Abstract
Near-infrared (NIR) spectroscopy is a useful technique for the non-destructive analysis of fruit quality. The key quality parameters of table grapes (Vitis vinifera) that affect consumer preference are the soluble solids content (SSC), pH, firmness, and seedlessness. This research focused on using NIR spectroscopy for assessing the quality of ‘Kyoho’ table grapes, as a non-destructive analysis under laboratory and field conditions. NIR spectra for each sample were acquired in the wavelength range of 400–1000 nm, using a visible/NIR spectrometer with fibre optics in the interactance mode. Partial least-square regression was used to calibrate the NIR spectral data with all the measured properties of table grapes. The best prediction model for firmness was the Savitzky–Golay first derivative (SGD1) with a coefficient of determination (R2prediction ) of 0.7427 in the laboratory, and 0.7804 in the field. The R2prediction values for pH in the laboratory and the field was 0.6276 using multiplicative scatter correction (MSC), and 0.7676 using SGD1, respectively. These values were similar to the R2prediction values of SSC, which were 0.6926 using MSC, and 0.8052 using the Savitzky–Golay second derivative, respectively. In both analyses the R2 of the calibration model was between 0.6944 and 0.8877. The partial least-square discriminant analysis was used to classify the percentage of seedlessness, which was 93.10% in the laboratory using SGD1 or MSC, and 79.31% in the field using MSC. Therefore, NIR spectroscopy is an efficient non-destructive technique for rapidly analysing Japanese table grape qualities in laboratory and field settings.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The key quality parameters of table grapes are the soluble solids content (SSC), titratable acidity, and pH (Piazzolla et al. 2017). The conventional methods for the analysis of grape quality are time-consuming, expensive, involve sample destruction, and require complicated preparation procedures. Near-infrared spectroscopy (NIRS) is an alternative technique for non-destructive fruit quality assessment. It can evaluate the internal starch, SSC, oil content, water content, dry matter content, acidity, firmness, stiffness factor, and other physiological properties of fruit and vegetable products (Slaughter et al. 2003; Nicolai et al. 2007; Shao and He 2007), such as watermelon (Sun et al. 2010), passionfruit (Maniwara et al. 2014), paddy rice (Siriphollakul et al. 2015), apple (Bobelyn et al. 2010), jujube (Wang et al. 2011), and mango (Saranwong et al. 2004). It can also determine the harvest times of table grapes (Piazzolla et al. 2017), classify the phytosanitary of wine grapes (Giovenzana et al. 2017), and monitor the ripening process of wine grapes whilst they are still on the vine (González-Caballero et al. 2012).
Chemometrics allows the development of calibration and validation models from near-infrared (NIR) spectra for predicting and managing the quality of fruit products. Giovenzana et al. (2017) applied partial least-square–discriminant analysis (PLS-DA) to grape spectra in order to test the performance of visible/NIR spectroscopy in classifying healthy and infected bunches. Interestingly, the non-destructive analysis of Japanese table grapes by NIRS techniques has not yet been reported. Therefore, this study aimed to develop a non-destructive method for the rapid analysis of Japanese table grapes for quality management using the NIRS technique.
2 Materials and methods
2.1 Raw materials
Experiments were carried out at the Niigata Agricultural Research Institute, Horticultural Research Centre, Niigata, Japan. Grape inflorescences were selected in the vineyard for both seeded and seedless treatments. The seedless treatment was applied by spraying 25 ppm GA3 twice during the flowering stages and then 14 days later, while there was no spraying for the seeded treatment. After that, three grapes berries from three positions on the fruit bunch (top, middle, and bottom) from 15 bunches of seeded and seedless treatments were collected on three harvesting dates: 28 August 2018 (79 days after flowering [DAF]), 3 September 2018 (85 DAF) and 11 September 2018 (93 DAF). Each berry was analysed by NIRS in the field (Fig. 1), and then NIRS analysis and chemometric analysis were performed in the laboratory (Fig. 2) at the Faculty of Environmental Science for Agriculture and Forestry, Niigata University (Niigata, Japan).
2.2 Analysis methods
2.2.1 NIRS analysis
Interactance spectra were recorded in the laboratory and the field using a NIRS spectrometer equipped with a PICL-NEX light source, WaveView software, a Handy Lambda II detector, and a fibre optic probe. Each spectrum was acquired in the wavelength range of 400–1000 nm, with a resolution of approximately 3 nm (total of 181 wavelengths).
2.2.2 Chemical analysis
The SSC and pH reference data for each sample were determined using a digital refractometer (PR-101α, Atago, Japan) and a pH meter (HM-30P, Japan), respectively.
2.2.3 Physical analysis
The firmness was obtained by texture analysis using a texture analyser (Shimpo, Japan), and the seedlessness was determined by cutting and counting. The percentage of seedlessness classification was calculated as follows:
where Ss is the number of correctly detected or predicted as seedless berries, and St is the total number of berry samples.
2.2.4 Chemometric analysis
Chemometric analysis was undertaken to develop the calibration model (57 samples) and prediction model (29 samples), based on the relationship between the NIRS data and all of the measured properties (SSC, pH, firmness, and seedlessness). The pre-treatments, including the Savitzky–Golay first derivative (SGD1), Savitzky–Golay second derivative (SGD2), and multiplicative scatter correction (MSC), were studied using partial least-square regression (PLSR) and partial least-square–discriminant analysis (PLS-DA) using Unscrambler software (version 10.5; Camo, Oslo, Norway).
Moreover, in this study the model’s efficiency was evaluated using the following statistical values: coefficient of determination (R2), standard error of calibration (SEC), and standard error of prediction (SEP), which are defined in Eqs. (2)–(4).
where xi is the predicted value of firmness, SSC, or pH from each observation; yi is the measured value; and np is the number of observations used in the validation set.
3 Results and discussion
3.1 Chemical and physical analyses
The firmness, pH, and SSC data of the seedless and seeded ‘Kyoho’ grape berries recorded at 79, 85, and 93 DAF (Table 1) were used in the calibration and prediction sets. Throughout the fruit development, the seeded grape was less firm than the seedless grape, presenting values of 9.39, 5.88, and 7.74 N versus 13.62, 9.59, and 13.38 N at 79, 85, and 93 DAF, respectively. In contrast, the SSC and pH of the seeded grapes were higher than the seedless grapes in the context of the maturity of the grape berry. The seeded grape will develop faster than the seedless grape because the exogenous GA3 application interferes with seed development and inhibits the growth of the seeds and the berry in seeded grapes, such as ‘Kyoho’ and ‘Red Globe’ cultivars (Cheng et al. 2013). In conventional analyses of the chemical and physical qualities, berries are typically destroyed. The analysis can only be carried out with random sampling. Conversely, NIRS can determine the aforementioned quality parameters and the berries can remain intact and continue to develop.
3.2 Raw spectrum
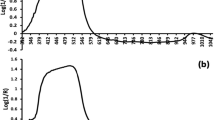
The raw spectra (400–1000 nm) of the ‘Kyoho’ grape were pre-processed in the laboratory and the field, as shown in Fig. 3. In both locations, the spectra showed the same peak at around 720–950 nm, but the original spectrum acquired in the field was noticeably higher compared with that recorded in the laboratory. There were several environmental factors affecting the in-field spectral wavebands. Variations in the air humidity and berry temperature, as well as instrumental noise significantly affected the spectral information, especially for the near infrared region. Such variations were addressed with suitable data pre-processing techniques thereafter (Ji et al. 2015).
3.3 Calibration and prediction analyses
The raw spectra of the ‘Kyoho’ grape both in the laboratory and the field, along with the physical parameters (firmness, pH, and SSC) were analysed and modified by PLSR using three different pre-treatments: SGD1, SGD2, and MSC. A total of 57 samples was used for developing the calibration model, and 29 samples were used for developing the prediction model. The results are displayed in Table 2 and Fig. 4.
From the analysis, the R2 of prediction and SEP of firmness were 0.7427 and 1.6101 N in the laboratory, and 0.7804 and 1.4870 N in the field, respectively, when pre-treated by SGD1. For the calibration model, the R2 and SEC of firmness were 0.8249 and 1.3902 N in the laboratory, and 0.8877 and 1.1135 N in the field, respectively. There were no notable differences between the values recorded in the laboratory and in the field, thus suggesting that NIRS can be applied in the field as a decision-making tool for determining harvesting date and quality management. These R2 values were greater than 0.64, which indicates that the models were suitable for screening the physical attributes (SSC, pH, and firmness) of the grapes (R2 = 0.64–0.81) and produced reliable predictions (R2 = 0.81–0.90) (Williams 2007). Fu et al. (2008) verified the usefulness of NIRS in predicting the firmness of white peach using the PLSR method. In that work, 120 peaches were used for calibration, and 60 for prediction. For each sample, reflectance spectra were acquired in the range of 800–2500 nm. The best results were obtained with MSC spectra pre-treatment (R2calibration = 0.948 and R2validation = 0.864) (Fu et al. 2008).
The R2prediction of pH in the laboratory and the field were 0.6276 and 0.7676 while the SEP were 0.0699 and 0.0552, respectively. The R2calibration of pH in the laboratory and the field were 0.7198 and 0.8099 when pre-treated using MSC and SGD1, respectively. Thus, this PLSR model was acceptable. A similar result was found when analysing the NIR reflectance spectra (350–2500 nm) of Satsuma mandarin (Citrus reticulata) (Gómez et al. 2006). For pH, the R2calibration was 0.865, and the SEC was 0.106 (Gómez et al. 2006). When the calibration model was used to predict the data for 30 unanalysed mandarins, the prediction result was reasonable (r = 0.81), and the SEP was 0.18 with a bias of –0.028 (Gómez et al. 2006). The PLS approach appeared to be acceptable since seven factors were used in the calibration model (Gómez et al. 2006).
For SSC in the laboratory and the field, the R2prediction were 0.6926 and 0.8052, the SEP were 0.8129% and 0.6452%, and the R2calibration were 0.6944 and 0.7613 by MSC and SGD2, respectively. The performance of these models was somewhat comparable to that developed for the SSC of the ‘Nebbiolo’ wine grape, which showed an R2prediction of 0.82, SEP of 7.31%, R2calibration of 0.72, and SEC of 5.89% (Guidetti et al. 2010). These values were based on data obtained using NIRS (380–1650 nm) on 25 wine grapes (red and white) whilst they were ripening on the vine. Cross-validation results indicated that the NIRS technique provided excellent precision for SSC (R2 = 0.94). This confirms that the NIRS technique is well-suited for evaluating the internal quality characteristics related to firmness, pH, and SSC. Moreover, according to González-Caballero et al. (2011), this approach enables the non-destructive quantification of chemical changes that take place during on-vine ripening and helps with the decision regarding the optimal timing for harvesting.
3.4 Loading weight analysis
The R2prediction of the best pre-treatment of each parameter was analysed by the loading weight. The results are shown in Table 3 and Fig. 5 The SGD1 was the best pre-treatment for firmness in both the laboratory and the field, which both showed the peak at 693.1 nm was similar to that at 769.7–773.0 nm. The best pre-treatment for pH was MSC in the laboratory and SGD1 in the field. The peak wavelength was 931.4–934.7 nm. The best pre-treatment for SSC was MSC in the laboratory and SGD2 in the field, the peak wavelengths were 928.1–924.9 and 934.7 nm, respectively. From the R2 and peak wavelength data, the parameters (firmness, pH, and SSC) were quite similar between the laboratory and the field. This suggests that in-field NIRS analysis will provide results that are comparable to those obtained from analysis in the laboratory. When evaluating the loading weight vectors from the PLS-DA of wine grape NIR spectra (1600–2400 nm) between ripening stages, the sugar peaks were around 1750 and 2067 nm, and the water peaks were around 1900 and 1970 nm (González-Caballero et al. 2012).
3.5 Percentage of seedlessness classification
The seedlessness of the ‘Kyoho’ table grape, which was exposed to GA3 applications during cultivation, is very important for obtaining a premium price in Japan. It is difficult to detect the presence or absence of seeds without destroying the sample. In this context, NIRS analysis is an interesting approach. The NIR spectrum of the ‘Kyoho’ grape and the seedlessness data in the laboratory and the field were analysed using the PLS-DA prediction model. A total of 57 grape samples on the last harvesting date (93 DAF) were analysed by Unscrambler software for constructing the calibration model, and 29 samples were used for the prediction model.
Table 4 shows the percentage of seedlessness classification using PLS-DA, the best result was obtained from the laboratory with 93.1% accuracy using the SGD1 spectral pre-processing technique. In contrast, a percentage of seedlessness classification of 79.3% was obtained in the field test upon MSC pre-treatment. This is the first study to report a seedlessness analysis for Japanese table grapes using NIRS. In the PLS-DA models developed by Guidetti et al. (2010), for 77.1% of both ripe and unripe wine grapes their SSC was correctly classified by the calibration model and 68.8% was correctly classified by the validation model.
4 Conclusion
The results from this study confirmed that the quality of Japanese table grapes can be efficiently predicted both in the laboratory and in the field. Interactance measurements of wavebands from 400 to 1100 nm with spectral acquisition of PLSR are considered promising techniques for predicting berry firmness, juice pH levels, and SSC, as well as the percentage of seedlessness classification. Some modifications of the optical apparatus for NIR measurements are still needed in order to increase prediction accuracy and reproducibility.
References
Bobelyn E, Serban A, Nicu M, Lammertyn J, Nicolai BM, Saeys W (2010) Postharvest quality of apple predicted by NIR-spectroscopy: study of the effect of biological variability on spectra and model performance. Postharvest Biol Technol 55:133–143. https://doi.org/10.1016/j.postharvbio.2009.09.006
Cheng C, Xu X, Singer SD, Li J, Zhang H, Gao M, Wang L, Song J, Wang X (2013) Effect of GA3 treatment on seed development and seed-related gene expression in grape. PLoS ONE 8(11):e80044. https://doi.org/10.1371/journal.pone.0080044
Fu X, Ying Y, Zhou Y, Xie L, Xu H (2008) Application of NIR spectroscopy for firmness evaluation of peaches. J ZHEJIANG UNIV-SC B 9(7):552–557. https://doi.org/10.1631/jzus.B0720018
Giovenzana V, Beghi R, Brancadoro L, Guidetti R (2017) Classification of wine grape based on different phytosanitary status by using visible/near infrared spectroscopy. Chem Eng Trans 58:331–336. https://doi.org/10.3303/CET1758056
Gómez AH, He Y, Pereira AG (2006) Non-destructive measurement of acidity, soluble solids and firmness of Satsuma mandarin using Vis/NIR-spectroscopy techniques. J Food Eng 77:313–319. https://doi.org/10.1016/j.jfoodeng.2005.06.036
González-Caballero V, Pérez-Marin D, López M, Sánchez M (2011) Optimization of NIR spectral data management for quality control of grape bunches during on-vine ripening. Sensors 11(6):6109–6124. https://doi.org/10.3390/s110606109
González-Caballero V, Sánchez M, Fernández-Novales J, López M, Pérez-Marin D (2012) On-vine monitoring of grape ripening using near-infrared spectroscopy. Food Anal Methods 5:1377–1385
Guidetti R, Beghi R, Bodria L (2010) Evaluation of grape quality parameters by a simple vis/NIR system. Biol Eng Trans 53(2):1–8. https://doi.org/10.13031/2013.29556
Ji W, Viscarra Rossel RA, Shi Z (2015) Accounting for the effects of water and the environment on proximally sensed vis-NIR soil spectra and their calibrations. Eur J Soil Sci 66:555–565. https://doi.org/10.1111/ejss.12239
Maniwara P, Nakano K, Boonyakiat D, Ohashi S, Hiroi M, Tohyama T (2014) The use of visible and near infrared spectroscopy for evaluating passion fruit postharvest quality. J Food Eng 143:33–43. https://doi.org/10.1016/j.jfoodeng.2014.06.028
Nicolai BM, Beullens K, Bobelyn E, Peirs A, Saeys W, Theron KI, Lammertyna J (2007) Nondestructive measurement of fruit and vegetable quality by means of NIR spectroscopy: a review. Postharvest Biol Technol 46(2):99–118. https://doi.org/10.1016/j.postharvbio.2007.06.024
Piazzolla F, Amodio ML, Colelli G (2017) Spectra evolution over on-vine holding of Italia table grapes: prediction of maturity and discrimination for harvest times using a Vis-NIR hyperspectral device. J Agric Eng 48:109–116. https://doi.org/10.4081/jae.2017.639
Saranwong S, Sornsrivichai J, Kawano S (2004) Prediction of ripe-stage eating quality of mango fruit from its harvest quality measured nondestructively by near infrared spectroscopy. Postharvest Biol Technol 31:137–145. https://doi.org/10.1016/j.postharvbio.2003.08.007
Shao Y, He Y (2007) Nondestructive measurement of the internal quality of bayberry juice using Vis/NIR spectroscopy. J Food Eng 79:1015–1019. https://doi.org/10.1016/j.jfoodeng.2006.04.006
Siriphollakul P, Kanlayanarat S, Rittiron R, Wanitchang J, Suwonsichon T, Boonyaritthongchai P, Nakano K (2015) Pasting properties by near-infrared reflectance analysis of whole grain paddy rice samples. J Innov Opt Health Sci 8(6):1550035
Slaughter DC, Thompson JF, Tan ES (2003) Nondestructive determination of total and soluble solids in fresh prune using near infrared spectroscopy. Postharvest Biol Technol 28:437–444. https://doi.org/10.1016/S0925-5214(02)00204-1
Sun T, Huang K, Xu H, Ying Y (2010) Research advances in nondestructive determination of internal quality in watermelon/melon: a review. J Food Eng 100:569–577. https://doi.org/10.1016/j.jfoodeng.2010.05.019
Wang J, Nakano K, Ohashi S (2011) Nondestructive evaluation of jujube quality by visible and near-infrared spectroscopy. LWT- Food Sci Technol 44:1119–1125. https://doi.org/10.1016/j.lwt.2010.11.012
Williams P (2007) Applications to agricultural and marine products: grains and seeds. In: Ozaki Y, McClure WF, Christy AA (eds) Near-infrared spectroscopy in food science and technology. Wiley, Hoboken, pp 165–218
Acknowledgements
This study was performed under the Double Degree Program between Niigata University and Chiang Mai University; and the Niigata Agricultural Research Institute, Horticultural Research Centre, Niigata, Japan.
Funding
This study was not funded by any company or organisation.
Author information
Authors and Affiliations
Contributions
Chaorai Kanchanomai is the main researcher who implemented the research, analysed the results, and wrote the manuscript with support from Phongkrit Maniwara. Shintaroh Ohashi, Daruni Naphrom, and Kazuhiro Nakano are advisors who designed, commented, and proofed the experiment together with Chaorai Kanchanomai. Wakana Nemoto is an assistant researcher who supported the operation in the field and the laboratory and also interpreted the data with the other authors.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical standards
All the procedures performed in studies that involved human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
Human and animal participants
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Communicated by Heakeun Yun, Ph.D.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kanchanomai, C., Ohashi, S., Naphrom, D. et al. Non-destructive analysis of Japanese table grape qualities using near-infrared spectroscopy. Hortic. Environ. Biotechnol. 61, 725–733 (2020). https://doi.org/10.1007/s13580-020-00256-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13580-020-00256-4