Abstract
The characteristics of internal quality attributes (firmness, soluble solids content and color values) of the Tokaloglu apricot cultivar (Prunus armeniaca L.) were predicted nondestructively using Fourier Transform-Near Infrared (FT-NIR) spectroscopy. Calibration methods were developed between the physical parameters, which were measured using standard methods, and the spectral measurements (in reflectance mode between 780 and 2500 nm) using Partial Least Squares method (PLS). Good correlations were obtained in calibration and validation procedures for Magness-Taylor (MT) maximum force, with a coefficient of determination (R2) of 0.82 (RMSEE = 4.45) in calibration and 0.80 (RMSECV = 4.68) in validation for multiple-harvest (MH) apricot group. The coefficient of determination (R2) for predicting MT slope was 0.79 (RMSEE = 0.83) in calibration and 0.77 (RMSECV = 0.88) in validation for the MH apricot group while it was 0.56 (RMSEE = 0.69) in calibration and 0.47 (RMSECV = 0.80) in validation for single-harvest (SH) apricot group. Good correlations were obtained for MT area with the coefficient of determination (R2) of 0.75 (RMSEE = 20.1) in calibration and R2 = 0.71 (RMSECV = 21) in validation for MH group. Good prediction values were obtained for soluble solids content for both applications (MH and SH) using FT-NIR spectroscopy: the best coefficient of determination was obtained for MH application with 0.77 (RMSEE = 1.45) in calibration and 0.75 (RMSECV = 1.51) in validation. Correlation values for prediction of chroma and hue were low for MH application, with R2 = 0.55 (RMSECV = 3.38) for chroma and with R2 = 0.16 (RMSECV = 0.49) for hue. The results showed that NIR spectroscopy has a good potential to predict internal quality of apricots non-destructively, however it has a limited ability to predict color features.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Apricot (Prunus armeniaca L.) is a very important product with its growing potential for Turkey. Sorting agricultural products manually are costly and time consuming, and may result false decisions about the quality of the produce. Sorting apricots nondestructively based on their internal and external physical properties is very attractive for the most efficient evaluation of the product. Soluble solids content (SSC) and firmness are important properties which mostly determine consumer perception for internal quality indices of apricot fruit; however, measurements of SSC and firmness are still destructive. Destructive firmness measurement techniques are not acceptable in automated fruit classification systems which evaluate every single fruit for its quality.
Some researchers have attempted to use optical techniques to develop a non-destructive means for assessing fruit and vegetable quality. Near-infrared spectroscopy (NIRS) is such a technique that has been used to nondestructively measure internal quality in a wide range of fruits and vegetables such as mango [22], kiwifruit [18, 23], apple [7, 12–14, 16], peach [9], apricot [4–6, 17, 19], cherries [6], tomato [21, 26] and pork [2]. Besides, the near-infrared (NIR) process is to be fast and cheap for measuring the quality of agricultural products.
Lu [15] predicted firmness and sugar content of sweet cherries using NIR diffuse reflectance spectroscopy with good correlation values for both quality features (with r values of 0.80, SEP = 0.79 N for firmness and of 0.95, SEP = 0.71 for Brix). Flores et al. [8] estimated internal quality parameters of intact tomatoes using NIR spectroscopy, developing correlation models for predicting SSC with R2 between 0.63 and 0.82, and TA with R2 between 0.41 and 0.71. Liu and Ying [13] used FT-NIR to evaluate the influence of the distance between the light source/detection probe and the fruit for measuring the sugar content of Fuji apples. Yongni et al. [26] measured the quality characteristics of tomato including fruit firmness, soluble solid contents (SSC) and acidity (pH) with Vis/NIRS. The best correlation coefficient (R2 = 0.90) was obtained for SSC in tomatoes. Lammertyn et al. [12] used nondestructive infrared spectroscopy technique with the wavelength range of 380–1650 nm to measure quality characteristics (pH, firmness, SSC) of Jonagold apples. Quality parameters such as pH, firmness and soluble solids content were successfully predicted with R2 values of 0.93, 0.90 and 0.82, respectively.
Gómez et al. [10] detected the internal quality of Satsuma mandarin using NIR reflection mode in the wavelength range of 350–2500 nm. The measurements were obtained at three different equidistant positions of the fruit. SSC (R2 = 0.94) was the best correlation compared to the firmness (R2 = 0.83) and pH (R2 = 0.81).
Slaughter and Crisosto [23] predicted fructose and glucose contents in kiwifruit using NIR spectroscopy. PLS analysis of the kiwifruit spectra indicated that 800–1000 nm was the best subset of the 700–1100 nm NIR region scanned for predicting fructose and glucose. PLS calibration model resulted good predictions for fructose and glucose contents of intact kiwifruit, with R2 = 0.96.
Although apricot was studied in various limited works such as by Bureau et al. [4], Carlini et al. [6], and Camps and Christen [5], the effect of data groups on the success of prediction models has not been investigated thoroughly in terms of variation and cultivar. Mostly multiple cultivars have been included in similar studies allowing wide variation in data groups.
Overall objective of this work was to predict quality parameters of soluble solids content, firmness and color of apricot fruit non-destructively using FT-NIR spectroscopy. In addition using a single cultivar in the experiments and two data groups with different variations in the physical constituents of the samples may be attributed as prominent contributions of this study: With this aim, it was expected to see if the variations in data groups would have any effect on the success of the prediction models developed using partial least square (PLS) method.
Materials and methods
Tokaloglu apricot (Prunus armeniaca L.) variety was used in this study. Hand harvested samples were obtained from a commercial apricot orchard in Canakkale region in 2007. Two apricot groups were included in the study. In the first one, apricots were harvested periodically (with 2 days intervals) from the same tree starting early in maturation stage (June 8) and ending in late maturation stage (June 28) (multiple-harvest, MH). The second apricot group was obtained by harvesting apricots only once in the beginning of their maturation stage (June 11) and keeping them in the cold storage (single-harvest, SH). A total of 440 apricots were harvested from the selected tree for the MH application while a total of 400 apricots were harvested (from the same tree with MH) and stored at cold storage (±0.5 °C, 90–95 % relative humidity) for SH application. With these two data groups, which were the results of applying two different harvesting schemes, it was expected to create two apricot groups (MH, SH) with different variations of the physical properties. For the first group (MH), around 50 samples were harvested periodically every other day from the same tree. Spectral and physical measurements of MH apricots were performed on the same day of harvest. Similarly, for the second group (SH), 50 samples were removed from cold storage and kept in room conditions (24 °C) for about 14 h to equilibrate their temperature before the measurements.
Spectroscopic measurements
Spectral measurements of all the samples (MH and SH) were performed in reflectance mode using a Bruker MPA (Multi-Purpose Analyzer) FT-NIR spectrometer (Bruker Optik, GmbH, Ettlingen Germany) equipped with an InGaAs detector (TE-InGaAs) and a 20-W high intensity NIR light source (tungsten-halogen). Spectral measurements were performed in a dark room to avoid the effect of ambient light. Reflectance measurements obtained via the fiber optic probe were in the wavelength range of 780–2500 nm. Thirty-two scans were performed for each reflectance spectrum in about 15.32 s. Resolution was 8 cm−1. Instrument control and spectra analysis were performed using OPUS software (Bruker Optik, GmbH, Ettlingen Germany).
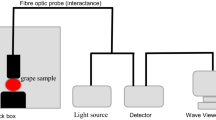
Reflectance spectra were obtained from the reference (Spectralon®, Bruker Optik, GmbH, Ettlingen Germany) and the sample consecutively for each sample measurement. Fiber optic probe was placed directly on the cheek of the fruit, which represents the fruit color the most, during spectral measurements. The fiber optic probe used had a bifurcated optical configuration which guided the light to the sample by the source fibers and received the reflected light with the detector (TE-InGaAs) fibers (Fig. 1). In the measuring head of the fiber optic probe, the source and detector fibers were mingled randomly forming a sensing area of about 11.7 mm2.
Physical quality measurements of apricots
Measurements of the physical properties of apricots were performed following the spectral measurements. These measurements were color, weight, size (diameter and length), firmness, and SSC (Table 1).
Color was measured after the spectral measurements using a colorimeter (Model CR-200, Minolta, Japan). Color was measured twice from the two opposite equatorial surfaces of the apricots. Color readings were recorded in the format of CIE XYZ color space (also known as CIE 1931 color space). They were then converted into Lab color space (CIE L*a*b*) which is an absolute color space. Chroma (\(\sqrt{a{{*}^{2}}+b{{*}^{2}}}\)) and hue (arc tan [b*/a*]) were derived from Lab color space.
Apricot firmness was measured using a Chatillon penetrometer, model DFS-500 (John Chatillon Sons, Inc. New York, NY, USA) with a flat-head stainless-steel cylindrical probe having a 6 mm diameter and without removing the skin. Velocity in application of the probe was around 0.75 mm s−1. Firmness measurements were performed on both cheeks of the fruit placing the probe close to the area where spectroscopic measurement was done. Penetration depth of the probe into the flesh of an apricot was between 6 and 8 mm. Strain energy (i.e., the area under the force/deformation curve until the maximum force, N-mm), slope (N/mm, ratio of force to the deformation until the maximum force) and maximum force (N) were extracted from the force/deformation curves and were considered to be the measures of fruit firmness [11].
SSC was measured using a digital refractometer (model RF-104 BP, 32-10, Atago Honcho, Itabashi-ku, Tokyo, Japan) which had an accuracy of 0.2 % and a scale range of 0–32 %. Refractometer readings were performed after squeezing couple of juice drops from each sample.
Spectra analysis
Relative spectra of the samples were obtained directly from the software as a result of the following calculation:
The wavelength range between 780 and 2500 nm was analyzed to find the optimal sub-wavelength ranges for the best correlations between physical quality parameters and spectral measurements. Calibration and validation models were developed using the partial least squares (PLS) with leave-one-out cross validation technique for predicting quality parameters of firmness, SSC, hue and chroma. In leave-one-out cross validation technique, calibration was made by leaving one sample out and using that sample in validation. This process is repeated until all the samples have been used in validation for once. PLS analysis was done for the common wavelength ranges in the full spectrum of the samples to determine the optimal sub-wavelength ranges using OPUS software. The performance of the calibration model was determined from the averages of the repeated procedures separately in calibration and validation [11].
Different processing techniques were performed on apricot spectra for possible improvements in the performance of the calibration models in PLS analysis in addition to testing different wavelength ranges. For this aim, three different spectrum processing techniques were applied to the spectra of apricots. In the first approach, no processing was applied to the spectra except smoothing. In the second approach, following spectrum processing techniques were applied on the spectra one at a time: constant offset elimination, min–max normalization, vector normalization, straight line subtraction, first derivative, second derivative, multiplicative scattering correction. In the last approach, two processing techniques, one after another, were applied on the apricot spectra: first derivative and straight line subtraction, first derivative and vector normalization, and first derivative and multiplicative scattering correction. Mean centering was applied on all the spectra, processed or not. The OPUS software (Bruker Optic, GmbH, Ettlingen, Germany) was used for establishing calibration and validation models. The coefficient of determination, the root mean squared error of estimation (RMSEE) and the root mean squared error of cross validation (RMSECV) were used to evaluate the performances of the calibration models [11].
Results and discussion
As seen in Table 1, although average MT slope and MT area firmness values of the two apricot groups (MH and SH) are close to each other, SD values were higher for MH data group. On the other hand, MT maximum force firmness measure and SD value related with this feature were higher for MH apricot group compared to SH group. These findings can highly be due to earlier harvests performed for MH group. Also, higher SD in firmness values of MH group compared to SH group may be explained with higher variation in firmness values of the MH group which was periodically harvested during a period of 18 days. In Fig. 2, MH firmness values decreased sharply during the harvest period while the decrease in SH firmness values occurred in a narrower range. Similarly, SSC values for MH apricot group increased quite steeply along with the harvest days. On the other hand, SSC values for SH apricot group did not show the same pattern with MH group; SSC values of the SH apricot group increased during the first couple of days of cold storage and then started to decrease for about a week and then it started to increase again toward the end of the storage period (Fig. 2). The difference between the SSC values of the two apricot groups is likely due to keeping the SH apricots in cold storage while letting the MH apricots to continue growing on the tree. Robertson et al. [20] reported a similar change pattern for SSC of peaches kept at cold storage at two different maturation levels. They explained this wavy change pattern of SSC of peaches at cold storage with decreasing trend of sucrose during storage despite the increases in glucose and fructose contents. Some of the apricots treated differently and kept in cold storage showed similar SSC change patterns as in this study [3]. Chroma color values of the MH apricots were slightly higher compared with SH apricots. The hue values were the same for both applications. There were slightly more change in the color of the MH apricots as they matured more rapidly on the tree and this may explain higher chroma and SD values for both color indices of MH apricots.
Firmness prediction
Good prediction was achieved for firmness (MT, maximum force) for MH apricot group (R2 = 0.80, RMSECV = 4.68) while it was quite low for SH apricot group compared to MH group (R2 = 0.41, RMSECV = 5.11) (Table 2; Fig. 3a, b). Similarly, when MT slope and MT area were considered as the firmness measures, predictions of firmness were successfully performed for MH apricot group (R2 = 0.77, RMSECV = 0.88, R2 = 0.71, RMSECV = 21, respectively) while the prediction success was poor for SH group (Table 2).
Effective wavelength ranges and peaks for optimal firmness, SSC and color predictions for MH and SH apricot sample groups are given in Table 3. The effective wavelength ranges for MH apricot group were between 800 and 1836 and between 2173 and 2355 nm which contained peaks at 975, 1166, 1443, 1783, 1926 and 2380 nm (Table 3). Effective wavelength range for SH group included all absorption peaks, which were between 800 and 2355 nm (Table 3). Zude et al. [27] pointed out that due to water and carbohydrate overtones absorption occurred around 960 nm in NIR, which is in agreement with the findings in this work. In addition, Mcglone and Kawano [18] reported the same absorption peaks in their study on postharvest quality assessment of kiwifruit.
Prediction of soluble solids content (SSC)
Good correlation results were obtained between the NIR spectra and SSC values for both apricot groups (MH and SH) (Table 2): Prediction results of SSC for MH apricot group (R2 = 0.75, RMSECV = 1.51) were slightly better than the results obtained for SH apricot group (R2 = 0.74, RMSECV = 1.36) (Table 2; Fig. 4a, b). Also the SSC prediction results obtained for calibration and validation were close to each other which shows that it is a good model for predicting SSC. Although the change patterns of the SSC for both apricot groups (MH and SH) were not completely the same, they overall looked alike; in another explanation, SSCs for both MH and SH apricot groups increased first and then decreased drastically for a longer time for SH group while it decreased slightly and for a shorter time for MH group. SSC for MH group increased steeply after a short decrease while it started to increase after around a week of decrease for SH group (Fig. 2).
The effective wavelengths in predicting SSC were between 1333 and 1836 nm for both MH and SH apricot groups, while the wavelength range of 2173–2300 nm was also effective for the SH group (Table 3). The effective peaks were at 1926, 1783 and 1166 nm for both applications for the prediction of SSC constitution. Effective wavelengths of 951, 1017, 1053, 1067, 1117, 1153 and 1191 nm were important for predicting SSC content of post-storage apricots [17]. Abu-Khalaf and Bennedsen [1] obtained a good correlation coefficient (79.9 %) and RMSEP (1.56) for predicting SSC in plums. The most important NIR ranges for modeling SSC prediction were 778–810, 902–935 and 960–990 nm for plums.
Color prediction
Color prediction results were shown in Table 2 for both apricot groups. The best chroma prediction was obtained for MH apricot group with R2 = 0.55 (RMSECV = 3.38) while the prediction obtained for SH group was low with R2 = 0.40 (RMSECV = 2.73), (Table 2; Fig. 5a, b). Hue prediction results were extremely lower compared to chroma prediction. The effective wavelength ranges for predicting chroma were 800–1836 nm and 2174–2355 nm for MH apricot group, while they were 1333–1639 nm and 1835–2355 nm for SH apricot group (Table 3). The effective peaks occurred at 1926, 1783, 1443, 1166 and 975 nm for both apricot groups. The β-carotenes content is a very important constituent of apricot color and wavelengths from 1111 to 2500 nm includes the above mentioned peaks for prediction of color. In a previous study, β-carotenes content was predicted with a high correlation for calibration (R2 = 0.94) compared to a low correlation for cross-validation (R2 = 0.26) [19].
Reflectance measurements
In average relative absorbance spectra of MH apricot group, differences between early, middle and late harvests of apricots can be seen clearly in Fig. 6. Important peaks were at 975, 1165, 1444, 1783, 1926 and 2380 nm in the average spectra of MH apricot group.
Similar absorption peaks were shown in Fig. 7 for SH apricot group in reflectance mode. The peaks at approximately 1700 nm were defined as protein and sugar peaks; peak at 1734 nm was approved as the protein band [23]. The water absorption peaks were specified at 975, 1168, 1443 and 1926 nm [25].
Conclusion
According to the results, FT-NIR spectroscopy provides successful predictions of internal quality of apricots. Firmness (MT maximum force, slope and area) predictions were achieved with superior results for MH apricot group (R2 = 0.80, 0.77, 0.71, respectively) compared the SH apricot group (R2 = 0.41, 0.47, 0.36). Having superior results for MH apricot group compared to those of SH group for firmness prediction models may explain the effect of variation in the data groups examined. As was expected, better prediction results were obtained for MH apricot group which had wider variations in firmness and SSC values.
Prediction of SSC was successfully performed for both apricot groups (MH and SH) resulting following determination of coefficients (R2) of 0.75 and 0.74, respectively. Prediction of SSC was better than firmness prediction for the SH apricot group. As a result good prediction results for SSCs were obtained for both data groups with slightly superior results for MH group which had a higher SD.
However, poor results were obtained for the prediction of apricot color compared to other constituents. Consequently, FT-NIR spectroscopy has a good potential to be used for determining the internal quality attributes of fruits such as apricot non-destructively. Regarding to the effect of variation in the data group one can say that variation has a positive effect on the success of the prediction model. Firmness and SSC predictions for MH apricot group in this study may support this approach. However, further studies are needed to clarify this result. Future studies should be conducted including more cultivars and also with different variation levels.
References
N. Abu-Khalaf, B.S. Bennedsen, Plum-tasting using near infra-red (NIR) technology. Int. Agrophy. 16, 83–89 (2002)
J.M. Balage, S.L. Silva, C.A. Gomide, M.N. Bonin, A.C. Figueira, Predicting pork quality using Vis/NIR spectroscopy. Meat Sci 108, 37–43 (2015)
R. Botondi, A. Crisa, R. Massantini, F. Mencarelli, Effects of low oxygen short-term exposure at 15 C on postharvest physiology and quality of apricots harvested at two ripening stages. J. Hort. Sci. Biotechnol. 75(2), 202–208 (2000)
S. Bureau, D. Ruiz, M. Reich, B. Gouble, D. Bertrand, J.M. Audergon, C.M.G.C. Renard, Rapid and non-destructive analysis of apricot fruit quality using FT-near-infrared spectroscopy. Food Chem. 113, 1323–1328 (2009)
C. Camps, D. Christen, Non-destructive assessment of apricot fruit quality by portable visible infrared spectroscopy. Food Sci. Technol. 42, 1125–1131 (2009)
P. Carlini, R. Massantini, F. Mencarelli, Vis–NIR measurement of soluble solids in cherry and apricot by PLS regression and wavelength selection. J. Agric. Food Chem. 48, 5236–5242 (2000)
G. Fan, J. Zha, R. Du, L. Gao, Determination of soluble solids and firmness of apples by Vis/NIR transmittance. J. Food Eng. 93, 416–420 (2009)
K. Flores, M.T. Sanches, D. Perez-Martin, J.E. Guerrero, A. Gorrido-Varo, Feasibility in NIRS instruments for predicting internal quality in intact tomato. J. Food Eng. 91, 311–318 (2009)
X. Fu, Y. Ying, Y. Zhou, XIE L., H. Xu, Application of NIR spectroscopy for firmness evaluation of peaches. J. Zhejiang Univ. Sci. 9, 552–557 (2008)
H. Gómez, Y. He, A.G. Pereira, Non-destructive measurement of acidity, soluble solids and firmness of Satsuma mandarin using Vis/NIR-spectroscopy techniques. J. Food Eng. 77, 313–319 (2006)
M.B. Kavdir, R. Buyukcan, R. Lu, H. Kocabiyik, M. Seker, Prediction of olive quality using FT-NIR spectroscopy in reflectance and transmittance modes. Biosyst. Eng. 103, 304–312 (2009)
J. Lammertyn, B. Nicolai, K. Ooms, V. De Smedt, J. DeBaerdemaeker, Non-destructive measurement of acidity, soluble solids, and firmness of Jonagold apples using NIR-spectroscopy. Trans. ASAE 41, 1089–1094 (1998)
Y.D. Liu, Y.B. Ying, Measurement of sugar content in Fuji apples by FT-NIR spectroscopy. J. Zhejiang Univ. Sci. 5, 651–655 (2004)
R. Lu, D.E. Guyer, R.M. Beaudry, Determination of firmness and sugar content of apples using near-infrared diffuse reflectance. J. Texture Studies 31, 615–630 (2000)
R. Lu, Predicting firmness and sugar content of sweet cherries using near-infrared diffuse reflectance spectroscopy. Trans. ASAE 44, 1265–1271 (2001)
R. Lu, D. Ariana, A near-infrared sensing technique for measuring internal quality of apple fruit. Appl. Eng. Agric. 18, 585–590 (2002)
M. Manley, E. Joubert, L. Myburgh, E. Lotz, M. Kidd, Prediction of soluble solids content and post-storage internal quality of Bulida apricots using near-infrared spectroscopy. J. Near-Infrared Spectrosc. 15, 179–188 (2007)
V.A. McGlone, S. Kawano, Firmness, dry-matter and soluble-solids assessment of postharvest kiwifruit by NIR spectroscopy. Postharvest Biol. Technol. 13, 131–141 (1998)
D. Ruiz, M. Reich, S. Bureau, C. Renard, J.M. Audergon, Application of reflectance colorimeter measurements and infrared spectroscopy methods to rapid and nondestructive evaluation of carotenoids content in apricot (Prunus armeniaca L.). J. Agric. Food Chem. 56, 4916–4922 (2008)
J.A. Robertson, F.I. Meredith, R.J. Horvat, S.D. Senter, Effect of cold storage and maturity on the physical and chemical characteristics and volatile constituents of peaches (cv. Cresthaven). J. Agric. Food. Chem. 38(3), 620–624 (1990)
A.G. Saad, P. Jaiswal, S.N. Jha, Non-destructive quality evaluation of intact tomato using VIS–NIR spectroscopy. Int. J. Adv. Res. 2, 632–639 (2014)
S. Saranwong, J. Sornsrivichai, S. Kawano, Prediction of ripe-stage eating quality of mango fruit from its harvest quality measured nondestructively by near infrared spectroscopy. Postharvest Biol. Technol. 31, 137–145 (2004)
D. Slaughter, C.H. Crisosto, Nondestructive internal quality assessment of kiwifruit using near-infrared spectroscopy. Semin. Food Anal. 3, 131–140 (1998)
P.C. Williams, in Near Infrared Technology in the Agricultural and Food Industries, ed. by P. Williams, K. Norris, Implementation of near infrared technology, (American Association of Cereal Chemists, Minnesota, 2001), pp. 145
P. Williams, K. Norris, Near Infrared Technology in the Agriculture and Food Industries, 2nd edn. (American Association of Cereal Chemists, Minnesota, 2001), pp. 268
S. Yongni, H. Yong, H.G. Antihus, G.P. Annia, Q. Zhengjun, Z. Yun, Visible/Near-Infrared spectroscopic technique for nondestructive assessment of tomato ‘Heatwave’ (Lycopersicum esculentum) quality characteristics. J. Food Eng. 81, 672–678 (2007)
M. Zude, B. Herold, J.M. Roger, V. Bellon-Maurel, S. Landahl, Non-destructive tests on the prediction of apple fruit flesh firmness and soluble solids content on tree and in shelf life. J. Food Eng. 77, 254–260 (2006)
Acknowledgments
The authors acknowledge the financial support of Canakkale Onsekiz Mart University Council of Scientific Research Projects (COMU-BAP, project 2007/31) for this study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Buyukcan, M.B., Kavdir, I. Prediction of some internal quality parameters of apricot using FT-NIR spectroscopy. Food Measure 11, 651–659 (2017). https://doi.org/10.1007/s11694-016-9434-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11694-016-9434-9