production of phycocyanin—a pigment with applications in biology, biotechnology, foods and medicine. Appl. Microbiol. Biotechnol. 80, 1–14 (2008)
G. Penton-Rol, J. Martin-Prida, G. Pardo-Andreu, G. Martínez-Sánchez, E.F. Acosta-Medina, A. Valdivia-Acosta, N. Lagumersindez-Denis, E. Rodríguez-Jiménez, A. Llópiz-Arzuaga, P.A. López-Saura, G. Guillén-Nieto, E. Pentón-Arias, C-Phycocyanin is neuroprotective against global cerebral ischemia/reperfusion injury in gerbils. Brain Res. Bull. 86, 42–52 (2011)
Y. Ou, S. Zheng, L. Lin, Q. Jiang, X. Yang, Protective effect of C-phycocyanin against carbon tetrachloride-induced hepatocyte damage in vitro and in vivo. Chem. Biol. Interact. 185, 94–100 (2010)
Y. Ou, L. Lin, X. Yang, Q. Pan, X. Cheng, Antidiabetic potential of phycocyanin: Effects on K KAy mice. Pharm. Biol. 51(5), 539–544 (2013)
P.C. Dartsh, Antioxydant potential of selected Spirulina platensis. Phyother. Res. 22, 627–633 (2008)
L. Tomaselli, Morphology, ultrastructure and taxonomy, in Spirulina platensis (Arthrospira): Physiology, Cell-Biology and Biotechnology, ed. A. Vonshak (Taylor & Francis, London, 1997), pp. 1–15
C. Zarrouk, Contribution à l’étude d’une cyanophycée. Influence de divers facteurs physiques et chimiques sur la croissance et photosynthèse de Spirulina maxima. (Setchell et Gardner) Geitler. PhD Thesis, University of Paris, Paris, 1966, p. 455
F.F. Madkour, A.E. Kamil, H.S. Nasr, Production and nutritive value of Spirulina platensis in reduced cost media. Egypt. J. Aquat. Res. 38, 51–57 (2012)
P.J. Van Soest, Use of detergents in the analyses of fibrous feeds. II. A rapid method for the determination of fiber and lignin. J. Assoc. Off. Agric. Chem. 46, 829 (1963)
M. Dubois, K.A. Gilles, J.K. Hamilton, P.A. Rebers, F. Smith, Colorimetric method for determination of sugars and related substances. Anal. Chem. 28, 350–356 (1956)
J. Kjeldahl, A new method for the estimation of nitrogen in organic compounds. Anal. Chem. 22, 366 (1883)
X. Xu, J. Beardall, D.N. Hallam, Modification of fatty acid composition in halophilic Antarctic microalgae. Phytochemistry 49, 1249–1252 (1998)
Ö. Tokusoglu, M.K. Ünal, Biomass nutrient profiles of three microalgae: Spirulina platensis, Chlorella vulgaris, and Isochrisis galbana. J. Food. Sci. 68(4), 1144–1148 (2003)
J.P. Wolff, Manuel d’analyse des corps gras (Edit. Azoulay, Paris, 1968), p. 552
M.M. El-Sheekh, A.A. Fathy, Variation of some nutritional constituents and fatty acid profiles of Chlorella vulgaris Beijerinck grown under auto and heterotrophic conditions. Int. J. Bot. 5, 153–159 (2009)
H.K. Lichtenthaler, A.R. Wellburn, Determination of total carotenoids and chlorophyll a and b of leaf in different solvents. Biol. Soc. Trans. 11, 591–592 (1985)
P. Anamika, M. Sandhya, P. Richa, P.K. Ghosh, Purification and characterization of C-phycocyanin from Cyanobacterial species of marine and freshwater habitat. Protein Expr. Purif. 40, 248–255 (2005)
J.P. Jourdan, Cultivez votre spiruline: manuel de culture artisanale de spiruline (2011), p. 223
Y.Z. Cai, Q. Luo, M. Sun, H. Corke, Antioxidant activity and phenolic compounds of 112 traditional Chinese medicinal plants associated with anticancer. Life Sci. 74, 2157–2184 (2004)
V.L. Singleton, J.A. Rossi, Colorimetry of total phenolics with phosphomolybdic- phosphotungstic acid reagents. Am. J. Enol. Vitic. 16, 144–158 (1965)
P. Scheldeman, D. Baurain, R. Bouhy, M. Scott, M. Muhling, A.B. Whitton, A. Belay, A. Wilmotte, Arthrospira (Spirulina) strains from four continents are resolved into only two clusters, based on amplified ribosomal DNA restriction analysis of the internally transcribed spacer. FEMS. Microbiol. Lett. 172(2), 213–222 (1999)
R.B. Dhiab, H.B. Ouada, H. Boussetta, F. Franck, A. Elabed, M. Brouers, Growth, fluorescence, photosynthetic O2 production and pigment content of salt adapted cultures of Arthrospira (Spirulina) platensis. J. Appl. Phycol. 19(4), 293–301 (2007)
T. Ould Bellahcen, A. Bouchabchoub, M. Massoui, M. El Yachioui, Qualité nutritionnelle de Spirulina platensis en croissance dans les eaux usées domestiques. Larhyss. J. 14, 122–129 (2013)
A. Aouir, M. Amiali, T. K. Gachovska, A. Benchabane, A. Bitam, The effect of pulsed electric field (PEF) and ultrasoud (US) technologies on the extraction of phycopiliproteins from Arthrospira Platensis. IEEE Canada International Humanitarian Technology Conference (IHTC), IEEE Communications Letters, 2015, pp. 1–4
E.W. Becker, Microalgae Biotechnology and Microbiology (Cambridge University Press, Cambridge, 1994), pp. 1–293
M. Sorto, J. Gonnet, Pilot project for the development of the Dihé food chain in Chad. (GCP/CHD/029/EC), INRAN, Rome, 29 Nov 2007, pp. 1–15
S. Bensehaila, A. Doumandji, L. Boutekrabt, H. Manafikhi, I. Peluso, K. Bensehaila, A. Kouache, A. Bensehaila, The nutritional quality of Spirulina platensis of Tamenrasset. Algeria. Afr. J. Biotechnol. 14(19), 1649–1654 (2015)
M. Quillet, Research on carbohydrate substances produced by Spirulina. Ann. Nutr. Alim. 29, 553–561 (1975)
H. Franke, M. Springer, O. Pulz, U. Tietz, U. Mueller, Polyunsaturated fatty acids from microalgae. Int. Food Ingred. 4, 41–45 (1994)
B. Capelli, R.G. Cysewski, Potential health benefits of Spirulina microalgae. A review of the existing literature. Nutra Foods 9(2), 19–26 (2010)
T.G. Tornabene, T.F. Bourne, S. Raziuddin, A. Bem-Amotz, Lipid and lipopolysaccharide constituents of Cyanobacterium Spirulina platensis (Cyanophyceae, Nostocales). Mar. Ecol. Prog. Ser. 22, 121–125 (1985)
B.J.F. Hudson, I.G. Karis, The lipids of the alga Spirulina. J. Sci. Food Agric. 25, 759–763 (1974)
Z. Cohen, A. Vonshak, A. Richmond, Fatty acid composition of Spirulina strains grown under various environmental conditions. Phytochemistry 26, 2255–2258 (1987)
R.N. Bhakar, B.S. Pabb, Total lipid accumulation and fatty acid profiles of microalga Spirulina under different nitrogen and phosphorus concentrations. Egypt. J. Biol. 16, 57–62 (2014)
D.M. Orcutt, G.W. Patterson, Effect of light intensity upon Nitzschia closternium (Cylindrotheca fusiformis). Lipids 9, 1000–1003 (1974)
H. Tatsuzawa, E. Takizawa, M. Wada, Y. Yamamoto, Fatty acid and lipid composition of the acidophilic green alga Chlamydomonas sp. J. Phycol. 32, 598–601 (1996)
M. Piorreck, K.H. Baasch, P. Pohl, Biomass production, total protein, chlorophylls, lipids and fatty acids of freshwater green and blue-green algae under different nitrogen regimes. Phytochemistry 23(2), 207–216 (1984)
J.J. Falquet, P. Hurni, The nutritional aspects of Spirulina. Antenna Technologies (2006), p. 25, http://www.antenna.ch/documents/AspNutr2006.pdf
M.A.C.L. Oliveira, M.P.C. Monteiro, P.G. Robbs, S.G.F. Leite, Growth and chemical composition of Spirulina maxima and Spirulina platensis biomass at different temperatures. Aquac. Int. 7, 261–275 (1999)
C.Y. Chen, C.Y. Chen, H.C. Huang, C.C. Huang, W.L. Lee, J.S. Chang, Engineering strategies for enhancing the production of eicosapentaenoic acid (EPA) from an isolated microalga Nannochloropsis oceanica CY2. Bioresour. Technol. 147, 160–167 (2013)
C. Xue, Y. Hu, H. Saito, Z. Zhang, Z. Li, Y. Cai, C. Ou, H. Lin, A. Imbs, Molecular species composition of glycolipids from Spirulina platensis. Food Chem. 77, 9–13 (2002)
M. Kent, H.M. Welladsen, A. Mangott, Y. Li, Nutritional evaluation of Australian microalgae as potential human health supplements. PLoS ONE 10(2), 1–14 (2015)
L. Tomaselli, L. Giovannetti, G. Torzillo, Physiology of stress response in Spirulina spp., in Spirulina Algae of Life, ed. F. Doumenge, H. Durand-Chastel, A. Toulemont (Bulletin de l’Institut Océanographique, Spécial. 12, Monaco, 1993), pp. 65–75
K.P. Quoc, J.P. Dubacq, Effect of growth temperature on the biosynthesis of eukaryotic lipid molecular species by the Cyanobacterium Spirulina platensis. Biochim. Biophys. Acta 1346, 237–246 (1997)
E.A.T. Floreto, H. Hirata, S. Ando, S. Yamasaki, Effects of temperature, light intensity, salinity and source of nitrogen on the growth, total lipid and fatty acid composition of Ulva pertusa Kjellman (Chlorophyta). Bot. Mar. 36, 149–158 (1993)
J. Van Wagenen, T.W. Miller, S. Hobbs, P. Hook, B. Crowe, M. Huesemann, Effects of light and temperature on fatty acid production in Nannochloropsis salina. Energies 5, 731–740 (2012)
I.A. Guschina, J.L. Harwood, Lipids and lipid metabolism in eukaryotic algae. Prog. Lipid Res. 45, 160–186 (2006)
A.P. Simopoulos, The importance of the omega-6/omega-3 fatty acid ratio in cardiovascular disease and other chronic diseases. Exp. Biol. Med. 233, 674–688 (2008)
B.K. M’Baye, B. Lô, E. Bassene, Etude quantitative de quelques pigments de la Spiruline cultivée en Mauritanie en vue d’une valorisation nutritionnelle. Int. J. Biol. Chem. Sci. 5(5), 2035–2038 (2011)
C. Pierlovisi, L’Hommeetla Spiruline: Un avenir commun? Composition chimique, intérêts alimentaires et activités biologiques. Thèse de Doctorat, Paris V- René Descartes, Faculté des Sciences Pharmaceutiques et Biologiques, Paris, 2007, p. 162
M. Rito-Palomares, L. Nunez, D. Amador, Practical application of aqueous two-phase systems for the development of a prototype process for C-phycocyanin recovery from Spirulina maxima. J. Chem. Technol. Biotechnol. 76, 1273–1280 (2001)
D.A. Benahmed, Analyse des aptitudes technologiques de poudres de dattes (Phoenix-dactilifera L.) améliorées par la spiruline. Etude des propriétés rhéologique, nutritionnelles et antibactériennes. Thèse de Doctorat, faculté des sciences de l’ingénieur. Univ Mhamed Bougara-Boumerdes, Algeria, 2012, pp 1–188
T.U. Harwati, Cultivation of microalgae: lipid production, evaluation of antioxidant capacity and modeling of growth and lipid production. PhD Thesis, Faculty of Life Sciences at the Technical University Carolo-Wilhelmina at Brunswick, 2013, pp. 1–130
H.B. Li, K.W. Cheng, C.C. Wong, K.W. Fan, F. Chen, Y. Jiang, Evaluation of antioxidant capacity and total phenolic content of different fractions of selected microalgae. Food Chem. 102, 771–776 (2007)
A. Scalbert, I.T. Johnson, M. Saltmarsh, Polyphenols: antioxidants and beyond. Am. J. Clin. Nutr. 81, 215S–217S (2005)
Acknowledgements
The authors would like to thank all staff at the Food Technology and Human Nutrition Laboratory. Also, a special thanks to Dr. Belhasnat R of Ecole Nationale Supérieure des Sciences de la Mer et de l’Aménagement du Littoral (ENSSMAL, Algeria) for permission to use the laboratory.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Aouir, A., Amiali, M., Bitam, A. et al. Comparison of the biochemical composition of different Arthrospira platensis strains from Algeria, Chad and the USA. Food Measure 11, 913–923 (2017). https://doi.org/10.1007/s11694-016-9463-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11694-016-9463-4