Abstract
β-Carotene nanoemulsions (BC-NEs), as potential active ingredients for water-based food systems, were stabilized with different food proteins (whey protein isolate (WPI); or sodium caseinate (SC)). The surfactant Tween 20 was used for comparison or in combination with the food proteins. The influence of heating, freezing, pH, and salts on the physical and chemical stabilities of the BC-NEs was investigated. The BC-NEs were stable to aggregation against heating, NaCl, and neutral pH, but were physically unstable against CaCl2 (WPI: 3.4 µm at > 150 mM; SC: 2.6 µm at > 10 mM) and low pH (WPI: 2.8 µm, SC: 1.3 µm at pH 3). However, the combination of Tween 20 and the proteins effectively prohibited BC-NE droplet aggregation under CaCl2 and low-pH conditions. In the chemical stability tests, BC degradation was significantly slower in the WPI-stabilized BC-NEs (WPI: 52% BC at 8 weeks) than in the others (Tween 20, SC: < 35% BC at 8 weeks) and was fastest at the most acidic pH value (pH 3; < 33% BC at 2 weeks). Therefore, this study provides useful insights into the formulation of functional BC emulsions for the commercial food and beverage industries.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Carotenoids are a class of natural antioxidant pigments found mainly in fruits and vegetables, including carrots, yams, cantaloupes, mangoes, papaya, spinach, and kale. Recently, several potential health benefits of carotenoids have been widely recognized, and their intake has been associated with a reduction of risk in certain chronic diseases such as cancers, cardiovascular disease, age-related macular degeneration, and cataracts [1, 2]. In particular, β-carotene (BC), one of the most important carotenoids in human nutrition, has the highest pro-vitamin A activity [3]. Therefore, it should be a strong candidate for incorporation into functional foods. However, the incorporation of BC into foods and beverages has been limited because of its high hydrophobicity, elevated melting point, and significant susceptibility to chemical degradation [4]. BC is highly susceptible to degradation by light, singlet oxygen, heat, transition metals, and free radicals. Once BC degradation has been initiated, several secondary reaction products can be formed and their oxidation can be accelerated [5]. Consequently, the beneficial health properties of carotenoids may be lost if they undergo chemical degradation in foods and beverages. Since BC is highly hydrophobic (low water solubility), it can be difficult to incorporate it directly into aqueous-based formulations. Instead, BC must be incorporated into colloidal delivery systems such as emulsions, liposomes, and solid lipid nanoparticles.
Colloidal delivery systems for the encapsulation of bioactive lipophilic compounds have been reported in the food, pharmaceutical, cosmetic, and agricultural industries. Oil-in-water (O/W) emulsions are one of the most widely utilized colloidal delivery systems in food and beverage products because they can be produced easily and economically [6]. For example, bioactive lipophilic compounds such as carotenoids were incorporated into emulsion-based systems that were simply prepared by solubilizing the compounds within an oil phase and then homogenizing it with an aqueous phase containing a water-soluble emulsifier [6]. Recently, nanoemulsion (NE) systems have been examined in the food industry because of certain advantages over conventional emulsions for encapsulating and delivering bioactive lipophilic compounds [7]. These systems scatter light weakly and can be incorporated into optically transparent products; they are highly stable against particle aggregation, sedimentation, and gravitational separation; and they may increase the bioavailability of highly lipophilic substances [7,8,9]. Many studies have also shown that the bioavailability of carotenoids is increased when they are incorporated into O/W emulsions, which may enhance their health-promoting activities [10,11,12].
As one of the common knowledge in the formation of colloidal delivery systems, food emulsifiers determine emulsion stability [5, 7]. Generally, Tween emulsifiers are known as nonionic emulsifiers, and they have excellent emulsifying functions in preparation of O/W emulsions because of their high hydrophile–lipophile balance values, which facilitate the production of NEs with small particle sizes and narrow size distributions. However, the NEs prepared with Tweens have been shown to be significantly vulnerable to oxidation. Proteins are also excellent emulsifiers that can improve the stability of O/W emulsions [7, 13], while altering the properties of the emulsion droplet interface in a manner that increases the oxidative stability of the lipid core [14]. If these protein-stabilized emulsions are to be used to inhibit lipid oxidation in the food industry, they must be able to withstand the rigors of food-processing operations and remain stable during prolonged storage [14].
The main objective of this study was to determine whether an emulsion system could be developed as a food ingredient with beneficial properties for the delivery of BC into water-based food systems. To accomplish this goal, β-carotene nanoemulsions (BC-NEs) were prepared with Tween 20, whey protein isolate (WPI), and sodium caseinate (SC) under varying environmental conditions including heating, freezing, pH, and ionic strength. To commercially utilize an O/W emulsion containing BC, the effects of the emulsifier type and pH on oxidative stability were also examined.
Materials and methods
Materials
β-Carotene (BC, 30% in corn oil) was purchased from DSM Nutritional Products, Ltd. (Basel, Switzerland). Standard β-carotene (> 95%) was supplied from Sigma-Aldrich (St. Louis, MO, USA), and medium chain triglyceride (MCT) oil was provided by SHS International, Ltd. (Liverpool, UK). For the continuous phase systems, WPI, sodium caseinate, and polyoxyethylenesorbitan monolaurate (Tween 20) were obtained from Sungpoong (Asan, South Korea), Sigma Chemical Co. (St. Louis, MO, USA), and Junsei (Tokyo, Japan), respectively. Sodium azide (NaN3) as an antimicrobial agent was purchased from Samchun Pure Chemical (Seoul, South Korea). All other chemicals were of analytical grade.
Methods
Preparation of BC-NE
BC-NEs were prepared using MCT oil containing BC (30% in corn oil) as the dispersed phase and 0.05 M phosphate buffer solution (PBS, pH 7) containing various emulsifiers such as proteins (WPI, SC) and Tween 20 as the continuous phase. BC was first dissolved in MCT oil at 140 ± 0.5 °C for several seconds and then cooled at ambient temperature. The continuous phase consisting of 1% weight/weight (w/w) protein emulsifier and 0.02% NaN3 in PBS was mixed under magnetic stirring at 400 rpm (DAIHAN Scientific Co. Ltd., Seoul, South Korea) for approximately 3 h and kept overnight to ensure complete dispersion and dissolution. In addition, to study the combined effects of the proteins and Tween 20, each emulsifier was mixed at different emulsifier ratios (Tween 20 : proteins = 1:0, 4:1, 1:1, 1:4, or 0:1 (w/w)). The ratio of the dispersed phase to the continuous phase was set at 3:97 (w/w), and the final BC concentration was approximately 0.03% in the emulsions. The emulsions were homogenized using an Ultra-Turrax T18 high-speed homogenizer (IKA Works, Guagzhou, China) at 11,000 rpm for 3 min. Then, the pre-emulsions were passed three times through a microfluidizer (Model M-110L, Microfluidics, Newton, MA, USA) at 120 MPa. Each NE was stored in a screw-capped amber bottle at 25 ± 0.5 °C for 2 months. The NEs were sampled, and various physicochemical properties were investigated at certain times during the storage period.
Particle size, ζ-potential, and surface tension measurements
The mean particle sizes and ζ-potentials of the NEs were determined by dynamic light scattering using a Zetasizer (Nano ZS90, Malvern Instruments Ltd., Worcestershire, UK). The mean particle sizes of the NEs are described by the triplicate measured mean diameter (z-average), and the width of the droplet size distribution is indicated by the polydispersity index (PDI). The ζ-potential was determined from the distribution of the electrophoretic mobility of the particles and measured velocity using the Smoluchowski equation. The surface tension of the NEs was determined using a KSV Sigma 703D Tensiometer (Monroe, CT, USA). All measurements were obtained in triplicate.
Effect of environmental stress on BC-NEs
Heat processing
The BC-NEs (5 mL) were transferred into a glass test tube and heated in a water bath (DAIHAN Scientific Co. Ltd., Seoul, South Korea) at 60 ± 0.5 °C for 4 h. After heating, the BC-NEs were cooled to ambient temperature, and the particle sizes were measured on the following day.
Freeze processing
The BC-NEs (5 mL) were transferred into a cryogenic test tube and frozen in a freezer (− 20 ± 0.5 °C, LG Electronics, Seoul, South Korea) for 24 h. After freezing, the BC-NEs were thawed to room temperature (25 ± 0.5 °C), and the particle sizes were measured on the following day.
pH
The BC-NEs (1 mL) were diluted with various buffer solutions (3 mL) from pH 1 to 13. The pH buffer solutions were prepared from HCl, KCl buffer (pH 1), citric acid, NaOH buffer (pH 3), citric acid, sodium citrate buffer (pH 5), phosphate buffer (pH 7), tris(hydroxymethyl)aminomethane, HCl buffer (pH 9), Na2HPO4, NaOH buffer (pH 11), and NaOH, KCl buffer (pH 13). The samples were stored at room temperature (25 ± 0.5 °C) for 24 h prior to analysis.
Salts
To study the effects of Na+ and Ca2+ ions on particle stability, the BC-NEs (1 mL) were diluted with solutions of NaCl or CaCl2 (3 mL) in the concentration range 0–200 mM. The samples were stored at room temperature (25 ± 0.5 °C) for 24 h prior to analysis.
BC degradation
The freshly prepared BC-NEs and BC-NEs diluted in pH 3 or 7 solutions were stored at ambient temperature for 8 weeks. The chemical degradation of BC during storage was determined by color fading using a solvent extraction method. Extracted BC was analyzed by high-performance liquid chromatography (HPLC, Varian 900-LC, Harbor City, CA, USA) equipped with a Varian 900-LC auto sampler and an SP-930D pump system (Young-Lin Co., Ltd., Anyang, South Korea). The BC was first extracted with ethanol and n-hexane from the BC-NE, and then an n-hexane solution containing BC was appropriately diluted with n-hexane. Thereafter, the diluted n-hexane solution was removed under nitrogen gas and then dissolved in the mobile phase (1 mL, 7:3 (volume/volume) acetonitrile:ethanol). Samples were separated on a ZORBAX Eclipse Plus C18 column (4.6 × 250 mm, 5 µm particle size, Agilent Tech., California, USA) using a mobile phase at 40 °C. The flow rate was 1.6 mL/min, and the injection volume was 50 µL. Peak areas were recorded with a UV/Vis detector set at 450 nm and analyzed by Galaxie software (Varian, Inc.) for data acquisition and processing. A standard curve (concentration versus peak area) was fabricated by linear regression analysis. Injections in triplicate were made at each concentration for standards and samples. The calibration of the peak area versus BC concentration was linear in the concentration range from 0.00 to 50 mg/L (y = 1017.7x + 971.6, R2 = 0.9972).
Statistical analysis
The data were analyzed by ANOVA using the SAS 9.2 statistical program (SAS Institute, Cary, NC, USA). The significance of differences between groups was assessed using multiple comparisons and analysis of variance followed by the Tukey honest significant difference tests. Differences with p values less than 0.05 were considered statistically significant. All measurements were performed on at least three prepared samples and are reported as means and standard deviations.
Results and discussion
Characterization of BC-NEs based on emulsifier type and concentration
The protein type and concentration are known to influence emulsion droplet size, surface protein concentration, and storage stability [7, 15]. Figure 1 presents the surface tensions and particle size profiles, and ζ-potentials of the BC-NEs prepared with Tween 20, WPI, and SC at different concentrations (0.1, 0.5, 1.0, 1.5, 2.0, and 3.0% weight/weight (w/w)). In general, surface tension plays an important role in ensuring droplet stability. The surface tension of the Tween 20-stabilized BC-NEs (control) was 34.7 mN/m, which was lower than that of the protein-stabilized BC-NEs (Fig. 1a) (p < 0.05). For WPI, the increment of WPI concentration significantly diminished the surface tension of the BC-NEs. Meanwhile, the SC-stabilized BC-NEs exhibited the lowest surface tension at 1% SC (p < 0.05). During droplet formation, emulsifiers may be absorbed onto the interfaces of the growing droplets, which will reduce the interfacial tension at detachment and consequently decrease the droplet volume.
Characteristic surface tension (a), droplet size (b), and ζ-potential (c) values of β-carotene NEs stabilized with different concentrations of emulsifiers. “C” was prepared with 1% (w/w) Tween 20. Data are given as the means ± standard deviations (SDs). [a–eSignificant difference depending on concentration at p < 0.05, A–Bsignificant difference depending on protein type at p < 0.05]
The effect of protein concentration on the particle size of the BC-NEs is shown in Fig. 1b. The particle size of the Tween 20-stabilized BC-NEs (control) was 118.5 nm, which was smaller than any of the protein-stabilized BC-NEs (p < 0.05) (Fig. 1b). The particle sizes of the WPI- and SC-stabilized BC-NEs ranged from 175.7 to 374.9 nm and 180.8 to 239.7 nm, respectively. For these BC-NEs, the initial particle sizes dramatically decreased—from 374.9 to 179.3 nm for WPI and from 239.7 to 179.5 nm for SC—as the protein significantly increased in concentration from 0.1 to 0.5% (p < 0.05). At 0.1% protein concentration, the significant difference in particle size of the emulsion system between WPI and SC can be attributed by their adsorption ability. According to the study of Ho et al. [16], SC is more surface active in comparison to the WPI; SC could be more quickly adsorbed to the interface due to the relatively higher amount of nonpolar groups compared to WPI. Therefore, the adsorbed proteins affect the interfacial layer of oil droplets resulting to emulsion stabilization [16]. Except at the 0.1% protein concentration, all the protein-stabilized BC-NEs exhibited particle sizes below 200 nm. This is with similar to previously studied by Jo and Kwon; they also said that BC-NEs had superior storage stability at 1% protein concentration [17]. At 0.1% protein, the BC-NEs displayed relatively large particle sizes, with size distributions that were bimodal in shape (i.e., two peaks were observed for the 0.1% protein) and PDI values greater than 0.6 (not shown). Thus, the lipid droplet surfaces were not saturated with protein, even at the lowest protein concentration (0.1%). However, above 0.5% protein, the protein-stabilized BC-NEs showed no significant difference with respect to protein type and concentration (p > 0.05); their size distribution exhibited a monomodal shape (i.e., only a single peak was observed from 0.5 to 3.0%). Conceivably, this obtained because all oil droplets in the emulsion were fully covered by the protein molecules at a concentration of 0.5%, and the excess protein was not utilized [17].
The ζ-potential measurements indicated that the protein-stabilized BC-NEs had a negative charge at neutral pH. As shown in Fig. 1c, the ζ-potential values of protein-stabilized BC-NEs ranged from − 28.4 to − 43.8 mV. At 0.1% protein, the ζ-potential values of the WPI- and SC-stabilized BC-NEs were − 43.8 and − 40.3 mV, respectively. In particular, the negative ζ-potential values of the WPI-stabilized BC-NEs significantly decreased with increasing WPI concentration (p < 0.05). Previous research showed that a change in the ζ-potential could be attributed to pH dependence, and the pH of the protein-stabilized emulsion was decreased with increasing emulsifier concentration. At relatively high H+ concentrations (low pH), the amino groups are positively charged while the carboxyl groups are neutral; therefore, the net protein charge is positive [17]. In this study, WPI and SC concentrations of 1% were used for studies of oxidation stability and physical stability under environmental stress because these emulsions exhibited good physical stability for all proteins tested.
Physical properties of O/W NEs against environmental stress
Influence of heating
Figure 2a illustrates the changes in particle size of the Tween 20- and protein-stabilized BC-NEs before and after heating at 60 °C for 4 h. The temperature and duration of heat processing were designed to target pasteurization at low temperature in the beverage industry. These processing conditions are very important factors for commercial applications in beverage products. The BC-NEs were stable against droplet aggregation, demonstrating particle sizes below 250 nm and monomodal size distributions after heating. The SC-stabilized BC-NEs exhibited no significant differences upon heating compared with the WPI-stabilized BC-NEs (p > 0.05). SC is well known for its thermal stability due to its disordered structure, so visible changes in aggregation and sedimentation did not occur between emulsion droplets [13]. The WPI-stabilized BC-NE significantly increased (from 165.9 to 215.3 nm) after heating for 4 h (p < 0.05), A slight increase in the particle size may be due to the heat-induced unfolding of the globular whey protein (e.g., WPI) adsorbed on the surface of emulsion droplets [13]. However, visible aggregation and sedimentation changes between emulsion droplets also were not observed.
Changes in droplet size of the β-carotene NEs before and after heating (a at 60 °C for 4 h) and freezing (b at − 20 °C for 24 h). All NEs were prepared with 1% (w/w) emulsifiers [“C” (Tween 20), WPI, SC]. Data are given as the means ± standard deviations (SDs). [a–dSignificant difference depending on emulsifier type at p < 0.05, A–Bsignificant difference depending on processing (heating or freezing) at p < 0.05]
Influence of freezing
Figure 2b illustrates the changes in particle size of the Tween 20- and protein-stabilized BC-NEs before and after freezing at − 20 °C for 24 h. Neither the Tween 20- nor SC-stabilized BC-NE suffered a significant change in particle size after freezing. However, the particle size of the WPI-stabilized BC-NE was dramatically increased to 5 µm (p < 0.05) after freezing, and the size distribution was multi-modal in shape. This result can be explained by water crystallization, which occurs when emulsions are placed in the freezer. As more water crystallizes, the droplets are forced closer together, promoting droplet–droplet interactions [18, 19]. Further, the ice crystals formed during freezing may penetrate the oil droplets and disrupt their interfacial membranes, making them more prone to coalescence [18]. Moreover, when the crystallized emulsions are thawed to ambient temperature, emulsion droplets may undergo partial coalescence due to the penetration of a fat crystal from one droplet through the membrane of another droplet [20]. For these reasons, we can predict that droplets coated with a WPI membrane are less susceptible to water crystallization than droplets coated with SC during freezing and thawing.
Influence of pH
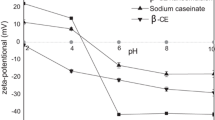
General beverage products range from acidic in soft drinks to slightly basic, and their visual stability without aggregation or sedimentation is very important [21]. Therefore, we investigated the influence of pH on the physical stability of the BC-NEs under different pH conditions (ranging from pH 1 to 13, Fig. 3). The BC-NEs were stable to droplet aggregation at pH 7, 9, 11, and 13, as indicated by the constant particle sizes (< 250 nm) and lack of visible phase separation. Notably, all BC-NEs showed the smallest particle size at neutral pH (pH 7). At that pH, droplets coated with proteins have a relatively high negative charge, which generates a substantial electrostatic repulsive force that contributes to emulsion stability. On the other hand, BC-NEs stored at pH 1, 3, and 5 were highly unstable to droplet aggregation, exhibiting large increases in particle size and visible evidence of phase separation. Large aggregates at low pH can be formed by protein denaturation, which causes structural changes and the eventual loss of emulsifying ability [13]. In addition, this can be attributed to the influence of pH on the electrostatic repulsion between globular protein-coated lipid droplets [7, 22]. In general, the major mechanism preventing droplet aggregation in protein-stabilized O/W emulsions is steric repulsion, and the surface charge of droplets also contributes to the physical stability of the emulsion. When a protein is absorbed onto the surface of a lipid droplet during the formation of an O/W emulsion, its conformation may change, exposing the amino acids to new environments and changing the isoelectric point. To determine if the isoelectric point of the protein was altered by its absorption onto the lipid droplet in the O/W emulsion, the electrical charge of the droplets (ζ-potential) was measured. When the pH is near the isoelectric point of 3–5, the surface net charge of the droplets is close to zero and the repulsive interactive forces between droplets are reduced, resulting in droplets that tend to aggregate [23]. Below the isoelectric point, the emulsion droplets have a relatively high positive surface because the protein amino groups are positively charged whereas the carboxyl groups are neutral [13]. As the pH approaches the isoelectric point, the positively charged droplets tend to balance the negatively charged emulsion droplets. The isoelectronic points of the 1% WPI- and 1% SC-stabilized BC-NEs are 3.45 and 4.50, respectively. Consequently, electrostatic repulsion is insufficient to overcome the van der Waals and hydrophobic attraction forces, leading to droplet aggregation [7]. This suggests that BC-NEs stabilized with proteins should not be incorporated into aqueous products that are weakly acidic (pH 3–5) since droplet aggregation and gravitational separation would be a problem. Once droplet aggregation occurs in a food system, its commercial value significantly drops. Nevertheless, this limitation may be resolved by using a combination of Tween 20 and protein during BC-NE preparation. As shown in Fig. 5a, BC-NEs were prepared with a combination of Tween 20 and protein at a weight ratio of one to one. The BC-NEs prepared with Tween 20 and the proteins had smaller particle sizes than the BC-NEs prepared with the proteins alone (p < 0.05).
Changes in droplet size (a) and isoelectric point (b ζ-potential) of the β-carotene NEs stabilized by Tween 20, WPI, and SC with pH. All NEs were prepared with 1% (w/w) emulsifiers (Tween 20, WPI, SC). Data are given as the means ± standard deviations (SDs). [a–cSignificant difference depending on pH at p < 0.05, A–Csignificant difference depending on emulsifier type at p < 0.05]
Influence of salts
The influence of salt type (NaCl or CaCl2) and concentration (0–200 mM) on protein-stabilized BC-NE stability is shown in Fig. 4. In general, minerals increase the ionic strength of the aqueous phase, which reduces the electrostatic repulsion between the particles through electrostatic screening and/or ionic binding and can lead to droplet aggregation [13, 24]. Moreover, the effect of minerals may also vary considerably in emulsified foods and beverage products. The Tween 20- and protein-stabilized BC-NEs exhibited better stability with NaCl (Fig. 4a). The BC-NEs had particle sizes of less than 200 nm except for the WPI-stabilized BC-NEs at 50 mM NaCl. The NaCl concentration may be insufficient to weaken the electrostatic repulsive forces for droplet aggregation. In other words, the addition of NaCl may have no influence on electrostatic attraction between droplets and Na+ ions [21]. In contrast, the protein-stabilized BC-NEs in CaCl2 solutions were relatively unstable toward droplet aggregation. The particle sizes of the WPI- and SC-stabilized BC-NEs ranged from 165.9 to 3422 nm and 184.5 to 8528 nm, respectively. As shown in Fig. 4b, the particle sizes of the protein-stabilized BC-NEs dramatically increased with increasing CaCl2 concentration (p < 0.05). The results also showed that the WPI-stabilized BC-NEs were relatively more stable than SC-stabilized BC-NEs in CaCl2 solution. A drastic increase in the SC-stabilized BC-NE particle size was observed at 10 mM CaCl2. The results agree with those of Chu, Ichikawa, Kanafusa and Nakajima [13], who reported that Ca2+ ions induced aggregation through ionic binding with caseins. Specific Ca2+ binding to the anionic phosphoseryl residues caused direct calcium bridge formation between casein molecules adsorbed on different particles. According to McClements [7] and Kulmyrzaev, Chanamai and McClements [24], the destabilization of the emulsions at high salt concentrations can be attributed to screening of the electrostatic repulsion between the protein-coated droplets by the salt ions. At relatively low salt levels, the electrostatic repulsion is still sufficiently strong to overcome the van der Waals and hydrophobic attraction forces, but above a critical salt level, this strength declines such that the attractive forces dominate, leading to droplet aggregation [21]. To overcome the droplet aggregation of protein-stabilized BC-NEs in CaCl2 solution, we examined the combined effects of Tween 20 and proteins at different Tween 20: protein ratios (Fig. 5b). The BC-NEs prepared with the Tween 20-protein mixtures exhibited smaller particles than those prepared with protein alone in CaCl2 solution (p < 0.05). Notably, the combined use of a protein with Tween 20 had a larger effect on reducing the particle size of the BC-NEs compared to protein alone in CaCl2 solution. For example, the particle sizes of BC-NEs prepared with 4:1 (w/w) Tween 20:WPI and :SC in 200 mM CaCl2 were 149.7 and 141.6 nm, respectively, compared 3422 and 1801 nm for the WPI- and SC-stabilized BC-NEs alone. Moreover, the higher the ratio of the Tween 20 in the mixture, the smaller the BC-NE particle size (p < 0.05).
Changes in droplet size of the β-carotene NEs stabilized by a combination of Tween 20 and protein (WPI, SC): at a pH 3 and b 200 mM CaCl2. Data are given as the means ± standard deviations (SDs). [aa−bSignificant difference depending on protein type at p < 0.05, A–B significant difference depending on addition of Tween 20 at p < 0.05; ba–bsignificant difference depending on ratio of Tween 20 and protein at p < 0.05, A–Bsignificant difference depending on protein type at p < 0.05]
BC degradation during storage
The chemical stability of the BC-NEs was evaluated based on the BC content over a storage period of 8 weeks at ambient temperature. First, the influence of the emulsifier type (Tween 20, WPI, or SC) on BC degradation in the NEs was examined (Fig. 6a). At the end of the storage period, the percentage of BC in the BC-NEs was reduced to a range of 25.9–51.7%, depending on the emulsifier. The BC in the WPI-stabilized BC-NEs was the most stable, while the lowest BC retention was observed in the Tween 20-stabilized BC-NEs. The BC retention performance decreased in the order WPI (51.7%) > SC (35.0%) > Tween 20 (25.9%) after 8 weeks (p < 0.05). According to the results of Mao, Xu, Yang, Yuan, Gao and Zhao [25] and Jo and Kwon [17], WPI is more effective at inhibiting the degradation of emulsified BC than non-ionic surfactants. There are three reasons for this phenomenon. One is WPI’s function as an antioxidant. Generally, food proteins are known to protect O/W emulsions from lipid oxidation by chelating transition metals or acting as free radical scavengers [21, 26]. Moreover, food proteins exhibit synergistic antioxidant activity in combination with other antioxidant compounds [17]. Second, a layer of absorbed β-lactoglobulin (a major constituent of WPI) at the oil–water interface may act as a physical barrier that prevents any pro-oxidants in the aqueous phase from contacting the BC present within the droplets [27]. Third, the oil–water interface is smaller in the protein-stabilized BC-NEs than the Tween 20-stabilized BC-NEs. When BC degradation occurs at the oil–water interface, a smaller surface area may lead to a slower reaction rate [25].
The influence of pH on the chemical stability of the BC-NEs was also examined with respect to their commercial application in food and beverage products. As mentioned above, the pH of the aqueous phase has significant implications because most beverage products range from acidic to slightly basic [21]. BC-NEs were prepared, diluted in different pH solutions (pH 3 or 7), and then stored at ambient temperature for 8 weeks (Fig. 6b). The rate of BC degradation was appreciably faster at pH 3 than at pH 7. After 2 weeks, the BC in BC-NEs dramatically decreased at pH 3 (p < 0.05), and the BC retentions in the Tween 20-, WPI-, and SC-stabilized BC-NEs of 22.0%, 33.2%, and 27.0%, respectively. After 6 weeks, the BC retention in all the BC-NEs was below 20%, except for the WPI-stabilized BC-NEs diluted at pH 7. Boon, McClements, Weiss and Decker [28] reported similar results, in which the rate of carotenoid degradation in O/W emulsions was higher at acidic pH values. According to Mortensen and Skibsted [29], carotenoids are protonated in the presence of acid and then undergo cis–trans isomerization and additional degradation reactions. This suggests that BC-NEs cannot be incorporated into acidic beverage products since BC degradation dynamically occurs.
Conclusions
In this study, the results highlight two important implications for the use of BC in processed foods. First, the physical stability of BC-NEs is responsive to environmental stresses during food processing, and it is influenced by the emulsifier type (Tween 20, WPI, SC). In particular, protein-stabilized BC-NEs (especially SC) were shown to be prone to droplet aggregation at acidic pH values (3–5) and Ca2+ ion strengths exceeding 10 mM. Second, BC degradation from an O/W emulsion system is influenced by emulsifier type and pH conditions. The results of this study demonstrate that WPI-stabilized BC-NEs are more stable toward chemical degradation than Tween 20-stabilized BC-NEs. Moreover, the rate of BC degradation at acidic pH (pH 3) is faster than at neutral pH (pH 7), regardless of emulsifier type. Current results indicated that proteins-stabilized BC-NEs are dependent on environmental stress, which in turn results could be high droplet aggregation and BC degradation under acidic pH values and high Ca2+ ion strengths. This would be useful information to make foods, especially functional beverage that are both healthy and sustainable. Further investigation is still required to evaluate the bioavailability and bioaccessibility of emulsion systems in order to apply this work to commercial technology in real food and beverage industries.
References
D. Albanes, Am. J. Clin. Nutr. 69, 1345s–1350 (1999) s
M.M.V. Naves, F.S. Moreno, Nutr. Res. 18, 1807–1824 (1998). https://doi.org/10.1016/S0271-5317(98)00137-7
D.-O. Ha, C.U. Park, M.-J. Kim, J. Lee, Food Sci. Biotechnol. 21, 607–611 (2012)
S. A.Hentschel, R.H. Gramdorf, T. Müller, Kurz, J. Food Sci. 73, N1–N6 (2008). https://doi.org/10.1111/j.1750-3841.2007.00641.x
L. Cornacchia, Y.H. Roos, J. Agric. Food Chem. 59, 7013–7020 (2011)
N. Garti, D.J. McClements, Encapsulation Technologies and Delivery Systems for Food Ingredients and Nutraceuticals, 1st edn. (Woodhead Publishing, UK, 2012), pp. 211–244
D.J. McClements, Food Emulsions: principles, Practices, and Techniques, 3rd edn. (CRC Press, Boca Raton, 2015), pp. 55–99
U. Buranasuksombat, Y.J. Kwon, M. Turner, B. Bhandari, Food Sci. Biotechnol. 20, 793–800 (2011)
M. Fathi, M.-R. Mozafari, M. Mohebbi, Trends Food Sci. Technol. 23, 13–27 (2012)
J. Rao, E.A. Decker, H. Xiao, D.J. McClements, J. Sci. Food Agric. 93, 3175–3183 (2013)
L. Salvia-Trujillo, C. Qian, O. Martín-Belloso, D. McClements, Food chem. 141, 1472–1480 (2013)
D. Xu, F. Yuan, Y. Gao, A. Panya, D.J. McClements, E.A. Decker, Food chem. 156, 374–379 (2014)
B.-S. Chu, S. Ichikawa, S. Kanafusa, M. Nakajima, J. Sci. Food Agric. 88, 1764–1769 (2008)
D. Djordjevic, D.J. McClements, E.A. Decker, J. Food Sci. 69, C356–C362 (2004)
M. Hu, D.J. McClements, E.A. Decker, J. Agric. Food Chem. 51, 1696–1700 (2003)
K.K. Ho, K. Schroën, M.F. San Martín-González, C.C. Berton-Carabin, Food Struct. 12, 34–42 (2017)
Y.-J. Jo, Y.-J. Kwon, Food Sci. Biotechnol. 23, 107–113 (2014)
T. Aoki, E.A. Decker, D.J. McClements, Food Hydrocoll. 19, 209–220 (2005)
H. Saito, A. Kawagishi, M. Tanaka, T. Tanimoto, S. Okada, H. Komatsu, T. Handa, J. Colloid Interface Sci. 219, 129–134 (1999)
T. Harada, K. Yokomizo, J. Am. Oil Chem. Soc. 77, 859–864 (2000)
C. Qian, E.A. Decker, H. Xiao, D.J. McClements, Food Chem. 135, 1440–1447 (2012)
K. Demetriades, J.N. Coupland, D.J. McClements, J. Food Sci. 62, 342–347 (1997)
D. Guzey, D.J. McClements, J. Agric. Food Chem. 55, 475–485 (2007)
A. Kulmyrzaev, R. Chanamai, D.J. McClements, Food Res. Int. 33, 15–20 (2000)
L. Mao, D. Xu, J. Yang, F. Yuan, Y. Gao, J. Zhao, Food Technol. Biotechnol. 47, 336–342 (2009)
C. Berton, M.-H. Ropers, M. Viau, C. Genot, J. Agric. Food Chem. 59, 5052–5061 (2011)
D.J. McClements, E.A. Decker, J. Food Sci. 65, 1270–1282 (2000)
C.S. Boon, D.J. McClements, J. Weiss, E.A. Decker, J. Agric. Food Chem. 57, 2993–2998 (2009)
A. Mortensen, L.H. Skibsted, J. Agric. Food Chem. 48, 279–286 (2000)
Acknowledgements
This research was supported by Basic Science Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT and Future Planning (No. 2014M3A7B4051898).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Jo, YJ., Choi, MJ., Hong, GP. et al. A comparison of physicochemical stabilities of β-carotene-loaded nanoemulsions prepared with different food proteins. Food Measure 13, 1373–1381 (2019). https://doi.org/10.1007/s11694-019-00053-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11694-019-00053-3