Abstract
In this study, variations in the biochemical composition of two Sargassum fusiforme strains (SF-1 and SF-2) with different vesicle shapes collected during harvest period were analyzed. Compared with several other algae, S. fusiforme (commonly known as Hijiki) can be recommended as a nutritious food for its high levels of minerals, polyunsaturated fatty acids, phlorotannins, fucoidan, and alginate. Comparison of the two strains indicated that there were significant differences between the contents and characteristics of some components. The alginate content of SF-1 (15.46–26.30%) was higher than that of SF-2 (14.97–24.40%), while the alginate with highest molecular weight and viscosity was obtained from SF-2 (3.77 × 106 g mol−1, 226.2 mPa s). The content of fucoidan of SF-2 (5.30–11.60%) was higher than that of SF-1 (6.11–7.87%). On the other hand, the phlorotannin content of SF-1 (17.55–48.91 mg g−1) was higher than that of SF-2 (28.28–39.76 mg g−1), and a higher purity of fucoxanthin could be obtained from SF-1 (80.59–92.40%) than that from SF-2 (72.50–84.14%). Analysis of fatty acid composition indicated that EPA and α-linolenic acid were more abundant in SF-1, whereas arachidonic acid was more abundant in SF-2. The highest content of various nutrients existed in different strains and different periods. So, strain and the time of harvest can be selected according to the purpose of utilization.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Seaweeds have been consumed as food traditionally in East Asian countries, especially in Japan, Korea, and China, and currently as well in countries in Europe, North America, and South America because they are delicious and contains nutritional components such as minerals and polysaccharides (Marinho-Soriano et al. 2006). Besides the application in food industry, they are also used for the extraction of the bioactive substances. However, the biochemical composition of seaweed is influenced not only by the species but also by its environmental conditions, its maturity, its reproductive type, and the season (Ito and Hori 1989; Murakami et al. 2011; Schiener et al. 2015). Thus, the determination of the nutrient composition and bioactive substances of seaweeds is essential for providing information on their potential application.
Brown algae have wide utilization in food processing and nutraceutical industries for their unique bioactive substances, i.e., fucoidan, alginate, phlorotannins, and fucoxanthin. Fucoidan is a family of sulfated hetero-polysaccharides whose structure depends primarily on the algal source (Albana et al. 2007; Zhao et al. 2016). Many of its biological activities have been reported, i.e., antioxidant, anticoagulant and antithrombotic, antiviral, anticancer, immunomodulating, anti-inflammatory, anti-fertilization, reducing blood lipids, and gastric protection activities (Li et al. 2008; Lorbeer et al. 2015). Alginates are also linear polysaccharides produced by marine brown algae, with a wide range of molecular sizes and viscosity (Mackie et al. 2016). They have been used extensively in the food processing industries as thickening or stabilizing and emulsifying agents. The application of the alginate largely depended on its viscosity (Hernández-Carmona et al. 1999). Phlorotannins are oligomers and polymers of phloroglucinol, biosynthesized by the acetate-malonate pathway. They have been evaluated in a wide range of experimental models to possess antihypertensive, anti-allergic, antibacterial, anticancer, antioxidant, neuroprotective, deodorizing, tyrosinase inhibitory, anti-diabetic, and anti-adipogenic properties (Kang et al. 2012; Liu and Gu 2012; Lee and Jeon 2013 Jung et al. 2014) and potential application in cosmetics (Liu et al. 2018). Fucoxanthin, a marine carotenoid, possesses an unusual allenic bond and a 5,6-monoepoxide which makes its structure unique (Zhang et al. 2014). It has been reported to have many health benefits, such as antioxidant (Zhang et al. 2014), anticancer (Kim et al. 2013), anti-obesity, and anti-diabetic activities (Miyashita et al. 2011; Wu et al. 2015).
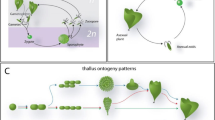
Sargassum fusiforme (Harvey) Setchell has been consumed as food for thousands of years in China, Korea, and Japan. It was also used as a traditional Chinese herbal medicine and documented in the book of Compendium of Materia Medica. In recent years, Chen et al. (2012) reported that polysaccharides purified from S. fusiforme showed anti-tumor properties in vivo and in vitro and improves the immune response in tumor-bearing mice. In addition, the antioxidant, antimicrobial, antihyperlipidemia, immunity enhancing, and neuroprotective properties of this seaweed have been studied (Chen et al. 2012; Jin et al. 2014; Wu et al. 2014a). These studies have indicated that S. fusiforme is a promising producer of raw materials for the nutraceutical industry. In China, S. fusiforme is widely distributed along the coastal areas of Liaodong Peninsula, Leizhou Peninsula, Fujian province, and Zhejiang province, while Dongtou of Zhejing province is the main cultivation area of S. fusiforme. The annual output of cultured S. fusiforme is around 1700 t (dry weight), and it is exported mostly to Japan with an annual incoming of around US$9 million. In this study, there are two S. fusiforme strains (SF-1 and SF-2) collected in Dongtou. SF-1 with slender vesicles is the traditional strain, while SF-2 with big round vesicles is a newly cultivated strain (Fig. 1). The big round vesicle strain brings in a higher yield because the higher weight; however, its chemical composition remains unknown. In order to understand the potential application of newly cultivated strain of S. fusiforme, there is a need to compare the bioactive substances and nutrient composition of these two strains.
The aim of this study is to analyze the biochemical composition of these two S. fusiforme strains, including moisture, ash, lipid, protein, and minerals, as well as the unique bioactive compounds, including alginate, fucoidan, fucoxanthin, and phlorotannins, during the harvest period of April, May, and June. The results provide information of the potential application and the time of harvest of these two cultivated S. fusiforme strains.
Materials and methods
Sampling of the seaweed
The Sargassum fusiforme samples were collected from April to June in 2014 in Dongtou (27°84′N, 121°12′E). The samples from each month were washed with distilled water, freeze dried, ground into powder, and stored at − 20 °C until chemical analysis.
Fatty acid analysis
Fatty acid composition was analyzed using a gas chromatograph (6890 N, Agilent). According to the method described by Nomura et al. (2013), dry lipids were mixed with 1 mL 2 N solution of methanolic sodium hydroxide and heated at 100 °C for 20 min. After cooling, 2 mL 1 N solution of methanolic hydrochloric acid was added and the mixture was heated at 100 °C for 10 min and then cooled again. Fatty acid methyl esters (FAMEs) were extracted by adding 1 mL hexane and dried with anhydrous Na2SO4.
FAMEs were analyzed on a gas chromatograph equipped with a flame ionization detector (FID) and an Inner Wax capillary column (30 mm × 0.25 mm i.d., 0.25 μm). Helium was used as the carrier gas at a split ratio of 1:3. The injector and detector temperatures were both set at 240 °C. The column temperature programming was as follows: from 170 to 210 °C at 3 °C min−1 and 24 min isothermally. Data were collected and analyzed using the GC Chem Station program (Agilent Technologies).
Elemental analysis
The concentrations of ten elements, i.e., calcium (Ca), magnesium (Mg), iron (Fe), manganese (Mn), copper (Cu), zinc (Zn), sodium (Na), potassium (K), phosphorus (P), and lead (Pb), were analyzed using ICP-AES.
Co-extraction of fucoidan and alginate
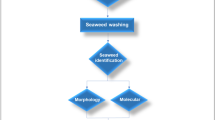
The co-extraction method (Fig. 2) of fucoidan and alginate from S. fusiforme was established in our previous research (Li et al. 2017). Briefly, the seaweed sample was treated with phosphate buffer containing 1% celluclast in an orbital shaker for about 12 h at 50 °C and then maintained at 70 °C for 3 h. After filtration, the supernatant was mixed well with 2% CaCl2 and the solution was kept at 4 °C overnight. The solution was then centrifuged at 4000×g for 15 min and the supernatant and precipitate A1 were collected. Ethanol was added to the supernatant to the concentration of 30%, and the solution was stored at 4 °C for 4 h. The obtained solution was centrifuged at 4000×g for 10 min to separate the supernatant and precipitate A2. Ethanol was added into the supernatant again to the final concentration of 60%, and the solution was kept at 4 °C for 8 h. The fucoidan was then precipitated by centrifugation at 4000×g for 10 min.
Alginate extraction was a sequential extraction carried out on the combination of algal residue and the precipitates A1 and A2. The mixture was soaked in 1% (w/w) sodium carbonate solution and extracted for 3 h at 60 °C in an orbital shaker. After filtering, alginate samples were separated from the crude extract by hydrochloric acid precipitation. The precipitate was washed three times with ethanol. Then, the alginic acid was converted into sodium alginate again by adding 40% sodium hydroxide solution to keep pH at 8 for 40 min. Extraction yields were calculated by the ratio of initial seaweed mass and obtained dry alginate mass.
Chemical composition analysis of fucoidan
Total carbohydrate of fucoidan was quantified using the phenol-H2SO4 method (Foley et al. 2011) and the total carbohydrate content was calculated by reference to the sugar standard (0–100 μg mL−1 fucose) at 490 nm. Sulfate content was estimated quantitatively using the BaCl2-gelatin turbidimetric method (Dodgson 1961). The concentration of sulfate present was determined by reference to a sulfate standard curve using K2SO4 (0–150 μg mL−1) at 550 nm.
Molecular weight and viscosity analysis of alginate
The molecular weights of the alginate samples were estimated by high-performance gel permeation chromatography (HPGPC) with a differential refractometer detector and a TSK-gel GM PWXL Column, eluted with 0.2 M NaCl at a flow rate of 0.5 mL min−1. The column temperature was kept at 35 °C. All samples were prepared as 0.1% (w/v) solutions, and 20 μL of solution was analyzed in each run. Dextran samples of Mw 5.21 × 105, 2.89 × 105, 1.1 × 105, 6.06 × 104, 1.26 × 104, and 4.32 × 103 g mol−1 were used as standards.
The viscosities were measured on a Rotary viscometer at 25 °C. The initial alginate solutions in a concentration of 10 mg mL−1 were prepared by sufficient stirring at room temperature.
Phlorotannin extraction and yield
Phenolic compounds were extracted in distilled water (2 g of algal powder in 50 mL distilled water) at 50 °C for 30 min. The extracts were centrifuged and the total phlorotannin content was determined using the Folin-Ciocalteu method with some modification (Wang et al. 2012a). A 0.2-mL aliquot of sample was mixed with 1.3 mL of distilled water and 0.5 mL of Folin-Ciocalteu reagent. Then, 1 mL of sodium carbonate (7.5% in distilled water) was added. The samples were incubated for 1 h at room temperature in the dark. The absorbance was measured at 770 nm. The phlorotannin content was calculated by using phloroglucinol (Sigma, USA) as a standard (0–100 μg mL−1), and the results were expressed as Milligram of phloroglucinol equivalent per gram of sample powder (mg PGE g−1).
Fucoxanthin purification
The co-extraction of lipids and pigments has been described previously. The extraction mixture was dissolved in the solution of hexane/acetone (ratio 6:4, v/v) and fractionated by silica column chromatography. The obtained fucoxanthin was analyzed by high-performance liquid chromatography (HPLC) with a UV-VIS detector and a Diamonsil C18 Column, eluted with 100% methanol at a flow rate of 1.0 mL min−1. The column temperature was kept at 30 °C. Fucoxanthin solutions (Sigma, USA) in a series of concentrations (0–300 μg mL−1) were used as standards.
Proximate analysis
Moisture content was measured using a Mettler MJ33 moisture analyzer (Mettler Toledo). Total ash content was determined by weight loss using an initial weight of 5.0 g of materials that were placed in an oven at 550 °C (Thiex et al. 2012). Crude protein content was determined using a Foss Kjeltec 8400 Automatic Kjeldahl analyzer and was calculated by multiplying nitrogen content with a factor of 6.25 (Nagappan and Vairappan 2014). Lipid and fucoxanthin were co-extracted using the solvent extraction method described by Gerasimenko et al. (2010) with a minor modification (Fig. 2). Briefly, sample powder was extracted with the mixture of acetone and ethanol (ratio 1:3, v/v) and then twice extracted with the mixture of chloroform and methanol (ratio 2:1, v/v) at 40 °C in an orbital shaker for 1 h. The combined filtrate was concentrated by rotary evaporation at 40 °C to estimate the total weight of extractable compounds in it. The lipid mass was calculated by subtracting the fucoxanthin mass from the total weight.
Statistical analysis
In order to eliminate the differences of different individuals, the samples from the same month were combined. Except the purification of the fucoxanthin and the determination of the molecular weight of alginate, each experiment was replicated three times, and analyses were performed three times. Data were expressed as the mean ± standard deviation (SD). Statistical analyses were performed using one-way ANOVA (SPSS V19.0). Group means were considered to be significantly different at p < 0.05.
Flowchart
Main processing for isolation of each component including the co-extraction of alginate and fucoidan, the simultaneous extraction of lipids and pigments, and the aqueous extraction of phlorotannins are summarized in Fig. 2.
Results
Proximate analysis (moisture, ash, proteins, lipids)
The significant semimonthly variations in the chemical composition of the two strains of S. fusiforme are shown in Table 1 (p < 0.05). The moisture contents of SF-1 ranged from 75.67 to 107.60 mg g−1 and those of SF-2 ranged from 84.40 to 107.50 mg g−1. The ash content of SF-1 increased to a maximum value of 351.70 mg g−1 in May 04 and decreased thereafter. The highest ash content for SF-2 was 377.63 mg g−1 in April 11 and the content generally decreased during the three harvest months. Total lipid content of both the two strains increased from April to May and decreased thereafter. The highest lipid contents were 46.10 and 42.57 mg g−1 for SF-1 and SF-2, respectively. The highest protein contents were 119.80 and 102.53 mg g−1 for SF-1 and SF-2, respectively.
Mineral elements
The semimonthly changes in contents of major mineral elements in two S. fusiforme strains are shown in Table 2. The content of major mineral elements of SF-1 and SF-2 showed similar variation. P was most abundant in both two strains, followed by K and Na of similar contents, then followed by Ca and Mg. The two strains showed similar contents of Ca and Na, while SF-1 showed higher contents of Mg, K, and P than those of SF-2.
The changes in contents of trace elements are shown in Table 3. Among trace elements detected, the most abundant element in the two strains was Fe, followed by Zn, Mn, and Cu. The contents of four trace elements of SF-1 were generally higher than those of SF-2. Furthermore, Cu was not detected in SF-2 during the three harvest months. Pb was also detected (Table 3) and ranged between 0.20 and 0.62 mg kg−1 in SF-1, and it varied from 0.10 to 0.84 mg kg−1 in SF-2.
Fatty acid composition
The semimonthly variations in the fatty acid composition of two S. fusiforme strains are shown in Tables 4 and 5. A total of 24 fatty acids were detected in SF-1 and 23 in SF-2 with the absence of C23:0. Two unknown fatty acids detected in both strains and, in accordance with the peak order, we hypothesized that they were nonadecylic acid (C19) and marked them as C19:a and C19:b, respectively.
The fatty acid composition of the two S. fusiforme strains was similar. In both strains, the most abundant fatty acid was palmitic acid (C16:0), followed by arachidonic acid (C20:4 n-6), α-linolenic acid (C18:3 n-3), eicosapentaenoic acid (EPA, C20:5 n-3), oleic acid (C18:1 n-9), and C19:a. α-Linolenic acid was the most abundant omega-3 PUFA followed by EPA, and arachidonic acid was the most abundant omega-6 PUFA. The contents of α-linolenic acid and EPA in SF-1 (7.30–12.45% and 7.23–10.04%) were higher than those in SF-2 (6.06–10.36 and 5.48–9.40%), while the levels of arachidonic acid and oleic acid in SF-2 (15.40–18.70 and 7.58–10.27%) were higher than those in SF-1 (12.45–18.45 and 5.87–9.88%). The highest PUFA levels were 46.10% (23.25 mg g−1 dry weight) and 50.44% (21.47 mg g−1 dry weight) in SF-1 (May 04) and SF-2 (May 13), respectively. The ratio of n-6/n-3 in SF-1 ranged from 0.95 to 1.45 and from 1.15 to 1.80 in SF-2.
Alginate yield, molecular weight, and viscosity
The semimonthly changes of the alginate content and characterization of the alginates from the two S. fusiforme strains are shown in Table 6. The highest alginate content was observed in SF-1 (June 15), whereas the alginate with maximum molecular weight and viscosity was obtained from SF-2 (May 04). The highest alginate contents were 262.97 mg g−1 and 244.05 mg g−1 for SF-1 and SF-2, respectively. The molecular weights of alginates extracted from SF-1 samples were highest in June 15 (9.49 × 105 g mol−1) and lowest in April 23 (3.55 × 105 g mol−1), while those from SF-2 were highest in May 04 (3.77 × 106 g mol−1) and lowest in April 11 (3.20 × 105 g mol−1). It was also observed that the viscosities of alginates were highly correlated with molecular weight (R2 = 0.98 for SF-1), and the highest values were 29.1 and 226.2 mPa∙s for SF-1 and SF-2, respectively.
Fucoidan yield and general analysis
The variations of the fucoidan contents of the two S. fusiforme strains are shown in Table 7. For both strains, the fucoidan contents of seaweeds in the intermediate stage of growth were lower than those of samples in other stages. The maximum fucoidan content of SF-2 (116.03 mg g−1) was higher than that of SF-1 (78.68 mg g−1).
Phlorotannin content
The semimonthly changes in the phlorotannins of the two S. fusiforme strains are shown in Table 8. Phlorotannins of the two strains increased from April reaching a maximum in May and decreasing thereafter. The maximum phlorotannin content of SF-1 (48.91 mg PGE g−1) was much higher than that of SF-2 (39.76 mg PGE g−1).
Fucoxanthin yield and purity
The purity and yield of fucoxanthin extracted from two S. fusiforme strains during harvest are listed at Table 9. More pure fucoxanthin could be obtained from SF-1 (80.59–92.40%) than from SF-2 (72.50–84.14%) by the method established in this study. Fucoxanthin purified from SF-1 collected in May 13, 29 and June 15 had relatively high purity (91.09–92.40%), and the sample collected in June 15 show a higher fucoxanthin yield than those of May 13 and 29.
Discussion
In China, Dongtou is known as the “hometown of Sargassum fusiforme”. Sargassum fusiforme cultivated in Dongtou grew fast in April and May and began breeding in June. There is distinct difference between the size of vesicles of SF-1 and SF-2. The size of vesicles of SF-1 is normal while that of SF-2 is much bigger. Although these two stains grew in the same culture condition, the results in this study indicated that the biochemical compositions and the trends of components variation were different between the two strains.
The comparison of the contents of ash, lipid, and protein in S. fusiforme with those of other seaweeds is summarized in Table 10. The contents of ash in S. fusiforme were found to be higher than those of some red seaweeds such as Porphyra purpurea (Taboada et al. 2013), Grateloupia turuturu (Denis et al. 2010), and Gracilaria cervicornis (Marinho-Soriano et al. 2006) and brown seaweed such as Sargassum oligocystum (Praiboon et al. 2018) and were similar to those of some brown seaweeds (Table 10) such as Costaria costata (Wu et al. 2014b), Eisenia arborea (Landa-Cansigno et al. 2017), and Sargassum horneri (Murakami et al. 2011). High ash content in seaweed is caused by its ability to absorb minerals from its environment (Pena-Rodriguez et al. 2011). The concentrations of major and trace minerals indicated that some trace minerals such as Fe, Zn, and Mn were of higher levels in S. fusiforme than those in many other seaweeds including S. oligocystum (Praiboon et al. 2018), Kappaphycus alvarezii (Yong et al. 2015), and Laminaria digitata and Laminaria hyperborea (Schiener et al. 2015).
Protein contents in young tissues were found to be higher (Table 1), and similar trends have been reported in S. horneri (Murakami et al. 2011) and Macrocystis pyrifera (Westermeier et al. 2011). The reason may be that tissue proteins are positively correlated with environmental supply and/or internal reserves of nitrogen, which are depleted or diluted during growth (Westermeier et al. 2011). As shown in Table 10, though the protein content of S. fusiforme was lower than those of some listed red seaweeds, its protein content was similar to those of many green seaweeds and brown seaweeds.
The lipid contents varied strongly according to the species (Table 10). The lipids content of two S. fusiforme strains were relatively higher than many Sargassum species such as S. wightii, S. horneri (harvested in southern Japan), S. oligocystum, and S. vulgare (Kumar et al. 2015; Murakami et al. 2011; Praiboon et al. 2018; Marinho-Soriano et al. 2006) but lower than S. horneri collected in the subarctic zone (Nomura et al. 2013). Compared seaweeds from other genera (Table 10), the lipid contents of the two S. fusiforme strains were higher than most other seaweeds, except Caulerpa sertularioides (Gosch et al. 2012), Cystoseira hakodatensis (Nomura et al. 2013), and Ulva lactuca (Yaich et al. 2011).
The major fatty acids in S. fusiforme were palmitic acid (C16:0), arachidonic acid (C20:4 n-6), α-linolenic acid (C18:3 n-3), EPA (C20:5 n-3), and oleic acid (C18:1 n-9), which was same as reported by Terasaki et al. (2009). Most seaweeds are reported to be rich in PUFA (Gosch et al. 2012). However, is some other Sargassum species such as S. oligocystum from Thailand and S. muticum from Malaysia, the main fatty acids were saturated fatty acids which composed of almost 50% of total fatty acids (Praiboon et al. 2018; Matanjun et al. 2009). Balboa et al. (2016) reported that seaweeds harvested in cold regions have higher polyunsaturated fatty acids than those harvested from tropical areas. The most abundant omega-3 PUFA in S. fusiforme was α-linolenic acid followed by EPA. Both are considered as essential nutrients in human health, especially in providing good protection against cardiovascular disease (Gosch et al. 2015). Arachidonic acid, the most abundant omega-6 PUFA in S. fusiforme, also plays an important role in biological systems, such as in the immune response, thrombosis, and brain function (Miyashita et al. 2013). In addition, EPA and α-linolenic acid were more abundant in SF-1, whereas arachidonic acid was more abundant in SF-2. However, diets with high n-6/n-3 ratio have been reported to cause some health problems, including inflammatory and autoimmune diseases, cardiovascular disease, and cancer (Gosch et al. 2015; Yong et al. 2015). As reported by Simopoulos (2002), it is recommended that the ratio of n-6/n-3 essential fatty acids should be 1:1 to 2:1. In the current study, the n-6/n-3 ratio in SF-1 varied from 0.95 to 1.45, and this in SF-2 varied from 1.15 to 1.80. Thus, both S. fusiforme strains could be considered as healthy food resources for fatty acids.
Lipids and pigments were co-extracted in this study by a method reported by Kim (2014) and Dos Santos et al. (2015) with a minor modification. Because fucoxanthin was a major carotenoid in this seaweed, it was further purified. High-purity fucoxanthin (92.40%) could be obtained on a silica gel from SF-1 sample harvested in June 15. The purity was higher than that obtained from SF-2 (84.14%), Sargassum binderi (90.7%), and Sargassum duplicatum (90.1%) (Noviendri et al. 2011).
In the traditional extraction process of fucoidan, the alginate precipitates were always discarded, and most of the alginates still remained in the algal residue in the form of insoluble alginate (Wu et al. 2014b). Thus, a co-extraction method of alginate and fucoidan was used in this study. Alginate was isolated from the combination of algal residue and the precipitates during the isolation of fucoidan. This method has many advantages including time saving, cost reduction, and recovery promoting, so it has potential industrial application.
In the current study, it was observed that alginate levels in both strains generally increased during the three harvest months. This may be due to the increasing in intensity of sunshine from April to June and the growth of vesicles which allows the algae to float on the surface of seawater receiving sufficient sunshine. Alginate contents in both strains of S. fusiforme (140.41–262.97 mg g−1 dw for SF-1 and 149.72–244.05 mg g−1 dw for SF-2) were higher than in Saccharina japonica (142–225 mg g−1 dw) (Chen et al. 2009), which is known as the most important resource for alginate production in the world. Thus, S. fusiforme, especially SF-1, could be developed as a new raw material for alginate production. It has been reported that the high contents of alginate could give flexibility and mechanical resistance to cells and tissues (Balboa et al. 2013), and plants growing in more turbulent water usually contain more alginate than the same species growing in calmer water (Schiener et al. 2015). Thus, the off-shore raft culture of S. fusiforme in Dongtou might contribute to the relatively high level of alginate. For alginate extracted from S. fusiforme, the viscosity was highly correlated with molecular weight (R2 = 0.9333) in accordance with the previous studies (Clementi et al. 1998; Torres et al. 2007). Generally there are three main categories of alginate, i.e., alginates of high viscosity (above 800 mPa s), alginates of medium viscosity (400–800 mPa s), and alginates of low viscosity (below 400 mPa s) (Hernández-Carmona et al. 1999), and these have different applications. The viscosities in our study were relatively low (9.9–29.1 mPa s for SF-1, 8.9–226.2 mPa s for SF-2), as found previously for S. fusiforme alginate (Guo et al. 2003). Low-viscosity alginate can be used in paper making, fruit industry, and textile printing and dyeing (Hernández-Carmona et al. 1999).
Fucoidan is a complex sulfated polysaccharide found in the cell wall matrix of brown algae which has a variety of physiological and biological activities. Previous studies have clearly shown that the composition and complexity of fucoidans from different brown seaweeds can vary considerably (O’Connell et al. 2008; Wijesinghe and Jeon 2012). It is known that other than fucose, fucoidan also contains additional monosaccharides such as mannose, galactose, glucose, xylose, and glucuronic acid, and many different sulfation patterns occur in these molecules (Wang et al. 2012b). In our present study, fucoidan yields in SF-2 were higher than those of SF-1 and other seaweeds except Undaria pinnatifida (Table 11). Fucoidan from S. fusiforme has been reported to have many biological activities including anticoagulant (Dobashi et al. 1989), antioxidant (Choi et al. 2010), anti-dementia (Hu et al. 2016), and anti-inflammation activities (Lee et al. 2015). The high content of fucoidan indicated that S. fusiforme could be developed as a useful raw material for the production of fucoidan.
Phlorotannins are integral structural components of the cell wall in brown algae and also play many secondary ecological roles such as protection from UV radiation and defense against grazing (Heffernan et al. 2015). In our current study, the contents of phlorotannins in SF-1 and SF-2 were higher than those of many other Sargassum spp., including S. muticum (Tanniou et al. 2013), S. horneri (Yin et al. 2015), and S. oligocystum (Praiboon et al. 2018). Moreover phlorotannins were extracted with distilled water instead of an organic solution, so that the phlorotannins could be safely and conveniently used in the pharmaceutical, cosmeceutical, and nutraceutical industries. However, only a few researches have been undertaken to evaluate the biological activities, types, and structures of phlorotannins from S. fusiforme (Wang et al. 2014; Yang et al. 2013) and further studies are necessary.
For algae and its products, Pb is the only heavy metal contaminant mentioned in National Food Safety Standards of China, and the maximum residue level is 1 mg kg−1 dry weight (Ministry of Health of the People’s Republic of China 2013). The levels of Pb in all samples detected in our study were below 1 mg kg−1 meaning that the two S. fusiforme strains cultivated in Dongtou can be considered as safe for food consumption.
In conclusion, the chemical composition of the two S. fusiforme strains extensively varied during harvest. This research improves our knowledge of the nutritional value of this species and the difference between the two strains. Sargassum fusiforme can be recommended as a nutritious food, based on its high levels of minerals, PUFAs, phlorotannins, fucoidan, and alginate of low viscosity. It also can be used as a raw material in pharmaceutical, cosmeceutical, and nutraceutical industries for extraction of bioactive substances. Because the highest content of various nutrients existing in different strains and periods, the strain and the time of harvest can be selected to satisfy different utilization requirements. The main morphological differences of the two strains are vesicle shapes, which may explain their biochemical differences.
References
Aguilera-Morales M, Casas-Valdez M, Carrillo-Domínguez S, González-Acosta B, Pérez-Gil F (2005) Chemical composition and microbiological assays of marine algae Enteromorpha spp. as a potential food source. J Food Compos Anal 18:79–88
Albana C, Natalia AU, Marina EP, Armida DI, Antonio P, Licia T, Nicola T, Galina EM, Albert EB, Maria I, Bilan AIU, Nadezhda EU, Alexey AG, Craig JS, Maeve K, Gabriel AR, Stefano I, Nikolay EN (2007) A comparative study of the anti-inflammatory, anticoagulant, antiangiogenic, and antiadhesive activities of nine different fucoidans from brown seaweeds. Glycobiology 17:541–541
Balboa EM, Conde E, Moure A, Falque E, Dominguez H (2013) In vitro antioxidant properties of crude extracts and compounds from brown algae. Food Chem 138:1764–1785
Balboa EM, Gallego-Fábrega C, Moure A, Domínguez H (2016) Study of the seasonal variation on proximate composition of oven-dried Sargassum muticum biomass collected in Vigo Ria, Spain. J Appl Phycol 28:1943–1953
Chen LM, Li DM, Yushan Z, Naihao Y (2009) Content and gelling properties of alginate from different region of Laminaria. China Food Addit 2:124–142
Chen X, Nie W, Yu G, Li Y, Hu Y, Lu J, Jin L (2012) Antitumor and immunomodulatory activity of polysaccharides from Sargassum fusiforme. Food Chem Toxicol 50:695–700
Clementi F, Mancini M, Moresi M (1998) Rheology of alginate from Azotobacter vinelandii in aqueous dispersions. J Food Eng 36:51–62
Denis C, Morancais M, Li M, Deniaud E, Gaudin P, Wielgosz CG, Barnathan G, Jaouen P, Fleurence J (2010) Study of the chemical composition of edible red macroalgae Grateloupia turuturu from Brittany (France). Food Chem 119:913–917
Dobashi K, Nishino T, Fujihara M, Nagumo T (1989) Isolation and preliminary characterization of fucose-containing sulfated polysaccharides with blood-anticoagulant activity from the brown seaweed Hizikia fusiforme. Carbohydr Res 194:315–320
Dodgson KS (1961) Determination of inorganic sulphate in studies on the enzymic and non-enzymic hydrolysis of carbohydrate and other sulphate esters. Biochem J 78:312–319
Dos Santos RR, Moreira DM, Kunigami CN, Aranda DA, Teixeira CM (2015) Comparison between several methods of total lipid extraction from Chlorella vulgaris biomass. Ultrason Sonochem 22:95–99
Foley SA, Szegezdi E, Mulloy B, Samali A, Tuohy MG (2011) An unfractionated fucoidan from Ascophyllum nodosum: extraction, characterization, and apoptotic effects in vitro. J Nat Prod 74:1851–1861
Gerasimenko NI, Busarova NG, Moiseenko OP (2010) Seasonal changes in the content of lipids, fatty acids, and pigments in brown alga Costaria costata. Rus J Plant Physiol 57:205–211
Gosch BJ, Magnusson M, Paul NA, de Nys R (2012) Total lipid and fatty acid composition of seaweeds for the selection of species for oil-based biofuel and bioproducts. GCB Bioenergy 4:919–930
Gosch BJ, Paul NA, de Nys R, Magnusson M (2015) Seasonal and within-plant variation in fatty acid content and composition in the brown seaweed Spatoglossum macrodontum (Dictyotales, Phaeophyceae). J Appl Phycol 27:387–398
Guo F, Xu S, Lin Z (2003) Study on decoloration, purification and structure of sodium alginate from Sargassum fusiforme. J Wuxi Univ Light Ind 22:52–56
Heffernan N, Brunton NP, Fitzgerald RJ, Smyth TJ (2015) Profiling of the molecular weight and structural isomer abundance of macroalgae-derived phlorotannins. Mar Drugs 13:509–528
Hernández-Carmona G, McHugh DJ, López-Gutiérrez F (1999) Pilot plant scale extraction of alginates from Macrocystis pyrifera. 2. Studies on extraction conditions and methods of separating the alkaline-insoluble residue. J Appl Phycol 11:493–502
Hu P, Li Z, Chen M, Sun Z, Ling Y, Jiang J, Huang C (2016) Structural elucidation and protective role of a polysaccharide from Sargassum fusiforme on ameliorating learning and memory deficiencies in mice. Carbohydr Polym 139:150–158
Hwang PA, Chien SY, Chan YL, Lu MK, Wu CH, Kong ZL, Wu CJ (2011) Inhibition of lipopolysaccharide (LPS)-induced inflammatory responses by Sargassum hemiphyllum sulfated polysaccharide extract in RAW 264.7 macrophage cells. J Agric Food Chem 59:2062–2068
Ito K, Hori K (1989) Seaweed: chemical composition and potential food uses. Food Rev Int 5:101–144
Jin W, Zhang W, Wang J (2014) A study of neuroprotective and antioxidant activities of heteropolysaccharides from six Sargassum species. Int J Biol Macromol 67:336–342
Jung HA, Jung HJ, Jeong HY, Kwon HJ, Ali MY, Choi JS (2014) Phlorotannins isolated from the edible brown alga Ecklonia stolonifera exert anti-adipogenic activity on 3T3-L1 adipocytes by downregulating C/EBPα and PPARγ. Fitoterapia 92:260–269
Jurković N, Kolb N, Colić I (1995) Nutritive value of marine algae Laminaria japonica and Undaria pinnatifida. Die Nahrung 39:63–66
Kang SM, Cha SH, Ko JY, Kang MC, Kim DK, Heo SJ, Kim JS, Heu MS, Kim YT, Jung WK, Jeon YJ (2012) Neuroprotective effects of phlorotannins isolated from a brown alga, Ecklonia cava, against H2O2-induced oxidative stress in murine hippocampal HT22 cells. Environ Toxicol Pharmacol 34:96–105
Kim JC (2014) Solvent extraction of fucoxanthin from Phaeodactylum tricornutum. Sep Sci Technol 49:410–415
Kim KN, Ahn G, Heo SJ, Kang SM, Kang MC, Yang HM, Dk K, Roh SW, Kim SK, Jeon BT, Park PJ, Jung WK, Jeon YJ (2013) Inhibition of tumor growth in vitro and in vivo by fucoxanthin against melanoma B16F10 cells. Environ Toxicol Pharmacol 35:39–46
Kordjazi M, Shabanpour B, Zabihi E, Faramarzi MA, Feizi F, Gavlighi HA, Feghhi MA, Hosseini SA (2013) Sulfated polysaccharides purified from two species of Padina improve collagen and epidermis formation in the rat. Int J Mol Cell Med 2:156–163
Kumar S, Sahoo D, Levine I (2015) Assessment of nutritional value in a brown seaweed Sargassum wightii and their seasonal variations. Algal Res 9:117–125
Landa-Cansigno C, Hernández-Carmona G, Arvizu-Higuera DL, Muñoz-Ochoa M, Hernández-Guerrero CJ (2017) Bimonthly variation in the chemical composition and biological activity of the brown seaweed Eisenia arborea (Laminariales: Ochrophyta) from Bahía Magdalena, Baja California Sur, Mexico. J Appl Phycol 29:2605–2615
Lee SH, Jeon YJ (2013) Anti-diabetic effects of brown algae derived phlorotannins, marine polyphenols through diverse mechanisms. Fitoterapia 86:129–136
Lee DG, Park SY, Chung WS, Park JH, Hwang E, Mavlonov GT, Kim IH, Kim KY, Yi TH (2015) Fucoidan prevents the progression of osteoarthritis in rats. J Med Food 18:1032–1041
Li B, Lu F, Wei X, Zhao R (2008) Fucoidan: structure and bioactivity. Molecules 13:1671–1695
Li L, Ma W, Zhou G (2012) The chemical analysis of S. kjellmanianum in Shandong peninsula. Adv Mater Res 554–556:1884–1888
Li YJ, Fan JX, Cheng LK, Xu JC, Gao X, Fu XT (2017) Co-extraction and characterization of alginate and fucoidan from two Sargassum fusiforme strains. Sci Technol Food Ind 38:90–95
Liu H, Gu L (2012) Phlorotannins from brown algae (Fucus vesiculosus) inhibited the formation of advanced glycation endproducts by scavenging reactive carbonyls. J Agric Food Chem 60:1326–1334
Liu N, Fu X, Duan D, Xu J, Gao X, Zhao L (2018) Evaluation of bioactivity of phenolic compounds from the brown seaweed of Sargassum fusiforme and development of their stable emulsion. J Appl Phycol 30:1955–1970
Lorbeer AJ, Lahnstein J, Fincher GB, Su P, Zhang W (2015) Kinetics of conventional and microwave-assisted fucoidan extractions from the brown alga, Ecklonia radiata. J Appl Phycol 27:2079–2087
Mackie AR, Macierzanka A, Aarak K (2016) Sodium alginate decreases the permeability of intestinal mucus. Food Hydrocoll 52:749–755
Marinho-Soriano E, Fonseca PC, Carneiro MA, Moreira WS (2006) Seasonal variation in the chemical composition of two tropical seaweeds. Bioresour Technol 97:2402–2406
Marudhupandi T, Kumar TTA (2013) Antibacterial effect of fucoidan from Sargassum wightii against the chosen human bacterial pathogens. Int J Curr Pharm Res 2:155–158
Matanjun P, Mohamed S, Mustapha NM, Muhammad K (2009) Nutrient content of tropical edible seaweeds, Eucheuma cottonii, Caulerpa lentillifera and Sargassum polycystum. J Appl Phycol 21:75–80
Men'shova RV, Ermakova SP, Rachidi SM, Al-Hajje AH, Zvyagintseva TN, Kanaan HM (2012) Seasonal variations of the composition, structural features, and antitumor properties of polysaccharides from Padina pavonica (Lebanon) as a function of composition. Chem Nat Compd 47:870–875
Ministry of Health of the People’s Republic of China (2013) National food safety standards on maximum levels of contaminants in foods. Standardization Administration of the People's Republic of China, Beijing
Miyashita K, Nishikawa S, Beppu F, Tsukui T, Abe M, Hosokawa M (2011) The allenic carotenoid fucoxanthin, a novel marine nutraceutical from brown seaweeds. J Sci Food Agric 91:1166–1174
Miyashita K, Mikami N, Hosokawa M (2013) Chemical and nutritional characteristics of brown seaweed lipids: a review. J Funct Foods 5:1507–1517
Murakami K, Yamaguchi Y, Noda K (2011) Seasonal variation in the chemical composition of a marine brown alga, Sargassum horneri (Turner) C. Agardh. J Food Compos Anal 24:231–236
Nagappan T, Vairappan CS (2014) Nutritional and bioactive properties of three edible species of green algae, genus Caulerpa (Caulerpaceae). J Appl Phycol 26:1019–1027
Nomura M, Kamogawa H, Susanto E, Kawagoe C, Yasui H, Saga N, Hosokawa M, Miyashita K (2013) Seasonal variations of total lipids, fatty acid composition, and fucoxanthin contents of Sargassum horneri (Turner) and Cystoseira hakodatensis (Yendo) from the northern seashore of Japan. J Appl Phycol 25:1159–1169
Noviendri D, Jaswir I, Salleh HM, Taher M, Miyashita K, Ramli N (2011) Fucoxanthin extraction and fatty acid analysis of Sargassum binderi and S. duplicatum. J Med Plant Res 5:2405–2412
O’Connell E, Murray P, Piggott C, Hennequart F, Tuohy M (2008) Purification and characterization of a N-acetylglucosaminidase produced by Talaromyces emersonii during growth on algal fucoidan. J Appl Phycol 20:557–565
Pena-Rodriguez A, Mawhinney TP, Ricque-Marie D, Cruz-Suarez LE (2011) Chemical composition of cultivated seaweed Ulva clathrata(Roth) C. Agardh. Food Chem 129:491–498
Praiboon J, Palakas S, Noiraksa T, Miyashita K (2018) Seasonal variation in nutritional composition and anti-proliferative activity of brown seaweed, Sargassum oligocystum. J Appl Phycol 30:101–111
Rioux LE, Turgeon SL, Beaulieu M (2007) Characterization of polysaccharides extracted from brown seaweeds. Carbohydr Polym 69:530–537
Schiener P, Black KD, Stanley MS, Green DH (2015) The seasonal variation in the chemical composition of the kelp species Laminaria digitata, Laminaria hyperborea, Saccharina latissima and Alaria esculenta. J Appl Phycol 27:363–373
Simopoulos AP (2002) The importance of the ratio of omega-6/omega-3 essential fatty acids. Biomed Pharmacother 56:365–379
Taboada MC, Millán R, Miguez MI (2013) Nutritional value of the marine algae wakame (Undaria pinnatifida) and nori (Porphyra purpurea) as food supplements. J Appl Phycol 25:1271–1276
Tanniou A, andanjon L, Incera M, Leon ES, Husa V, Grand JL, Nicolas JL, Poupart N, Kervarec N, Engelen A, Walsh R, Guerard F, Bourgougnon N, Stiger-Pouvreau S (2013) Assessment of the spatial variability of phenolic contents and associated bioactivities in the invasive alga Sargassum muticum sampled along its European range from Norway to Portugal. J Appl Phycol 26:1215–1230
Terasaki M, Hirose A, Narayan B, Baba Y, Kawagoe C, Yasui H, Saga N, Hosokawa M, Miyashita K (2009) Evaluation of recoverable functional lipid components of several brown seaweeds (Phaeophyta) from Japan with special reference to fucoxanthin and fucosterol contents. J Phycol 45:974–980
Thiex N, Novotny L, Crawford A (2012) Determination of ash in animal feed: AOAC official method 942.05 revisited. J AOAC Int 95:1392–1397
Torres MR, Sousa AP, Silva Filho EA, Melo DF, Feitosa JP, de Paula RC, Lima MG (2007) Extraction and physicochemical characterization of Sargassum vulgare alginate from Brazil. Carbohydr Res 342:2067–2074
Wang T, Jonsdottir R, Liu H, Gu L, Kristinsson HG, Raghavan S, Olafsdottir G (2012a) Antioxidant capacities of phlorotannins extracted from the brown algae Fucus vesiculosus. J Agric Food Chem 60:5874–5883
Wang PP, Zhao XL, Lv YJ, Liu YN, Lang YZ, Wu JD, liu X, Li MM, Yu GL (2012b) Analysis of structural heterogeneity of fucoidan from Hizikia fusiforme by ES-CID-MS/MS. Carbohydr Polym 90:602–607
Wang C, Liu Y, Lin M (2014) Antitumor effect of phlorotannins from Sargassum fusiforme in S180 sarcoma-bearing mice. J Guangdong Med College 32:614–616
Westermeier R, Murúa P, Patiño DJ, Muñoz L, Ruiz A, Müller DG (2011) Variations of chemical composition and energy content in natural and genetically defined cultivars of Macrocystis from Chile. J Appl Phycol 24:1191–1201
Wijesinghe W, Jeon YJ (2012) Biological activities and potential industrial applications of fucose rich sulfated polysaccharides and fucoidans isolated from brown seaweeds: a review. Carbohydr Polym 88:13–20
Wu X, Jiang W, Lu J, Yu Y, Wu B (2014a) Analysis of the monosaccharide composition of water-soluble polysaccharides from Sargassum fusiforme by high performance liquid chromatography/electrospray ionisation mass spectrometry. Food Chem 145:976–983
Wu X, Wang G, Fu X (2014b) Variations in the chemical composition of Costaria costata during harvest. J Appl Phycol 26:2389–2396
Wu MT, Su HM, Cui Y, Windust A, Chou HN, Huang CJ (2015) Fucoxanthin enhances chain elongation and desaturation of alpha-linolenic acid in HepG2 cells. Lipids 50:945–953
Yaich H, Garna H, Besbes S, Paquot M, Blecker C, Attia H (2011) Chemical composition and functional properties of Ulva lactuca seaweed collected in Tunisia. Food Chem 128:895–901
Yang X, Lu H, Li Y, Liu Y (2013) Antioxidative activities of phlorotannins with different molecular weight extracted from Sargassum fusiforme. Mar Sci 37:47–51
Yin S, Woo H-C, Choi J-H, Park Y-B, Chun B-S (2015) Measurement of antioxidant activities and phenolic and flavonoid contents of the brown seaweed Sargassum horneri: comparison of supercritical CO2 and various solvent extractions. Fish Aquat Sci 18:123–130
Yong YS, Yong WTL, Ng SE, Anton A, Yassir S (2015) Chemical composition of farmed and micropropagated Kappaphycus alvarezii (Rhodophyta, Gigartinales), a commercially important seaweed in Malaysia. J Appl Phycol 27:1271–1275
Zhang YP, Fang H, Xie QL, Sun JP, Liu R, Hong Z, Yi RZ, Wu H, Yi RZ (2014) Comparative evaluation of the radical-scavenging activities of fucoxanthin and its stereoisomers. Molecules 19:2100–2113
Zhao XL, Liu XX, Li CX, Yu GL (2016) In vitro and in vivo hypoglycemic effects of brown algal fucoidans. Int J Biol Macromol 82:249–255
Acknowledgements
We especially thank Culture Collection of Seaweed at the Ocean University of China for their help in providing S. fusiforme.
Funding
This work was founded by the grant of International Science and Technology Cooperation Program of China (No. 2015DFG32290), the grant of Public Science and Technology Research Funds Projects of Ocean (No. 201405040), and Jiangsu Provincial Key R & D Project (BE2015335).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Li, Y., Fu, X., Duan, D. et al. Comparison study of bioactive substances and nutritional components of brown algae Sargassum fusiforme strains with different vesicle shapes. J Appl Phycol 30, 3271–3283 (2018). https://doi.org/10.1007/s10811-018-1543-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-018-1543-x