Abstract
Purpose
Research on gill monogeneans of Characiform has made it possible to find two new species belonging to the genus Annulotrema. The purpose of this paper is to carry out their morphological description.
Methods
Specimens of Brycinus macrolepidotus were captured in the Nyong River at Akonolinga. Each monogenean was mounted between slide and cover slip in a drop of glycerin ammonium picrate mixture. The sclerotized parts of their haptor and reproductive organs were then drawn and measured.
Results
Two new species of Annulotrema were described. Annulotrema ngombiensis n. sp. is morphologically close to Annulotrema tenuicirra Paperna, 1973 and Annulotrema pikei Price, Peebles and Bamford, 1969. However, the new species differs from these other two mainly by the characteristic well-marked terminal cap and the tubiform prostatic reservoir of the accessory part of its MCO, as well as by its dorsal bar with a characteristic triangular piece in the middle of the fork and its ventral bar without filaments. Annulotrema nkengfacki n. sp. is distinguished from all other species previously described as well as from its closest congeners, Annulotrema helicocirra Paperna, 1973 and Annulotrema bouixi Birgi, 1988 by the structure of its MCO whose accessory part is composed of a trapezoid structure surmounted by another which is forked-shaped and by its arched-shaped ventral bar with an inner lining.
Conclusion
Brycinus macrolepidotus from Cameroon can harbour two species of Annulotrema, both described in the current study. This result contributes to the knowledge of the species diversity of this genus in the Nyong Basin.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Monogenea Carus, 1863, is one of the most species-rich classes of fish parasites, which are commonly found on gills and skin [1]. They are generally host specific; one species of parasite occurs in a single species or closely related species of hosts [2]. Although research on monogeneans biodiversity has intensified recently in Africa, there are indices that this taxon remains underestimated. African tetras are known to harbour three genera of monogeneans of which, Annulotrema Paperna and Thurston, 1969, is the most species-rich, and exclusively parasitizes host belonging to the families Characidae, Hepsetidae and Distichodontidae [3,4,5,6,7,8,9,10,11,12,13,14]. The first studies relating to the monogeneans of African tetras in Cameroon (Table 1) are those made by Birgi [9, 15]. Since then, systematic research has led to the description of several new species of monogeneans on various fish hosts in Cameroon [16,17,18,19,20,21,22,23]. Despite this intense sampling efforts, no monogeneans were collected from the gills of Brycinus macrolepidotus Valenciennes, 1849, which is one of the common fish species, widely distributed in the southern Cameroon waters. However, studies conducted elsewhere on the monogeneans of this fish species have described Annulotrema alberti Paperna, 1973, Annulotrema longipinnis, Paperna, 1973, Annulotrema tenuicirra Paperna, 1973, Annulotrema elongata Paperna, 1969 (re described by Kičinjaová et al. [13], Annulotrema alestesnursi Paperna, 1973 (re-described by Kičinjaová et al. [13], and Annulotrema helicocirra Paperna, 1973.
Research work on the Characiform populations from the Nyong River in Akonolinga has made it possible to undertake an inventory of the monogeneans of the hosts collected. This study led to finding of two species belonging to the genus Annulotrema and provides the first evidence for monogenean species on the gills of B. macrolepidotus in Cameroon. The purpose of this paper is to carry out the morphological description of both species.
Materials and Methods
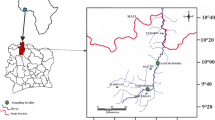
A total of 32 specimens of Brycinus macrolepidotus, 70–180 mm standard length were caught with gillnets in the Nyong River (Akonolinga, centre Cameroon) on February 2019 at three localities: main bridge (3°46′ 10.53’’N; 12°14′ 49.062 E), Melan (3°46′ 11.424’’N; 12°16′ 22.236’’E) and Sombo (3°46′ 13.548’’N; 12°16′ 27.612’’E) (Fig. 1). The captured fish were kept in icebox with ice inside and then, transported to the Laboratory of Parasitology and Ecology of the University of Yaoundé I where they were transferred to the freezer until they were examined for monogeneans.
During examination, the gills from both sides were removed and isolated in Petri dishes containing tap water. Under a stereomicroscope, each monogenean was dislodged from the gill lamellae with fine needle and mounted between slide and coverslip in a drop of glycerin ammonium picrate mixture (GAP) [24]. Two days later, cover-slips were sealed with nail varnish and observations were made using the Yvymen optical microscope with an integrated camera. Identification of monogeneans was based on the morphology and size of the sclerotized parts of the haptor and the copulatory complex. Scheme measurements (Fig. 2) were made according to Kičinjaová et al. [13] and Gussev [25]. The nomenclature adopted at ICOPA IV [26] was used to distinguish the seven pairs of marginal hooks (in Roman numerals). Drawings of the sclerotized parts were made using the Corel Draw X4 software (Version 14. 0.0.701; Corel Corporation). The various measurements are given in micrometres (µm) and presented as follows: average ± standard deviation (minimum value-maximum value). Holotypes and paratypes were deposited in the Helminthological collection of the Muséum National d’Histoire Naturelle, Paris, France (MNHN). The remaining specimens have been kept in the collection of Laboratory of Parasitology and Ecology, University of Yaoundé I, Cameroon.
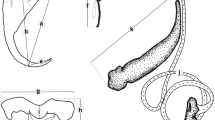
General scheme of measurements for sclerotized parts of haptor and reproductive organs (1) anchor: a inner length, b outer length, c inner root length, d outer root length, e point length; (2) ventral bar: f total length, g total width, h median width; (3) dorsal bar: i total length, j total width, k median width, l length of median sclerotized part; (4) marginal hooks: m total length; (5) MCO: n total length, o tube trace length, p basal length, q basal width, r cap length, s tube trace length of prostatic reservoir; (6) vagina: t tube trace length
Results
The two monogeneans species collected from the gills of the 32 specimens of B. macrolepidotus examined are all comply with the description of members of Annulotrema by having the following characteristics: haptor armed with four anchors (the inner and the outer roots generally form a U on the ventral anchors and a V on the dorsal ones) [4, 9], two bars with or without filaments and 14 marginal hooks [3]. Male copulatory organ with an accessory piece and one or two prostatic reservoirs [9]. Vagina in the lateral position [3, 7]. Gill parasites of African characids (Characiform).
Annulotrema ngombiensis n. sp.
Type Host: Brycinus macrolepidotus Valenciennes, 1849.
Infection site: Gills.
Type locality: Akonolinga-Centre near the Nyong Bridge (3°46′ 10.53’’N; 12°14′ 49.062 E) Others localities: Sombo (3°46′ 13.548’’N; 12°16′ 27.612’’E) and Melan (3°46′ 11.424’’N; 12°16′ 22.236’’E).
Specimens studied: 40 individuals mounted in Malmberg’s solution.
Type specimens: Holotype: MNHN HEL 1646 and Paratypes: MNHN HEL 1647–1648 deposited at the Muséum National d’Histoire Naturelle (Paris).
Etymology: The name of this species is inspired by that of a small village called Ngombi located in the district of Akonolinga where we had fortuitously captured its host for the first time.
Prevalence: 87.5% (28 infested hosts).
Body length haptor included 533 ± 85 (314–790), width 49 ± 9 (24–69) at the level of the pharynx and 66 ± 13.4 (49–86) at the level of the ovaries. Ventral anchor, with inner root robust and approximately three times longer than the outer root, blade straight in its middle part, and ends by a short point; inner length = 37 ± 1.9 (34–41), outer length = 35 ± 2.0 (30–39), inner root length = 12 ± 1.7 (7–16), outer root length = 4 ± 0.9 (3–7) and point length = 8 ± 0.8 (6–10). Dorsal anchor less robust than the ventral one, inner root with a bump at it median part and incurved at it terminal part; outer root reduced, blade small incurved ends with a short point: inner length = 42 ± 2.3 (36–46); outer length = 35 ± 2.3 (30–43); inner root length = 15 ± 2.2 (5–18); outer root length = 5 ± 0.9 (3–7) and point length = 7 ± 0.7 (5–9). Ventral bar arched and slightly bulging at its extremities: total length = 24 ± 1.9 (20–27), total width = 6 ± 0.9 (5–9) and median width = 4 ± 0.8 (3–7). Dorsal bar forked with two branches almost equal and sinuous, small triangular piece in the middle of the fork: total length = 23 ± 1.5 (20–28), total width = 16 ± 2.4 (10–21); median piece length = 9 ± 2.2 (7–11) and median width = 10 ± 1.3 (6–12). Marginal hooks 7 pairs with filament; and dissimilar in size, marginal hook lengths: Pair I = 12 ± 1.5 (9–15); Pair II = 13 ± 1.5 (10–17); Pair III = 16 ± 2.5 (12–24); Pair IV = 18 ± 2.0 (13–22); Pair V = 21 ± 0.6 (17–25); Pair VI = 20 ± 2.1 (15–25); Pair VII = 17 ± 0.4(13–21).
The male copulatory organ, with a long tubular prostatic reservoir, starts with piriform base following by an elongated accessory piece and ends by a cap which embraces the terminal part of copulatory tube: total length 48 ± 6.7 (34–60); tube trace length 62 ± 6.1 (45–71); base length 7 ± 0.9 (5–8); base width 5 ± 0.6 (4–6); cap length 18 ± 2.8 (11–23) and prostatic reservoir tube trace length 35 ± 6.0 (26–49). The vagina tubiform and sinuous forms a number 6 knot to third length and ends with an opening at one extremity; tube trace length 26 ± 3.6 (21–33).
Remarks
Annulotrema ngombiensis n. sp. has some similarities with A. tenuicirra drawn by Paperna [5] and redrawn by Christison [27] in having forked dorsal bar, MCO with a visible prostatic reservoir attached to its basal part, elongated copulatory tube, elongated accessory piece whose terminal part forms a spatula or a cap which embraces the distal part of the copulatory tube and same sizes of their ventral and dorsal anchors (34–41 vs 32–35 and 36–46 vs 35–37, respectively). However, A. ngombiensis n. sp. has dorsal bar with round base, ventral bar forming a well-marked V, dorsal anchor with a short point, whereas A. tenuicirra presents dorsal bar with triangular base, linear ventral bar and dorsal anchor with very curved point. Also, in A. ngombiensis n. sp., the terminal part of the copulatory tube remains inside the spatula; the prostatic reservoir is tubiform while in A. tenuicirra, the terminal part of the copulatory tube comes out of the spatula and the prostatic reservoir clearly visible forms a small oval mass attached to the base of the MCO.
Annulotrema ngombiensis n. sp. and A. elongata have an elongated MCO with an accessory piece that runs up the copulatory tube and ends in a tip. Moreover, the dorsal bar in both species forms a fork with two branches of almost equal length. Despite these similarities, the accessory piece of A. ngombiensis n. sp. forms a well-marked cap in its distal part while that of A. elongata becomes thinner; the ventral bar of A. elongata has posterior extensions and saddle-like while that of A. ngombiensis n. sp. does not have any but is thin and U-shaped; the size of ventral and dorsal anchors is higher in A. elongata than in A. ngombiensis n. sp. (34–41 vs 45–49 and 36–46 vs 48–54, respectively).
Annulotrema ngombiensis n. sp can be classified in the same morpho-group as A. pikei parasite of Hydrocynus forskahlii and the two species share the same shape of the elongated MCO with a pear-shaped base and an elongated accessory piece which ends in a cap that surmounts the terminal part, although this cap is wider in A. ngombiensis n. sp. In addition, the dorsal bar of each species is forked with a thin median piece and their ventral and dorsal anchors have similar sizes (34–41 vs 36–43 and 36–46 vs 35–43, respectively). Nonetheless, the fork formed by the dorsal bar is less marked in A. pikei and the branches less developed than in A. ngombiensis n. sp. The ventral bar of A. pikei has posterior extensions in the original description [6] and in the re-description of the sclerotized parts made by Řehulková et al. [3]. This bar seems to form a saddle composed of several joined pieces in A. pikei while it appears single and concave in A. ngombiensis n. sp. The accessory piece of A. pikei begins at the level of the copulatory tube and extends all along the latter, whereas in A. ngombiensis n. sp., this piece starts at the level of the basal part of the male copulatory organ, outlines the copulatory tube before to ascend. It is important to note that the samples provided for the re-description of A. pikei are not of good quality and as reported by Řehulková et al. [3]; this species requires a re-description on better quality samples. Despite these drawbacks, we cannot consider the species A. ngombiensis n. sp as a synonym of A. pikei.
Annulotrema nkengfacki n. sp.
Type host: Brycinus macrolepidotus Valenciennes, 1849.
Infection site: Gills.
Type locality: Akonolinga-Centre near the Nyong Bridge (3°46′ 10.53’’N; 12°14′ 49.062 E). Others localities: Sombo (3°46′ 13.548’’N; 12°16′ 27.612’’E) and Melan (3°46′ 11.424’’N; 12°16′ 22.236’’E).
Specimens studied: 15 individuals mounted in Malmberg’s solution.
Type specimens: Holotype: MNHN HEL 1649 and Paratypes: MNHN HEL 1650–1651 deposited at the Muséum National d’Histoire Naturelle (Paris).
Etymology: This species was named in honour of Professor Nkengfack Augustin Ephrem, Head of Department of Organic Chemistry of the University of Yaoundé I.
Prevalence: 50% (16 infested hosts).
The total body length haptor included 303 ± 50.6 (232–426), width 42 ± 8.6 (27–57) at the level of the pharynx; 68 ± 15.7 (36–96) at the level of the ovaries. Ventral anchor with robust inner root, approximately two times as long as outer root with which it forms an angle of approximately 90°, blade well arched ends with a curved point: inner length = 30 ± 1.6 (28–32); outer length = 29 ± 1.0 (28–31); inner root length = 10 ± 1.0 (8–12); outer root length = 5 ± 1.0 (3–7) and point length = 3 ± 0.6 (2–4). Dorsal anchor little less robust than ventral anchor, inner root twice as long as the outer root with which it forms a more or less acute angle, blade fine and ends with very short point: inner length = 31 ± 1.9 (28–35); outer length = 26 ± 2.2 (24–30), inner root length = 11 ± 1.5 (9–14); outer root length = 5 ± 1.0 (4–7) and point length = 2 ± 0.3 (2–3). Ventral bar V-shaped, with one of the branches more robust and slightly curvy, middle part with trapezoidal structure with an extension on each end of its large base: total length = 17 ± 3.0 (13–20); total width = 9 ± 1.4 (7–12) and median width = 5 ± 1.1 (3–7). Dorsal bar arched in its lower part with ends depressed in a cup: total length = 20 ± 1.2 (18–22); total width = 8 ± 1.9 (6–12) and median width = 4 ± 0.8 (3–5). Marginal hooks 7 pairs with filament and dissimilar in size: Pair I = 12 ± 1.3 (9–14); Pair II = 11 ± 0.8 (10–13); Pair III = 18 ± 0.8 (17–20); Pair IV = 17 ± 1.5 (15–20); Pair V = 17 ± 0.9 (15–19); Pair VI = 16 ± 1.0 (14–18); Pair VII = 15 ± 1.5 (13–18).
Male copulatory organ with ovoid basal piece, following by a tubular penis which forms one turn in clockwise, accessory piece complex with lower part trapezoid and surmounted by a fork in which the copulatory tube slides: total length = 21 ± 2.0 (16–24); tube trace length = 56 ± 10.1 (36–69); base length = 5 ± 0.4 (4–6) and base width = 4 ± 0.5 (3–5). The vagina sclerotized tubiform with a small opening at its terminal end: tube trace length = 6 ± 3.0 (5–7).
Remarks
Annulotrema nkengfacki n. sp., from the morphological point of view presents similarities with A. helicocirra in having arched ventral bar, ventral anchor with the blade well-curved and similar sizes (28–32 vs 32–37) spirally coiled MCO. Nonetheless, the dorsal bar which is filiform in A. helicocirra with a triangular piece attached to the base is rather curved in A. nkengfacki n. sp.; MCO in the species being described presents one turn of spire when there are two in A. helicocirra. Although having morphological similarities and exploiting the same host, the differences observed in A. nkengfacki n. sp. and A. helicocirra lead to separate them clearly.
Annulotrema nkengfacki n. sp. is also similar to A. bouixi in sharing the same morphology of the MCO with the tube which describes a loop and an accessory piece that forms a blade into which the end of the copulatory tube penetrates. As with A. nkengfacki n. sp., the ventral and dorsal bars of A. bouixi form a V which is wider in the dorsal bar. Moreover, the sizes of the ventral and dorsal anchors are similar in both species (28–32 vs 24–32 and 28–35 vs 30–32, respectively). Despite these similarities, the accessory piece of the male copulatory organ in A. bouixi starts at the level of the base and forms a characteristic terminal hook while that of A. nkengfacki n. sp. begins at the level of the copulatory tube and ends in an opening which frames the terminal part of the copulatory tube of the MCO; the ventral bar forms a V in both species, but in A. bouixi the base of the V is distinguished by a characteristic T-shaped expansion which has not been observed in A. nkengfacki n. sp. Note that, our work on B. kingsleyae monogeneans (In press) allowed us to have several samples of A. bouixi and thus, to confirm the separation with A. nkengfacki n. sp.
Discussion
This study supports the presence of two new monogenean species of Annulotrema on B. macrolepidotus from the Nyong River. They are well distinguished by features of the haptor and genital sclerotized structures especially, the shape of the bars, the size and shape of the anchors, the general shape of the MCO and of the vagina, which are meaningful for discrimination of Annulotrema species [9, 10, 13]. These species are different from each other and from all their previously described congeners despite some morphological similarities with some of them.
Several studies have focused on the systematics of monogeneans of Alestidae fish and have contributed based on morphological criteria, to the identification of 48 species of Annulotrema [3, 4, 8,9,10, 13, 14]. Based on the same criteria, the two species described in this article are different from the others and should be considered new. With these species, there are now 50 Annulotrema species and this is the first record of monogeneans on B. macrolepidotus from Cameroon.
Data availability
The datasets generated and analyzed during the current study are available from the corresponding author on reasonable request.
References
Rohde K, Heap M, Hayward CJ, Graham KJ (1992) Calitotyle australiensis n. sp. And Calitotyle sp. (Monogenea, Monopisthocotylea) from the rectum and rectal glands and Rugogaster hycholagi Shell, 1973 (Trematoda, Apisdogastrea from the rectal glands of holocephalans off the coast of southeastern Autralia. Syst Parasitol 21:69–79
Combes C (1995) Interactions durables. Ecologie et évolution du parasitisme. Paris France, Masson 524 p. (Coll. d’écologie, n° 26)
Řehulková E, Musilová N, Gelnar M (2014) Annulotrema (Monogenea: Dactylogyridae) from Hydrocynus brevis (Characiformes: Alestidae) in Senegal, with descriptions of two new species and remarks on Annulotrema pikei. Parasitol Res 113(9):3273–3280. https://doi.org/10.10007/s00436-014-3990-x
Paperna I (1969) Monogenetic trematodes of the fish of the Volta Basin and South Ghana. Bull I F A N 3:840–880
Paperna I (1973) Lymphocystis in fish from East Africa Lakes. J Wildl Dis 9(4):331–335. https://doi.org/10.7589/0090-3558-9.4.331
Paperna I (1979) Monogenea of inland water fish in Africa. Ann Mus Roy Afr Centr 8 Sci Zool 226:1–131
Paperna I, Thurston JP (1969) Annulotrema n. gen., a new genus of monogenetic trematodes (Dactylogyridae, Bychowsky, 1933) from African characid fish. Zool Anz 226:1–131
Thurston JP (1970) The incidence of Monogenea and parasitic Crustacea on the gills of fish in Uganda. Rev Zool Bot Afr 82(1/2):111–130
Birgi E (1988) Les monogènes des Characoidea de la zone forestière Camerounaise. Ann Fac Sc Bio Biochim 3(5):591–611
Ergens R (1988) Four species of the genus Annulotrema Paperna et Thurston, 1969 (Monogenea: Ancyrocephalinae) from Egyptian freshwater fish. Folia Parasitol 35:209–215
Guégan JF, Lambert A (1990) Twelve new species of Dactylogyrids (Platyhelminthes, Monogenea) from West African barbels (Teleostei, Cyprinidae), with some biogeographical implications. Syst Parasitol 17(3):153–181
Guégan JF, Lambert G, Birgi E (1988) Some observations on the branchial parasitism from chracid fishes, genus Hydrocynus in West Africa. Description of a new species, Annulotrema pikoides n. sp. (Monogenea, Ancyrocephalidae) from Hydrocynus vittatus (Castelnau, 1861). Ann Parasitol Hum Comp 63:91–98. https://doi.org/10.1051/parasite/1988632091
Kičinjaová ML, Blažek R, Gelnar M, Řehulková E (2015) Annulotrema (Monogenea: Dactylogyridae) from the gills of African tetras (Characiformes: Alestidae) in Lake Turkana, Kenya, with descriptions of four new species and a redescription of A. elongata Paperna and Thurston, 1969. Parasitol Res 114:4107–4120. https://doi.org/10.1007/s00436-015-4682-x
Kičinjaová ML, Barson M, Gelnar M, Řehulková E (2018) Two new species of Annulotrema (Monogenea: Dactylogyridea) from Hydrocynus vittatus (Characiformes: Alestidae) in Lake Kariba. Zimbabwe J Helminthol 92(4):467–476. https://doi.org/10.1017/s0022149x1700058x
Birgi E (1987) Monogènes parasites branchiaux de poissons d’eau douce au Tchad et au Sud-Cameroun. Université des Sciences et Techniques du Languedoc (Montpellier II), Taxonomie et Essai de Biogéographie comparée. Thèse Doctorat d’Etat, p 196p
Bilong Bilong CF, Euzet L, Birgi E (1994) Deux nouveaux Eutrianchoratus Paperna, 1969 (Monogenea, Ancyrocephalidae), parasites branchiaux de Parachanna obscura (Günther, 1861) (Teleostei, Channidae), au Cameroun. Parasite 1:357–362. https://doi.org/10.1051/parasite/1994014357
Pariselle A, Bitja Nyom AR, Bilong Bilong CF (2013) Check-list of the ancyrocephalids (Monogenea) parasitizing Tilapia species in Cameroon, with the description of three new species. Zootaxa 3599(1):78–86. https://doi.org/10.11646/zootaxa.3599.1.7
Nack J, Bilong Bilong CF, Euzet L (2005) Monogènes parasites de Clariidae (Teleostei, Siluriformes) au Cameroun: I. Description de deux nouvelles espèces du genre Gyrodactylus dans le bassin du Nyong. Parasite 12:213–220
Nack J, Bitja Nyom AR, Pariselle A, Bilong Bilong CF (2016) New evidence of a lateral transfer of monogenean parasite between distant fish hosts in Lake Ossa, South Cameroon: the case of Quadriacanthus euzeti n. sp. J Helminthol 90:455–459. https://doi.org/10.1017/S0022149X15000577
Bahanak D, Nack J, Pariselle A, Bilong Bilong CF (2016) Description of three new species of Quadriacanthus (Monogenea: Ancyrocephalidae) gill parasites of Clarias submarginatus (Siluriformes: Clariidae) from Lake Ossa (Littoral region, Cameroon). Zoologia 33(4):1–8. https://doi.org/10.1590/S1984-4689zool-20160044
Bassock Bayiha ED, Nack J, Pariselle A, Bilong Bilong CF (2016) Two new species of gill parasites assigned to Protoancylodiscoides (Monogenea, Ancyrocephalidae) from Chrysichthys spp. (Siluriformes, Claroteidae) in River Sanaga (Cameroon). Zootaxa 4170(1):178–186. https://doi.org/10.11646/zootaxa.4170.1.11
Akoumba JF, Pariselle A, Tombi J, Bilong Bilong CF (2017) Description of two new Ancyrocephalid (Quadriacanthus and Bagrobdella) monogenea from the gills of Auchenoglanid catfishes (Pisces, Siluriformes, Claroteidae) in Cameroon. Vie et Milieu 67(2):59–64
Mbondo JA, Nack J, Pariselle A, Bilong Bilong CF (2017) The diversity of monogenean gill Parasites of two Synodontis species (Siluriformes, Mochokidae) with the description of two new species assigned to Synodontella. Vie et Milieu 67(2):75–80
Malmberg G (1957) On the occurrence of Gyrodactylus on Swedish fishes. Skr Utg Södra Sver Fisk Föreningen 1956:19–76 (In Swedish with English abstract and species descriptions)
Gussev AV (1962) Monogenoidea. In: Bychovskaya-Pavlovskaya IE et al (eds) Key to parasites freshwater fish of the USSR. Moscow-Leningrad. Academya Nauk SSSR, p 919
Euzet L, Prost M (1981) Report of the meeting on Monogenea: Systematics, biology and ecology. In: Slusarski (ed) Review of advances in parasitology Warsawa PWN. Polish Scientific Publishers, p 1
Christison KW (1998) Branchial monogenean parasites (Monogenea: Dactylogyridae) of characin fishes from the Okavango river and Delta, Botswana. Dissertation (MSc. (Zoology))- University of the Orange Free State, 251p
Acknowledgements
We thank the Professor Jean-Lou Justine (National Museum of Natural History of Paris) to his help in the conservation of type specimens in the Helminthological collection. We thank the Professors of the Joint Institutional Review Board for Animal & Human Bioethics (JIRB) for the Ethical Clearance established for this study. The manuscript initial version was benefitted from the criticism of Maria Lujza Kičinjaová.
Funding
No funding was received for conducting this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no conflict of interest.
Ethical Approval
For this study, fish were sampled using gillnets in accordance with national regulations.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ndongo, I., Akoumba, J.F., Tombi, J. et al. Two New Species of Annulotrema (Monogenea: Dactylogyridae) Gill Parasites of Brycinus macrolepidotus Valenciennes, 1849 from Nyong River, Cameroon. Acta Parasit. 68, 257–265 (2023). https://doi.org/10.1007/s11686-022-00645-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11686-022-00645-y