Abstract
Purpose
To describe three new species of Cosmetocleithrum in the gills of Trachelyopterus galeatus (Siluriformes, Auchenipteridae) from Aguapeí River, Upper Paraná River basin, São Paulo State, Brazil.
Methods
Fifty-three specimens of T. galeatus were captured in the mouth of the Aguapeí River from August 2013 to June 2014. Monogeneans were mounted unstained in Hoyer’s and Gray and Wess’s medium.
Results
Cosmetocleithrum spathulatum sp. n., Cosmetocleithrum baculum sp. n., and Cosmetocleithrum galeatum sp. n. differ from all known congeneric species mainly in the morphology of the accessory piece (i.e. spatulate-shaped, claviform, and a straight rod with hook-shaped distal portion, respectively). Also, the three new species share hooks with different sizes with hooks pairs 5 and 7 bigger than others and with an erect delicate point, inconspicuous thumb, longer shaft, and slender shank.
Conclusions
To date, 18 species of Cosmetocleithrum were recognised parasitizing siluriforms in the Neotropical region. The present study expands the number to 21 species, however, despite this increase, the number of known taxa of monogeneans in neotropics is far from representing the ideal situation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Freshwater fishes of the order Siluriformes present a rich fauna of gill monogeneans in the Neotropical region [1,2,3]. Among Siluriformes, fishes of the family Auchenipteridae harbour dactylogyrids from three genera in Brazil and Peru: Cosmetocleithrum Kritsky, Thatcher and Boeger, 1986, Demidospermus Suriano, 1983, and Vancleaveus Kritsky, Thatcher and Boeger, 1986 [4].
Cosmetocleithrum was erected by Kritsky et al. [5] to accommodate dactylogyrids parasitizing siluriforms in the Neotropical region. Species of this genus are characterized mainly by having a dorsal bar with two submedial projections. To date, 18 valid species of Cosmetocleithrum are known to parasitize freshwater fishes of the families Pimelodidae, Doradidae, and Auchenipteridae [3, 5,6,7,8,9,10] (Table 1). Among them, four species parasitize hosts of the family Auchenipteridae: Cosmetocleithrum striatuli Abdallah, Azevedo and Luque, 2012 from Trachelyopterus striatulus (Steindachner, 1877) and Auchenipterus nuchalis (Spix and Agassiz, 1829), Cosmetocleithrum laciniatum Yamada, Yamada, Silva and Anjos, 2017 from Trachelyopterus galeatus (Linnaeus, 1766), Cosmetocleithrum berecae Cohen, Justo, Gen and Boeger, 2020, and Cosmetocleithrum nunani Cohen, Justo, Gen and Boeger, 2020 from Auchenipterus nuchalis, all species described in Brazil.
During a parasitological survey of freshwater fishes from an environmental protection area, Aguapeí River, Upper Paraná River basin, São Paulo State, Brazil, were retrieved specimens of monogeneans on the gills of T. galeatus. Herein, we describe three new species of Cosmetocleithrum in this fish host.
Materials and Methods
Fifty-three specimens of T. galeatus (standard length in cm: 13.66 ± 1.61 [mean ± sd] and 11–17 [range] were captured in the mouth of the Aguapeí River (21° 03′03.16″ S; 51° 45′58.16″ W) (the map of the study area is available in [11]) from August 2013 to June 2014. The hosts were collected under the license number 577/2015 IBAMA (Brazilian Institute of Environment and Renewable Natural Resources) and SP/538/88 DEFOP (Department of the Development of Fishery and Inspection). All animal procedures were performed in full compliance with the Animal Experimentation Ethics Commission (Authorisation No.120-CEEA of the São Paulo State University—UNESP). In the laboratory, fishes were identified according to Graça and Pavanelli [12].
After capture, some hosts were frozen and later examined in the laboratory, whereas some freshly killed hosts were examined in situ, having the monogeneans recovered and fixed with a glycerin–ammonium picrate (GAP) mixture to study their sclerotized structures [13]. In the laboratory, gills were removed and placed in Petri dishes with tap water, and then examined under a stereomicroscope. Monogenean specimens were mounted unstained in Hoyer’s and Gray and Wess’s medium [5].
The parasites were analysed using a V3 Leica Application Suite (LAS) computerised system for image analysis adapted in a microscope with differential interference contrast. Drawings were obtained with the aid of a camera lucida mounted on a Leica DMLS microscope equipped with phase-contrast optics. Measurements are in micrometers and expressed as the mean is followed by the range and the number of specimens measured in parentheses. Landmark definition and morphometric measurements of haptor sclerotized parts and copulatory complex were taken according to Gussev [14] and Řehulková et al. [15] (Fig. 1). The numbering and distribution of hook pairs follow Mizelle [16]. Specific terminology of the genus Cosmetocleitrum follows Kritsky et al. [5]. The prevalence and mean intensity of infection were calculated following Bush et al. [17].
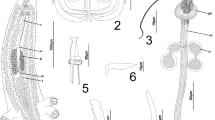
Scheme of measurements for the sclerotized structures of the haptor and copulatory complex of Cosmetocleitrum spp.: a total length of anchor, b length of base, c length of superficial root, d length of deep root, e length of point, f total length of hook, g total length of bar, h bar height, i bar width, j total curve length of MCO (dash line), k accessory piece length
Type and voucher specimens were deposited in the Helminthological Collection of the Instituto Oswaldo Cruz (CHIOC), Rio de Janeiro, Brazil. Other paratypes were deposited in the Helminthological Collection of the Institute of Biosciences (CHIBB), Botucatu, São Paulo State, Brazil.
Results
Dactylogyridae Bychowsky, 1933.
Ancyrocephalinae Bychowsky, 1937.
Cosmetocleithrum Kritsky, Thatcher and Boeger, 1986.
Cosmetocleithrum spathulatum sp. n.
(Fig. 2a–h).
Cosmetocleithrum spathulatum sp. n. from the gills of Trachelyopterus galeatus (Linnaeus, 1766). a Whole mount in ventral view (composite); b Copulatory complex (ventral view); c Hook pairs 1–4 and 6; d Hook pairs 5 and 7; e Ventral bar; f Dorsal bar; g Ventral anchor; h Dorsal anchor. Scale bars: a = 100 µm; b–h = 20 µm
Taxonomic Summary
Type-host: Trachelyopterus galeatus (Linnaeus, 1766) (Siluriformes: Auchenipteridae).
Type-locality: Mouth of the Aguapeí River, Upper Paraná River, São Paulo State, Brazil (21° 03′03.16″ S and 51° 45′58.16″ W).
Site of infestation: Gill lamellae.
Type-material: Holotype, CHIOC 39420a; Paratypes, CHIOC 39420b, 39421a, 39421b and CHIBB 607L, 608L, 609L, 610L, 611L, 612L, 613L, 614L.
Prevalence and mean intensity of infestation: 94% (50 of 53 fishes examined) and 21.54 parasites per parasitized host.
Etymology: The specific name derives from Latin (spathula = “spatula”) and refers to the shape of the base of the accessory piece.
Description
[Based on 25 specimens: seven fixed in GAP, five stained with Gray and Wess´s medium, and 13 stained with Hoyer's medium]. Body fusiform 385 (286–527, n = 10) long, 126 (71–192, n = 9) wide near mid-length (Fig. 2a). Three bilateral pairs of head organs poorly developed. Eye-spot granules subspherical, scattered throughout cephalic region. Pharynx spherical 26 (20–33, n = 8) in diameter, muscular, glandular; esophagus short. Two intestinal caeca confluent posteriorly to testis, lacking diverticula. Gonads in tandem, germarium pre-testicular 36 (31–45, n = 3) long, 26 (24–27, n = 3) wide. Testis posterior to germarium 53 (48–62, n = 3) long, 25 (18–32, n = 3) wide; vas deferens looping left intestinal caecum. Seminal vesicle a dilation of vas deferens; prostatic reservoir spherical, anterior to seminal vesicle. Copulatory complex (Fig. 2b) comprising male copulatory organ (MCO) 56 (50–64, n = 17) long, sclerotized, coiled, with about 1½ counterclockwise ring, a sclerotized base bulb-shaped; and an accessory piece 22 (18–27, n = 18) long, not articulated with MCO, spatula-shaped, distally bifurcate, robust, distal portion serving as guide from middle portion of MCO. Vagina single, muscular, with thickened wall opening on left margin of body. Peduncle narrow; haptor subhexagonal 89 (78–104, n = 7) long, 80 (65–98, n = 9) wide, with seven pairs of hooks, with ancyrocephaline distribution. Ventral bar with enlarged ends (Fig. 2e): (g) 28 (22–34, n = 20); (h) 5 (3–8, n = 19); (i) 3 (2–5, n = 21). Dorsal bar yoked-shaped, with two submedial projections (Fig. 2f): (g) 27 (21–33, n = 19); (h) 5 (4–7, n = 19); (i) 4 (3–7, n = 20). Anchors similar, with well-developed roots, tapered superficial root, conspicuous deep root, evenly curved shaft and elongated point. Ventral anchor (Fig. 2g): (a) 27 (25–29, n = 19); (b) 23 (20–25, n = 19); (c) 10 (8–11, n = 19); (d) 2 (1–3, n = 19); (e) 12 (8–14, n = 19). Dorsal anchor (Fig. 2h): (a) 25 (24–27, n = 20); (b) 22 (19–24, n = 20); (c) 10 (7–11, n = 20); (d) 2 (2–3, n = 20); (e) 11 (8–14, n = 20). Hooks pairs 1–4 and 6 (Fig. 2c) 15 (14–16, n = 22) long, with FH loop about 60% shank length, slender uniform shank, expanded tip, posteriorly directed thumb, straight shaft, and evenly curved point. Hooks pairs 5 and 7 (Fig. 2d)16 (16–17, n = 8) long, with FH loop about 50% shank length, erect shaft, straight shank, erect delicate point, and inconspicuous thumb. Oviduct, ootype, seminal receptacle, uterus, and egg not observed. Vitelline follicles dense, dispersed throughout trunk but absent in region of reproductive organs and MCO.
Remarks
Cosmetocleithrum spathulatum sp. n. differs from all previously known congeneric species in the morphology of its accessory piece, i.e. spatulate-shaped proximal region. The new species closely resembles Cosmetocleithrum parvum Kritsky, Thatcher and Boeger, 1986, Cosmetocleithrum phryctophallus Soares, Neto and Domingues, 2018, Cosmetocleithrum tortum Mendoza-Franco, Mendoza-Palmero and Scholz, 2016, and Cosmetocleithrum trachydorasi (Acosta, Scholz, Blasco-Costa, Alves and da Silva, 2018) Cohen, Justo, Gen and Boeger, 2020 by possessing a vaginal vestibule cup-shaped. Despite this similarity, only C. tortum presents a dextral vagina. Cosmetocleithrum spathulatum sp. n. resembles Cosmetocleithrum longivaginatum Suriano and Incorvaia, 1995, Cosmetocleithrum bifurcum Mendoza-Franco, Mendoza-Palmero and Scholz, 2016, C. tortum, and C. nunani which present hooks dissimilar in size. However, the new species can be distinguished from these species, by presenting members of hooks pairs 5 and 7 bigger than others and with an erect delicate point, inconspicuous thumb, longer shaft, and slender shank.
Cosmetocleithrum baculum sp. n.
(Fig. 3a–h).
Cosmetocleithrum baculum sp. n. from the gills of Trachelyopterus galeatus (Linnaeus, 1766). a Whole mount in ventral view (composite); b Copulatory complex (ventral view); c Hook pairs 1–4 and 6; d Hook pairs 5 and 7; e Ventral bar; f Dorsal bar; g Ventral anchor; h Dorsal anchor. Scale bars: a = 100 µm; b–h = 20 µm
Taxonomic Summary
Type-host: Trachelyopterus galeatus (Linnaeus, 1766) (Siluriformes: Auchenipteridae).
Type-locality: Mouth of the Aguapeí River, Upper Paraná River, São Paulo State, Brazil (21° 03′03.16″ S and 51° 45′58.16″ W).
Site of infestation: Gill lamellae.
Type-material: Holotype, CHIOC 39417; Paratypes, CHIOC 39418, 39419 and CHIBB 618L, 619L.
Prevalence and mean intensity of infestation: 17% (9 of 53 fishes examined) and 1.22 parasites per parasitized host.
Etymology: The specific name derives from Latin (baculum = “a little stick”) and refers to the clavate shape of the accessory piece.
Description
[Based on 18 specimens: 15 stained with Hoyer’s medium, one stained with Gray and Wess’s medium and two fixed in GAP]. Body fusiform and robust 667 (509–814, n = 11) long, 155 (117–198, n = 10) wide near midlength (Fig. 3a). Three pairs of head organs poorly developed. Eye-spot granules subspherical, scattered throughout cephalic area. Pharynx spherical 35 (26–44, n = 6) in diameter, esophagus short. Gonads in tandem, germarium pre-testicular 58 (44–67, n = 3) long, 50 (38–65, n = 3) wide. Testis posterior to germarium 93 (68–121, n = 3) long, 48 (37–58, n = 3) wide; vas deferens looping left intestinal caecum. Seminal vesicle a dilation of vas deferens; prostatic reservoir spherical, anterior to seminal vesicle. Copulatory complex (Fig. 3b) comprising MCO 126 (111–143, n = 17) long, with about 2½ counterclockwise rings with a sclerotized base; and an accessory piece 35 (24–46, n = 16) long not articulated to cirrus base, claviform. Vagina sinistral, marginal, and slightly sclerotized. Peduncle short, broad; haptor globose 153 (106–210, n = 12) long, 119 (94–152, n = 10) wide, with 7 pairs of hooks, with ancyrocephaline distribution. Ventral bar yoked-shaped, with an anterior protuberance at middle portion and with enlarged ends (Fig. 3e): (g) 45 (39–50, n = 12); (h) 11 (8–12, n = 10); (i) 9 (6–11, n = 12). Dorsal bar yoked-shaped, with two submedial projections (Fig. 3f): (g) 48 (41–53, n = 12); (h) 11 (9–13, n = 12); (i) 7 (5–10, n = 12). Ventral anchor, with well-developed roots, tapered superficial root, evenly curved shaft and short point (Fig. 3g): (a) 50 (47–53, n = 14); (b) 43 (38–47, n = 14); (c) 18 (14–21, n = 14); (d) 5 (3–6, n = 14); (e) 16 (13–21, n = 12). Dorsal anchor, triangular superficial root, deep root inconspicuous, evenly curved shaft and short point (Fig. 3h): (a) 44 (40–52, n = 14); (b) 38 (34–44, n = 14); (c) 15 (13–21, n = 14); (d) 4 (3–5, n = 13); (e) 13 (12–15, n = 13). Hooks dissimilar in size; each with slender uniform shank, expanded tip, posteriorly directed thumb, straight shaft, and evenly curved point. Hooks pairs 1–4 and 6 (Fig. 3c) 20 (18–25, n = 66) long, with FH loop about 70% shank length, slender uniform shank, expanded tip, posteriorly directed thumb, straight shaft, and evenly curved point. Hooks pairs 5 and 7 (Fig. 3d) 26 (24–26, n = 28) long, with FH loop about 60% shank length, erect shaft, straight shank, erect delicate point, and inconspicuous thumb. Oviduct, ootype, seminal receptacle, uterus, and egg not observed. Vitelline follicles dense, dispersed throughout trunk but absent in the region of reproductive organs and MCO.
Remarks
Cosmetocleithrum baculum sp. n. closely resembles C. longivaginatum, C. striatuli, C. laciniatum, and Cosmetocleithrum leandroi Soares, Neto and Domingues, 2018 by the shape of the dorsal (yoked-shaped) and ventral bar (straight with enlarged ends). The new species can be distinguished from all congeners in having MCO base with a single lateral flap, accessory piece clavate shaped, and ventral bar with anteromedial rotund projection. It is noteworthy that Cosmetocleithrum baculum sp. n. also possesses hooks with different sizes and similar morphology to Cosmetocleithrum spathulatum sp. n.
Cosmetocleithrum galeatum sp. n.
(Fig. 4a–h).
Cosmetocleithrum galeatum sp. n. from the gills of Trachelyopterus galeatus (Linnaeus, 1766). a Whole mount in ventral view (composite); b Copulatory complex (ventral view); c Hooks pairs 1–4 and 6; d Hooks pairs 5 and 7; e Ventral bar; f Dorsal bar; g Ventral anchor; h Dorsal anchor. Scale bars: a = 100 µm; b–h = 20 µm
Taxonomic Summary
Type-host: Trachelyopterus galeatus (Linnaeus, 1766) (Siluriformes, Auchenipteridae).
Type locality: Mouth of the Aguapeí River, Upper Paraná River, São Paulo State, Brazil (21° 03′03.16″ S and 51° 45′58.16″ W).
Site of infestation: Gill lamellae.
Type-material: Holotype, CHIOC 39414; Paratypes, CHIOC 39415, 39416 and CHIBB 622L, 623L, 624L, 625L, 626L.
Prevalence and mean intensity of infestation: 91% (48 of 53 fishes examined) and 5.04 parasites per parasitized host.
Etymology: The specific name galeatum refers to the specific name of the type-host.
Description
[Based on 25 specimens: 2 fixed in GAP, 3 stained with Gray and Wess’s medium, and 20 stained with Hoyer´s medium]. Body fusiform, robust, 652 (431–917, n = 17) long, 157 (115–230, n = 20) wide near mid-length (Fig. 4a). Cephalic lobes poorly developed. Four pairs of head organs. Eye-spot absent, granules scattered in the cephalic region. Pharynx spherical 39 (24–57, n = 11) in diameter, esophagus inconspicuous. Two intestinal caeca confluent posterior to gonads, lacking diverticula. Gonads in tandem, germarium pre-testicular 21 (n = 1) long, 11 (n = 1) wide. Testis posterior to germarium 46 (n = 1) long, 25 (n = 1) wide; vas deferens looping left intestinal caecum. Seminal vesicle a dilation of vas deferens; seminal receptacle anterior to germarium, prostatic reservoir present. Copulatory complex (Fig. 4b) comprising MCO 396 (317–471, n = 23) long, with 6 counterclockwise rings, with irregularly sclerotized base; and a straight rod accessory piece 36 (25–46, n = 19) with hook-shaped distal portion, guarding termination of MCO, and non-articulated. Vagina a poorly sclerotised elongate tube opening on left margin of body. Peduncle short, broad; haptor sub-square 128 (87–173, n = 13) long, 110 (84–183, n = 11) wide, with 7 pairs of hooks with ancyrocephaline distribution. Ventral bar yoked-shaped (Fig. 4e): (g) 43 (33–49, n = 20); (h) 10 (8–13, n = 20); (i) 7 (5–11, n = 20). Dorsal bar yoked-shaped, with two submedial projections, and a posteromedial protuberance (Fig. 4f): (g) 39 (30–46, n = 17); (h) 10 (7–12, n = 16); (i) 7 (4–9, n = 17). Anchors similar, each with tapering superficial root, short deep root, short shaft with the presence of grooves, elongate point. Ventral anchor, with well-developed roots, superficial root elongate, short and straight shaft, elongated point with slightly curved tip (Fig. 4g): (a) 48 (42–55, n = 22); (b) 40 (36–44, n = 22); (c) 19 (14–22, n = 22); (d) 5 (2–9, n = 22); (e) 17 (13–20, n = 22). Dorsal anchor, with well-developed roots, superficial root elongate, evenly curved shaft and short point with slightly curved tip (Fig. 4h): (a) 42 (38–46, n = 19); (b) 35 (29–39, n = 19); (c) 15 (12–19, n = 19); (d) 5 (3–6, n = 19); (e) 12 (9–16, n = 19). Hooks different in size. Hooks pairs 1–4 and 6 (Fig. 4c) 17 (16–19, n = 50) long, (smallest in length) with FH loop about 50% shank length, slender uniform shank, expanded tip, posteriorly directed thumb, straight shaft, and evenly curved point. Hooks pairs 5 and 7 (Fig. 4d) 21 (18–23, n = 25) long, (largest in length) morphologically distinct from remaining hooks with FH loop about 30% shank length, erect shaft, erect delicate point, straight shank, and inconspicuous thumb. Oviduct, ootype, uterus, prostatic reservoir, and egg not observed. Vitelline follicles dense, dispersed throughout trunk but absent in the region of reproductive organs and MCO.
Remarks
Cosmetocleithrum galeatum sp. n. closely resembles C. longivaginatum by sharing similar morphology of the MCO with six counterclockwise rings, and the shape of the vaginal canal, but differs from this species in having a straight rod accessory piece with a hook-shaped distal portion (a straight rod accessory piece with bifid proximal portion). The morphology of accessory piece of the new species is most similar to C. trachydorasi, however, Cosmetocleithrum galeatum sp. n. can be distinguished from this species by presenting MCO with six counterclockwise rings (a single counterclockwise ring in C. trachydorasi). This species resembles Cosmetocleithrum baculum sp. n. in the general morphology of the bars. Also, Cosmetocleithrum galeatum sp. n. possesses hooks with different sizes and similar morphology to Cosmetocleithrum spathulatum sp. n., and Cosmetocleithrum baculum sp. n.
Discussion
Currently, studies on monogenean diversity from freshwater fishes in the neotropics have progressively increased [4]. This finding is promoting due to an increasing number of studies mainly focused on fishes from Amazon and Paraná basins in South America [18,19,20]. In particular, freshwater fishes of the order Siluriformes present 14 genera of dactylogirids in the Neotropical region [1,2,3]. According to Cohen et al. [10], Demidospermus Suriano, 1983 and Cosmetocleithrum are the most representatives genera. Mendoza-Palmero et al. [21] consider catfish as a suitable group of host for an extraordinarily rich and diverse fauna of gill monogeneans, that represents an interesting model for phylogenetic studies in Neotropical region.
Cosmetocleithrum spp. were originally described parasitizing gills of doradid hosts from the Amazon basin [5]. To date, the majority species of Cosmetocleithrum is restricted to hosts of this family [3, 5]. Other congeners were described from the gills of pimelodids and auchenipterids. Cosmetocleithrum striatuli, C. laciniatum, C. berecae, C. nunani, and the three new species here described also have been found parasitizing host of Auchenipteridae. Cosmetocleithrum bulbocirrus Kritsky, Thatcher and Boeger, 1986 is the only species that have been reported in fishes of three families (Doradidae, Auchenipteridae, and Erythrinidae) [3]. Nevertheless, Graça et al. [22] considered the infestation with C. bulbocirrus in Hoplias aff. malabaricus (Bloch, 1794) (Characiformes, Erythrinidae) as accidental due to low prevalence (1.8%) and intensity of infestation (one single monogenean per infected fish). Recently, C. rarum and C. longivaginatum were reported by Yamada et al. [23] in T. galeatus. However, these species were reviewed in this study and correctly described as Cosmetocleithrum baculum sp. n. and Cosmetocleithrum galeatum sp. n., respectively.
Previously, Suriano and Incorvaia [6] reported the presence of hooks with similar morphology and a different size in C. longivaginatum from Pimelodus albicans Valenciennes, 1840 in Argentina. Afterward, Mendoza-Franco et al. [24] and Cohen et al. [10] pointed out differences in size and morphology of hooks in C. bifurcum, C. tortum, and C. nunani, respectively. In the present study, the three new species recovered from T. galeatus also showed these differences on hooks (Table 2). So, based on the fact that the original description of the genus by Kritisky et al. [5] did not mention hooks with different size and morphology, we propose these differences as an emended morphological diagnosis.
Cohen et al. [10] comparing Cosmetocleithrum gussevi Kritsky, Thatcher and Boeger, 1986 (type species of Cosmetocleithrum) and Paracosmetocleithrum trachydorasi, highlighted their similarity and, in this way, proposing a new combination to P. trachydorasi as C. trachydorasi. Also, these authors distinguished two groups among Cosmetocleithrum species according to bars and accessory piece morphologies: the first group comprises species with non-articulated bars and accessory piece distally bifid, and the second species with articulated bars with accessory piece variably. Cosmetocleithrum spathulatum sp. n., and Cosmetocleithrum galeatum sp. n. closely resembles members of the first group, however, Cosmetocleithrum baculum sp. n. possess non-articulated bars and a non-bifid accessory piece.
Conclusions
Considering the 3 new species here described, to date 21 species of Cosmetocleithrum are recognised parasitizing siluriforms in the Neotropical region. Notwithstanding, the number of known taxa of monogeneans in neotropics is far from representing the ideal situation. Future studies describing new taxa should be encouraged to understanding the evolutionary relationship in the parasite-host system.
Data Availability
The datasets of the present study are available from the corresponding author.
References
Braga MP, Araújo SBL, Boeger W (2014) Patterns of interaction between Neotropical freshwater fishes and their gill Monogenoidea (Platyhelminthes). Parasitol Res 113:481–490. https://doi.org/10.1007/s00436-013-3677-8
Acosta AA, Scholz T, Blasco-Costa I, Alves PV, Silva RJ (2018) A new genus and two new species of dactylogyrid monogeneans from gills of Neotropical catfishes (Siluriformes: Doradidae and Loricariidae). Parasitol Int 67:4–12. https://doi.org/10.1016/j.parint.2017.09.012
Soares GB, Neto JFS, Domingues MV (2018) Dactylogyrids (Platyhelminthes: Monogenoidea) from the gills of Hassar gabiru and Hassar orestis (Siluriformes: Doradidae) from the Xingu Basin, Brazil. Zool 35:1–16. https://doi.org/10.3897/zoologia.35.e23917
Cohen SC, Kohn A, Justo MCN (2013) South American Monogenoidea parasites of fishes, amphibian and reptiles. Oficina de Livros, Rio de Janeiro, p 663
Kritsky DC, Thatcher VE, Boeger WA (1986) Neotropical Monogenea. 8. Revision of Urocleidoides (Dactylogyridae, Ancyrocephalinae). ProcHelmintholSoc Wash 53:1–37
Suriano DM, Incorvaia IS (1995) Ancyrocephalid (Monogenea) parasites from siluriform fishes from the Paranean-Plateanichthyogeographical province in Argentina. Acta Parasitol 40:113–124
Abdallah VD, Azevedo RK, Luque LF (2012) Three new species of Monogenea (Platyhelminthes) parasites of fish in the Guandu river, southeastern Brazil. Acta Sci Biol Sci 34:483–490. https://doi.org/10.4025/actascibiolsci.v34i4.10466
Yamada POF, Yamada FH, Silva RJ, Anjos LAS (2017) A New Species of Cosmetocleithrum (Monogenea, Dactylogyridae), a gill parasite of Trachelyopterus galeatus (Siluriformes, Auchenipteridae) from Brazil, with notes on the morphology of Cosmetocleithrum striatuli. Comp Parasitol 84:119–123. https://doi.org/10.1654/1525-2647-84.2.119
Morey GAM, Cachique JCZ, Babilonia JJS (2019) Cosmetocleithrum gigas sp. n. (Monogenoidea: Dactylogyridae) from the gills of Oxidoras niger (Siluriformes: Doradidae) from the Peruvian Amazon. Biologia 74:1–4. https://doi.org/10.2478/s11756-019-00331-x
Cohen SC, Justo MCN, Gen DVS, Boeger WA (2020) Dactylogyridae (Monogenoidea, Polyonchoinea) from the gills of Auchenipterus nuchalis (Siluriformes, Auchenipteridae) from the Tocantins River, Brazil. Parasite 27:1–12. https://doi.org/10.1051/parasite/2020002
Acosta AA, Smita NJ, Silva RJ (2020) Diversity of helminth parasites of eight siluriform fishes from the Aguapeí River, upper Paraná basin, São Paulo state, Brazil. IJP Parasites Wildl 11:120–128. https://doi.org/10.1016/j.ijppaw.2020.01.003
Graça WJ, Pavanelli CS (2007) Peixes da planície de inundação do Alto rio Paraná e áreas adjacentes. Eduem, Maringá
Ergens R (1969) The suitability of ammonium picrate-glycerin in preparing slides of lower Monogenoidea. Folia Parasit 16:320
Gussev AV (1985) Monogenea. In: Bauer ON (ed) Key to parasites of the freshwater fish fauna of the USSR, 2nd edn. Nauka Publications, USSR, pp 87–99
Řehulková E, Mendlová M, Šimková A (2013) Two new species of Cichlidogyrus (Monogenea: Dactylogyridae) parasitizing the gills of African cichlid fishes (Perciformes) from Senegal: morphometric and molecular characterization. Parasitol Res 112:1399–1410. https://doi.org/10.1007/s00436-013-3291-9
Mizelle JD (1936) New species of trematodes from the gills of Illinois fishes. Am Midl Nat 17:785–806
Bush AO, Lafferty KD, Lotz JM, Shostak AW (1997) Parasitology meets ecology on its own terms: Margolis revisited. J Parasitol 83:575–583
Thatcher VE (2006) Amazon fish parasites, 2nd edn. Pensoft Publishers, Sofia-Moscow, p 508
Takemoto RM, Pavanelli GC, Lizama MAP, Lacerda ACF, Yamada FH, Moreira LHA, Ceschini TL, Bellay S (2009) Diversity of parasites of fish from the Upper Paraná River floodplain, Brazil. Braz J Biol 69:691–705. https://doi.org/10.1590/S1519-69842009000300023
Eiras JC, Takemoto RM, Pavanelli GC, Adriano EA (2011) About the biodiversity of parasites of freshwater fish from Brazil. Bull EurAssoc Fish Pathol 31:161–168
Mendoza-Palmero CA, Scholz T, Mendoza-Franco EF, Kuchta R (2012) New species and geographical records of dactylogyrids (Monogenea) of catfish (Siluriformes) from the Peruvian Amazonia. J Parasitol 98:484–497. https://doi.org/10.1645/GE-2941.1
Graça RJ, Ueda BH, Oda FH, Takemoto RM (2013) Monogenea (Platyhelminthes) parasites from the gills of Hoplias aff. malabaricus(Bloch, 1794) (Pisces: Erythrinidae) in the Upper Paraná River Floodplain, States of Paraná and Mato Grosso do Sul, Brazil. Check List 9:1484–1487. https://doi.org/10.15560/9.6.1484
Yamada POF, Yamada FH, Silva RJ, Anjos LA (2017) Ecological implications of floods on the parasite communities of two freshwater catfishes in a Neotropical floodplain. Acta Parasitol 62:312–318. https://doi.org/10.1515/ap-2017-0039
Mendoza-Franco EF, Mendoza-Palmero CA, Scholz T (2016) New species of Ameloblastella Kritsky, Mendoza-Franco and Scholz, 2000 and Cosmetocleithrum Kritsky, Thatcher and Boeger, 1986 (Monogenea: Dactylogyridae) infecting the gills of catfishes (Siluriformes) from the Peruvian Amazonia. SystParasitol 93:847–862. https://doi.org/10.1007/s11230-016-9671-7
Acknowledgements
We are so grateful to the São Paulo Energy Company (CESP) for the facilities offered for the development of this work.
Funding
The authors thank the Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP) for the scholarship granted to P.O.F.Y. #2013/25786-7 and F.H.Y. #2011/22603-3; 2014/14298-4. R.J.S. is supported by CNPq-PROTAX #440496/2015-2; FAPESP #2016/50377-1; and CNPq #309125/2017-0.
Author information
Authors and Affiliations
Contributions
POFY concept of the manuscript, data collection, collection, and preservation of the material, identified the parasites species, performing morphometrical analysis and wrote the manuscript; FHY identified the species, performing morphometrical analysis and editing the manuscript; and RJS concept of the manuscript, data collection, identified the parasites species and editing the manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
de Oliveira Fadel Yamada, P., Yamada, F.H. & da Silva, R.J. Three New Species of Cosmetocleithrum (Monogenea: Dactylogyridae) Gill Parasites of Trachelyopterus galeatus (Siluriformes: Auchenipteridae) in Southeastern Brazil. Acta Parasit. 66, 436–445 (2021). https://doi.org/10.1007/s11686-020-00282-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11686-020-00282-3