Abstract
Anxiety and depression are associated with altered communication within global brain networks and between these networks and the amygdala. Functional connectivity studies demonstrate an effect of anxiety and depression on four critical brain networks involved in top-down attentional control (fronto-parietal network; FPN), salience detection and error monitoring (cingulo-opercular network; CON), bottom-up stimulus-driven attention (ventral attention network; VAN), and default mode (default mode network; DMN). However, structural evidence on the white matter (WM) connections within these networks and between these networks and the amygdala is lacking. The current study in a large healthy sample (n = 483) observed that higher trait anxiety-depression predicted lower WM integrity in the connections between amygdala and specific regions of the FPN, CON, VAN, and DMN. We discuss the possible consequences of these anatomical alterations for cognitive-affective functioning and underscore the need for further theory-driven research on individual differences in anxiety and depression on brain structure.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
Affective disorders are not only related to modifications in localized brain regions involved in affective processing but are also associated with altered communication within global brain networks and changes in broad cognitive function. Notably, anxiety is presumed to impact four core brain networks involved in cognitive function, specifically the fronto-parietal network (FPN), cingulo-opercular network (CON), ventral attention network (VAN), and default mode network (DMN) (Sylvester et al. 2012; Liao et al. 2010a). Additionally, anxiety perturbs functional connectivity between the amygdala and key regions of these four networks at rest (Etkin et al. 2009), during emotion regulation (Etkin et al. 2010) and in response to masked threats (Monk et al. 2008). Similar deficits in network connectivity have been reported in depression (Sylvester et al. 2012; Cullen et al. 2014; Lu et al. 2012). Interestingly, it has been hypothesized that anxiety and depression are associated with overactivation of the CON and VAN (in case of anxiety) but underactivation of the FPN and DMN (Sylvester et al. 2012). However, structural evidence regarding greater or reduced integrity of brain white matter supporting such hypotheses is limited.
Perturbed functional activity and connectivity have been detected in different neural networks in anxiety disorders (Sylvester et al. 2012; Liao et al. 2010a); however, analyses examining the structural connectivity within these networks have been limited. Four general neural networks have been implicated in affective disorders (for the specific regions involved in each network please see Table 1). The CON, or salience network, is responsible for detecting errors and conflicts, although the dorsal anterior cingulate cortex of this network has also been reported to be involved in affect processing and cognitive control (Sylvester et al. 2012). The FPN is principally involved in the exertion of top-down cognitive control (Dosenbach et al. 2008), as opposed to the VAN which supports bottom-up stimulus-driven attention (Fox et al. 2006). In contrast to the other networks, which are hypothesized to operate bilaterally, the VAN is postulated to be predominantly right-lateralized (Fox et al. 2006). Finally, the DMN is involved in a broad array of functions such as future planning, self-referential activities, and emotion regulation (Raichle et al. 2001). These functional networks are constrained by anatomical white matter (WM) structure in the brain (Honey et al. 2009; Diez et al. 2015), yet few studies have reported relationships. Patterns in resting state activity in DMN and FPN have been linked to anatomical connectivity patterns, showing for example strong interconnections (i.e., connection density) between the precuneus and medial prefrontal cortex (PFC) of the DMN (Honey et al. 2009).
The amygdala has been a main point of interest in research for the past number of years due to its prominent role in anxiety and depression (e.g., Beesdo et al. 2009; Rauch et al. 2003); however, research on the connectivity between the amygdala and other parts of the brain such as the four core neural networks reported above has been more limited in scope (e.g., Kim and Whalen 2009; Tromp et al. 2012; Taylor et al. 2007). While structural connectivity studies have been increasing recently, they have, to date, only examined the connectivity between the amygdala and one other brain region or network. For instance, studies in trait anxiety (Kim and Whalen 2009), generalized anxiety disorder (Tromp et al. 2012), and major depression (Taylor et al. 2007; Liu et al. 2016) suggest that increased symptoms of affective disorders are associated with lower WM integrity (lower fractional anisotropy, FA) in the amygdala-PFC tracts (including regions of the CON, VAN, and DMN). However, opposite findings have found positive associations between FA values and trait anxiety in ventrolateral PFC of the VAN (Clewett et al. 2014) or uncinate fasciculus connection with PFC (Modi et al. 2013). Discrepant findings are also present in other WM regions of the brain (e.g., Ayling et al. (2012) for a review) and could be due to small sample sizes, dissimilar definitions of regions of interest, differences in clinical status of participants, or the use of different methods for the measurement of tract integrity. Taken together, the limited research available on the influence of affective disorders on structural WM integrity is contradictory and has insufficiently taken into account the relevant brain networks per se as well as their relation to the amygdala. Research on the influence of anxiety and depression on brain anatomy would greatly benefit from large-scale theory-driven studies using robust methods for the calculation of white matter integrity.
Therefore, this study investigated the extent to which trait anxiety and depression have an impact on the WM integrity of four critical brain networks involved in the top-down control of attention (FPN), error monitoring (CON), stimulus-driven attention (right-lateralized VAN), and default-mode and emotion regulation (DMN) and their relation to the amygdala using a comparatively large representative sample (the Human Connectome Project, HCP). Based on prior theoretical models (Sylvester et al. 2012), we anticipated 1) that more anxiety-depression would predict greater structural connectivity in the amygdala-FPN and amygdala-VAN paths but less structural connectivity in amygdala-CON and amygdala-DMN paths. Moreover, also based on prior work (Sylvester et al. 2012; Liao et al. 2010a), we hypothesized that 2) overactivation of CON and VAN in anxiety and depression would be associated with greater structural connectivity within structures of these networks whereas the underactivation of DMN and CON previously reported in relation to these disorders led us to expect reduced structural connectivity among the individual network structures.
Methods
Sample
The present study sample consisted of the HCP (S500 release) data. This release contained 543 participants of which 483 subjects (286 females) aged between 22 and 36 (M = 29.16; SD = 3.46; Table 2) could be used for analysis in the current study. A total of 60 HCP participants could not be included in this study due to missing or invalid diffusion data (n = 56), no Achenbach adult self-report scores (n = 3), or incomplete ethnicity data (n = 1). Relevant sample characteristics are presented in Table 2. For estimate IQ, Ravens progressive matrices correct score was used (Raven et al. 2003). While the majority of the sample had a white ethnic background (n = 356; 50 Hispanic), participants of African American (n = 102), Asian or Pacific (n = 9), and mixed (n = 6) or unknown (n = 10) ethnic background were also included. All data was handled in accordance with the HCP data use terms.
Achenbach adult self-report
The scale within the HCP that measures socio-emotional problems in the past six months is the Achenbach adult self-report (ASR; Achenbach 2009). Due to its large sample size, no diagnostic interview was available within this dataset. This self-report scale allows for the calculation of an anxiety-depression scale (range 0–36 points). While there was unfortunately no appropriate scale measuring anxiety and depression separately, these are highly comorbid disorders that appear to share a lot of underlying features, including network dysfunction (Sylvester et al. 2012; Korgaonkar et al. 2014). The presence of high comorbidity is supported by the significant correlation between the DSM depression and DSM anxiety measures (r(481) = .67, p < .001) in the ASR in this sample. Mean ASR anxiety-depression score in this sample was 5.64 (SD =5.33; Table 2) and only a small subsample suffered from anxiety or depression symptoms that reached clinical significance (14 participants or 2.90 % of the sample when using a cut-off of percentile 98). There was no gender difference in ASR anxiety-depression score (t(481) = 0.56, p = .58) .
MRI acquisition
All subjects were scanned at Washington University in St. Louis using a Siemens Skyra 3 T scanner with a customized SC72 gradient insert (i.e., the ‘Connectome Skyra’ which improves the quality of the diffusion imaging scans). High angular diffusion MRI was recorded (spin-echo EPI sequence, repetition time (TR) = 5520 ms, echo time (TE) = 89.5 ms, flip angle =78°, refocusing flip angle =160°, field of view (FOV) = 210 × 180 (RO x PE), matrix =168 × 144 (RO x PE), slice thickness = 1.25 mm, 111 slices, 1.25 mm isotropic voxels, multiband factor = 3, echo spacing =0.78 ms, bandwith =1488 Hz/Px, phase partial Fourier =6/8, and b-values of 1000, 2000, and 3000 s/mm2). SENSE was used for diffusion reconstruction (Sotiropoulos et al. 2013). The dMRI protocol was completed in 6 runs, with 3 gradient tables (with 90 directions and 6 B0 acquisitions) applied in both right to left and left to right phase encoding. A T1w structural image (TR = 2400 ms, TE = 2.14 ms, TI = 1000 ms, flip angle =8°, FOV = 224 × 224) sampled at the same resolution as the diffusion data was also included.
Regions of interest
The key brain regions of the networks of interest (i.e., FPN, CON, VAN, and DMN) will be used as seeds and targets in the subsequent analyses (Table 1). Since it was not feasible to manually draw the a priori ROIs individually in such a large sample and since standard masks based on existing atlases were mostly large and imprecise, we created spherical masks centered around the peak coordinate of activation. Peak coordinates were collected through a literature search on PubMed with the names of the networks or commonly used synonyms (such as “salience network” and “cognitive control network”) as key words. However, when these terms did not lead to satisfactory results, the names of the specific key regions were also used. Since there was no single study that provided coordinates for all a priori regions of interest (ROI), multiple studies were consulted and a list of coordinates was constructed. Subsequently, spheres of 10 mm radius were created around the coordinates (using fslmaths) to produce ROIs of approximately the same size, which were large enough to account for inter-individual differences and prevent false negatives. When multiple coordinates were found for a single region, the final ROI was selected based on: (1) the specificity (i.e., lack of overlap between different anatomical regions), (2) the nature of the study: meta-analyses were preferred over research articles, and (3) visual inspection which evaluated both accordance with the proposed location presented by Sylvester et al. (2012) and overlap with the relevant Brodmann areas.
The coordinates of the FPN originated from the study of Dosenbach et al. (2007) who applied graph theory to resting state functional connectivity MRI data. The coordinates of the CON were collected from a resting state MRI paradigm (Raichle 2011). For the VAN, we consulted an ALE meta-analysis of functional studies using attention and working memory tasks (Kollndorfer et al. 2013) as well as a meta-analysis on visual oddball effects (Kim 2014). Finally, the coordinates of the DMN were based on three studies: the resting state MRI study from Raichle (2011), a resting state PET study (Drevets et al. 1997), and a resting state functional MRI study (Greicius et al. 2003).
The final selection of coordinates was transformed from standard space to native space where they could be used as a basis for probabilistic fibertracking. The transformation matrices were created by registering the native image to the standard by use of linear (FSL FLIRT; Jenkinson et al. 2002) and non-linear (FNIRT; Andersson et al. 2007; Jenkinson et al. 2012) transformations and subsequently reversing the transformation matrix (by use of the FSL invwarp command). In subcortical areas, such as the amygdala, it is difficult to construct accurate standard masks. Therefore, individual amygdala masks were created with FSL FIRST (Patenaude et al. 2011). FSL FIRST uses learned models (based on manually segmented images) to search for the most probable shape of a subcortical structure given the observed intensities in the T1-weighted image of a participant.
Analysis of diffusion MRI
The HCP diffusion data used in this study had already undergone preprocessing by the Wu-Minn consortium (Andersson et al. 2003; Andersson et al. 2012): the b0 image intensity was normalized across runs; EPI distortions, eddy-current-induced distortions, and subject motion were removed; gradient-nonlinearities were corrected; and the diffusion data were registered with the structural image, brought into 1.25 mm structural space, and masked with the final brain mask. Preprocessing was performed using the FSL software (TOPUP, EDDY, and FLIRT tools; Jenkinson et al. 2012), further information on the preprocessing of the diffusion data can be found on the HCP website (http://www.humanconnectome.org/documentation/).
Diffusion parameters were calculated from the preprocessed data using the FSL-tool BedpostX (Behrens et al. 2007; Jbabdi et al. 2012). This tool uses Markov Chain Monte Carlo sampling to calculate the dominant fiber distributions in each voxel. In this dataset, three fiber distributions could be calculated per voxel. Subsequently, the FSL ProbtrackX-tool was used to calculate the tracts between the different regions of interest (Behrens et al. 2007). In accordance with the standard FSL DTI pipeline, 5000 samples were sent from each voxel in the seed region and a curvature threshold of 0.2 and step length of 0.5 mm was used. Furthermore, a midline exclusion mask was used when tracking within the networks since we did not have hypotheses regarding interhemispheric connectivity. Tracking was done in both directions (from A to B and from B to A) and subsequently averaged to increase the reliability of the tract between the two regions of interest (Clewett et al. 2014). The FSL DTIFIT tool was used to calculate FA, which is a good measure of WM integrity (e.g., Teipel et al. 2010). All brain analyses were performed on the high performance cluster of Ghent University because of the high computational demands of these analyses when performed on the high-quality HCP dataset.
A threshold was applied to the results of the fibertracking to reduce the chances that sporadic/erroneous connection paths drive the findings. Since there is no consensus about the optimal threshold, a relative threshold of 15 % of the maximum value was used to account for individual differences as well as to be stringent enough to optimize tract quality (see also Bennett et al. 2011; Nakamae et al. 2014; Khalsa et al. 2013). This thresholded path was subsequently used to mask the whole-brain FA image and the mean FA within each tract was calculated. Additionally, tract volume (in voxels) and connection probability (the number of streamlines or connections that connect the seed and the target regions) were calculated. While we are aware that these two measures might suffer from some limitations (Jones et al. 2013), the debate on the effectiveness of the different indices of white matter integrity is still ongoing and both connection probability and tract volume have been used in previous research with interesting results (e.g., Khalsa et al. 2013; Budisavljevic et al. 2016). Consequently, in the present study we used three parameters of interest that have been reported to represent different measures of white matter integrity (Peeva et al. 2013): 1) mean tract FA (representing WM directionality), 2) connection probability (i.e., WM connection strength between two regions), and 3) tract volume.
Statistical analysis
Unix-based scripts were executed on the high performance cluster to calculate and extract the mean FA, connection probability, and tract volume from all participants. The output was written in text files and consequently imported into SPSS (version 20, IBM, Chicago, IL, USA), together with the demographic information, for statistical analysis. Linear regression was performed to assess whether anxiety-depression could predict the integrity of the tracts connecting the key regions of the four neural networks with one another and the amygdala. A laterality effect was only expected in the VAN and therefore, the results of the left and right hemisphere were averaged for all other networks. The model consisted of the ASR anxiety-depression scores as our main independent variable of interest. In addition, other important factors that might influence brain connectivity were added as regressors, i.e., age, gender, ethnicity, intelligence, and intracranial volume (e.g., Clayden et al. 2012). Ethnicity was represented by 5 variables with a value of 0 or 1, as the 6th is redundant (since the majority of participants had a white ethnic background, this predictor was left out). Since ASR anxiety-depression correlated with whole-brain FA (r = −.16, p < .001) and we were only interested in network effects, whole-brain FA was added as an independent variable in the regression analysis. Finally, for the pathways between the amygdala and cortical structures, amygdala size was also added as a predictor. Amygdala volume significantly correlated with intracranial volume (r(483) = .55, p < .001). Data were screened for influential cases to prevent the results from being driven by a small subsample of (clinical) participants. For each regression influential cases were defined as having a Cook’s distance higher than 4/n (Bollen and Jackman 1990) and excluded from further analysis. Subsequently, outliers (over 3 SD from the mean of the dependent variable) were removed. We controlled for multiple comparisons (i.e., multiple ROIs) by adjusting the significant p-values for the anxiety variable using the step-down Finner procedure (p < .05 corrected, Finner 1990, 1993). Effect size for the regressions was Cohen’s f2.
Results
Regional fractional anisotropy (FA)
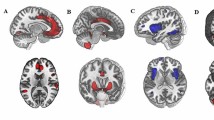
Higher anxiety-depression predicted lower FA in the tracts between the amygdala and key regions of the CON, DMN, and FPN. Specifically, greater symptoms relate to lower FA in the tracts between the amygdala and the dorsolateral PFC (dlPFC) within the FPN (β = -.12, t(440) = -3.11, corrected p = .01, R 2 = .30, f 2 = .43), the anterior PFC within the CON (β = -.09, t(439) = -2.28, corrected p = .05, R 2 = .30, f 2 = .43), and the parahippocampal gyrus (PHG) within the DMN (β = -.10, t(467) = -2.62, corrected p < .03, R 2 = .41, f 2 = .69) (Table 3). Figure 1 (left pane) provides a visual representation of the tracts between the amygdala and PFC.
Connection probability
The connection probability analyses also suggested that there was a negative influence of anxiety-depression on the connections between the amygdala and FPN. However, in this case the amygdala – inferior parietal lobe (IPL) tract showed a negative relationship with increasing symptoms (β = -.10, t(445) = -2.11, corrected p = .05, R 2 = .13, f 2 = .15; Table 4). Furthermore, anxiety-depression also predicted the connection probability of the amygdala and the temporal-parietal junction (TPJ) of the VAN (β = -.09, t(443) = -2.03, corrected p = .05, R 2 = .15, f 2 = .18; Table 4). Interestingly however, these two tracts appear to share a lot of voxels (Fig. 1, right pane).
Tract volume
Greater symptoms of anxiety and depression were negatively associated with tract volume in the amygdala – dlPFC tract of the FPN (β = -.10, t(439) = -2.14, corrected p = .05, R 2 = .17, f 2 = .20; Fig. 1; Table 5). No other effects were significant.
Discussion
This study examined to what extent trait anxiety-depression is represented in the WM integrity within core cognitive-affective networks and between these networks and the amygdala in a large healthy sample. Two main findings pertinent to the central hypotheses emerged. First, WM connectivity between the amygdala and the core networks was significantly affected by anxiety-depression. Specifically, higher anxiety-depression predicted lower WM integrity in the amygdala connections of all 4 different networks although we had expected heightened connectivity between the amygdala and FPN and VAN but lower connectivity between CON and DMN. Disrupted emotion-cognition interactions have been reported in both anxiety and depression (Banich et al. 2009), which is in accordance with the present results showing less WM integrity between a major “affective hub” of the brain and cognitive control regions. Second, against expectations, the current study did not detect altered WM integrity among structures of the four networks.
As predicted, anxiety-depression influenced amygdala connectivity with various networks involved in cognitive-affective function. Most interestingly, both key regions (dlPFC and IPL) of the FPN showed reduced amygdala connectivity in relation to anxiety-depression. The dlPFC – amygdala tract was characterized by reduced FA and reduced tract volume while the IPL displayed lower connection probability with the amygdala with increasing symptoms. The dlPFC – amygdala tract has received most attention in previous research on anxiety, nevertheless with rather mixed outcomes (e.g., Etkin et al. 2009; Eden et al. 2015). While some research reported heightened resting-state functional connectivity between these regions in generalized anxiety disorder (Etkin et al. 2009), others documented lower functional connectivity when viewing fearful faces in social anxiety disorder (Prater et al. 2013). In addition, Eden et al. (2015) did not find an effect of anxiety on the WM integrity of this tract in high trait anxiety. However, self-regulatory control of the FPN such as cognitive reappraisal has been linked to anxiety showing a positive relationship between emotion regulation ability and WM integrity (Eden et al. 2015) but reduced coactivation of the dlPFC during cognitive reappraisal in social anxiety disorder (Goldin et al. 2009). Furthermore, top-down functional connectivity from the dlPFC to the amygdala has been shown to be impaired in depression, indicating that the dlPFC is less effective in exerting cognitive control over the amygdala (Lu et al. 2012). Our findings are broadly consistent with such reports showing reduced structural WM integrity with greater anxiety-depression. An interesting hypothesis would therefore be that this reduction in WM integrity in the amygdala – dlPFC tract contributes to decreased recruitment of dlPFC subregions of the FPN necessary for cognitive control.
With regard to the salience and error detection network (CON), the WM between the anterior PFC (BA 10) and amygdala showed reduced integrity in relation to anxiety-depression. Here, our findings are consistent with the reduced fronto-limbic connectivity found in generalized anxiety disorder (Etkin et al. 2009), the lower functional coupling between amygdala and BA 10 associated with increasing social phobia severity (Laeger et al. 2014), and the weaker functional connectivity between BA 10 and amygdala elicited by negative stimuli that is associated with increasing severity of depression and anxiety in patients with major depression (Friedel et al. 2009). Etkin et al. (2009) speculate that reduced connectivity between the amygdala and the CON might be associated with dysfunctions in the modulation of the autonomic nervous system. This hypothesis receives some indirect support from the neurovisceral integration model, which states that the central autonomic network, the brain network responsible for the regulation of heart rate variability, comprises both prefrontal cortex (including BA 10) and the amygdala (Thayer and Brosschot 2005). However, future studies should directly investigate whether (WM) connectivity between amygdala and CON has implications for the autonomic nervous system. With regard to structural WM connectivity, evidence of an effect of anxiety and depression on anterior PFC – amygdala connections is rare. While lower uncinate fasciculus integrity has been reported in generalized anxiety disorder (Tromp et al. 2012) and major depressive disorder (Taylor et al. 2007; Liu et al. 2016), the present study extends this prior work by showing that individual differences in anxiety-depression in a large healthy cohort impact the specific connections between amygdala and anterior PFC as determined by tractography.
Similar to the frontal networks (FPN and CON), anxiety-depression also influenced amygdala connectivity to posterior networks (VAN) showing lower connection probability between the amygdala and TPJ in relation to anxiety-depression. The TPJ has been implicated in various functions including bottom-up attention processes (Corbetta and Shulman 2002; Carter and Huettel 2013) and social cognition (Carter and Huettel 2013). Bottom-up attention processes are known to be altered in anxiety as shown by a greater attentional bias to anxiety-relevant stimuli (Bar-Haim et al. 2007). A greater attentional bias to fearful stimuli has already been associated with changes in functional TPJ – amygdala coupling in healthy participants (Carlson et al. 2013). Yet, while Carlson et al. (2013) reported greater functional connectivity between the two regions, the current study observed lower structural WM connectivity with increasing anxiety-depression. It is, however, worth noting that anxiety or depression disposition was not taken into account in this previous work (Carlson et al. 2013). Taken together, few studies have examined TPJ involvement in anxiety and depression to date but the present structural findings, together with much behavioral work (for review see Bar-Haim et al. 2007) suggesting perturbed bottom-up processing of negative stimuli, would mandate future research effort.
Finally, connectivity between the amygdala and the DMN was also disrupted as shown by lower WM integrity in the amygdala – PHG tract associated with increasing anxiety-depression symptoms. Prior work in small samples of patients documents greater functional connectivity between the amygdala and PHG in anxiety (Liao et al. 2010b), while lower positive resting state functional connectivity between these regions has been reported in adolescent depression (Cullen et al. 2014). The PHG – amygdala connection is believed to constitute a crucial aspect of emotion regulation (Ochsner and Gross 2005) and it has been hypothesized that sustained emotion dysregulation could cause grey matter atrophy in the PHG in social anxiety disorder patients (Liao et al. 2011). Therefore, emotion regulation deficits might contribute to less WM connectivity between these structures. Clearly, more work is needed to disambiguate the effect that anxiety and depression might have on PHG structure and connectivity. Likewise, the relevance of the amygdala – PHG connections for emotion regulation deserves further investigation.
In contrast to the WM connections of the amygdala with the respective networks, WM connections within the networks could not be predicted by anxiety-depression. This finding was unexpected given the support for altered functional activity within these networks (e.g., Sylvester et al. 2012; Liao et al. 2010a; Korgaonkar et al. 2014). Perhaps, the influence of affective disorders on these networks, and the functions they represent, could be driven by altered, decreased connections with the amygdala. The involvement of the amygdala in anxiety and depression has been supported extensively by previous research (e.g., Davis and Whalen 2001) and it shares activation patterns with abundant and functionally heterogeneous regions of the brain (e.g., Bzdok et al. 2013). This amounts to a very large potential for the amygdala and its whole-brain WM connections to influence the functioning of brain networks. Hariri and Whalen (2011) indeed argue that the amygdala is very sensitive to different intrinsic and extrinsic factors and that it will use this information to influence the rest of the brain to guide our behavior. Pessoa (2008) goes further, proposing that it is not possible to separate affective and cognitive contributions to cognitive control functions. Therefore, the functions represented by the neural networks of interest in this study, such as attentional control, would be rooted in a constant interaction between the network’s key regions and the amygdala relaying emotion information. Taken together, previous research and theories support the notion that altered connections between amygdala and the cognitive networks could result in altered functioning of the networks even though within-network connections are unaffected.
In addition to anxiety-depression, other variables also emerged as significant predictors of tract integrity. First, the effect of amygdala size, which is mainly predictive of connection probability, is inherently related to the method of tracking used in this study. Since 5000 streamlines originated from each voxel of the seed mask, greater amygdala size should result in a higher number of streamlines arriving at the target region and therefore higher connection probability (see also Eden et al. 2015). Whole-brain FA also significantly predicted local WM integrity. This effect is in line with expectations and indicates that global and local FA were relatively consistent within participants. Finally, gender also predicted WM integrity, with male participants showing lower tract integrity than their female counterparts. While previous research suggests that men mostly have higher FA values than women, some white matter bundles also show greater FA in women as compared to men (e.g. the corpus callosum or fornix; Inano et al. 2011; Kanaan et al. 2014). Likewise, men also have higher whole-brain grey and white matter volume (Ruigrok et al. 2014). However, while the meta-analysis of Ruigrok et al. (2014) shows that the effect of gender displays a very diverse pattern in local grey matter, i.e. that men can have both higher and lower grey matter volume than women depending on the ROI, no localized WM analyses were reported. Taken together, the effect of gender on WM integrity and volume might not be uniform throughout the brain and deserves further research. The current study used three measures of tract integrity: tract FA, connection probability, and tract volume. Previous research suggests that all three measures represent different measures of white matter integrity; respectively WM directionality, WM connection strength between two regions, and tract volume (Peeva et al. 2013). However, the relationship among these three measures requires further enquiry.
This study has some limitations. First of all, in the HCP dataset no clinician-administered inventory for psychopathology was available and therefore the current study used the ASR questionnaire as a measure of anxiety and depression. However, in studies investigating neural correlates of anxiety and depression in a healthy normative sample, as opposed to a clinical sample, self-reported trait measures are commonly used (e.g., Etkin et al. 2004; Bishop 2009). Moreover, the use of a dimensional measure in a large general population provides much increased power and allows more interpretative strength regarding generalizability (in contrast to a comparison between a small sample with and without anxiety for example). However, the current study does not enable us to disentangle the effects of anxiety and depression given that the ASR problem scales do not have a separate anxiety and depression measure as well as the high correlation between these two symptom clusters. Thus, future research should investigate to what extent anxiety and depression would show distinct deficits in these networks. A second limitation is that changes in neurotransmitter systems might not be captured by diffusion MRI (Eden et al. 2015), and therefore the current results cannot inform on possible alterations in chemical communication between the regions of interest. Additionally, our analysis pipeline cannot account for artifacts originating from physiological noise (Walker et al. 2011; Jones et al. 2013). However, the implemented FSL pipeline is commonly used (e.g., Korgaonkar et al. 2014; Eden et al. 2015; Peeva et al. 2013) and can model three fiber directions per voxel as well as crossing fibers. Furthermore, while head movements can distort diffusion MRI findings (Yendiki et al. 2013), this cannot explain the effect of anxiety-depression in this study since the effects of head motion were removed in data preprocessing. Care has to be taken when interpreting null findings such as the lack of anxiety-related within-network WM changes. Since previous studies on the effect of anxiety on network functioning were mostly performed in small samples of clinically anxious participants (see also Sylvester et al. 2012), it is possible that the current large general population sample did not have the severity or specificity of symptoms to show these within network functional or structural dysfunctions. Furthermore, due to its correlational nature, the data do not presently allow any causal conclusions as to how anxiety-depression might perturb brain networks. While this study shows that anxiety-depression can predict WM integrity in the connection between the amygdala and certain structures of core brain networks, we can only speculate about the functional implications since we did not examine the relation to behavioral (performance) data. Future studies will need to elucidate relationship between structural WM alterations and functional deficits.
In conclusion, the current study applied probabilistic tractography in a large sample of healthy young adults to show that anxious and depressive feelings can predict WM integrity between four important neural networks and the amygdala. While these deficits could have important implications for emotion-cognition interactions in anxiety and depression, future studies are needed to determine the consequences of these deficits for cognitive-affective functioning and psychopathology.
References
Achenbach, T. M. (2009). The Achenbach system of empirically based Assessement (ASEBA): Development, findings, theory, and applications. Burlington, VT: University of Vermont Research Center for Children, Youth and Families.
Andersson, J. L. R., Skare, S., & Ashburner, J. (2003). How to correct susceptibility distortions in spin-echo echo-planar images: application to diffusion tensor imaging. NeuroImage, 20(2), 870–888. doi:10.1016/S1053-8119(03)00336-7.
Andersson, J. L. R., Jenkinson, M., & Smith, S. (2007). Non-linear registration aka spatial normalisation. https://www.fmrib.ox.ac.uk/analysis/techrep/tr07ja2/tr07ja2.pdf. Accessed 14 Mar 2016.
Andersson, J. L. R., Xu, J., Yacoub, E., Auerbach, E., Moeller, S., & Ugurbil, K. (2012). A comprehensive Gaussian process framework for correcting distortions and movements in diffusion images. Proceedings of the 20th Annual Meeting of ISMRM, Melbourne, 2426.
Ayling, E., Aghajani, M., Fouche, J. P., & van der Wee, N. (2012). Diffusion tensor imaging in anxiety disorders. Current Psychiatry Reports, 14(3), 197–202. doi:10.1007/s11920-012-0273-z.
Banich, M. T., Mackiewicz, K. L., Depue, B. E., Whitmer, A. J., Miller, G. A., & Heller, W. (2009). Cognitive control mechanisms, emotion and memory: a neural perspective with implications for psychopathology. Neuroscience and Biobehavioral Reviews, 33(5), 613–630. doi:10.1016/j.neubiorev.2008.09.010.
Bar-Haim, Y., Lamy, D., Pergamin, L., Bakermans-Kranenburg, M. J., & van, I. M. H. (2007). Threat-related attentional bias in anxious and nonanxious individuals: a meta-analytic study. Psychological Bulletin, 133(1), 1–24. doi:10.1037/0033-2909.133.1.1.
Beesdo, K., Lau, J. Y., Guyer, A. E., McClure-Tone, E. B., Monk, C. S., Nelson, E. E., et al. (2009). Common and distinct amygdala-function perturbations in depressed vs anxious adolescents. Archives of General Psychiatry, 66(3), 275–285. doi:10.1001/archgenpsychiatry.2008.545.
Behrens, T. E., Berg, H. J., Jbabdi, S., Rushworth, M. F., & Woolrich, M. W. (2007). Probabilistic diffusion tractography with multiple fibre orientations: what can we gain? NeuroImage, 34(1), 144–155. doi:10.1016/j.neuroimage.2006.09.018.
Bennett, I. J., Madden, D. J., Vaidya, C. J., Howard Jr., J. H., & Howard, D. V. (2011). White matter integrity correlates of implicit sequence learning in healthy aging. Neurobiology of Aging, 32(12), 2317.e1–2317.e12. doi:10.1016/j.neurobiolaging.2010.03.017.
Bishop, S. J. (2009). Trait anxiety and impoverished prefrontal control of attention. Nature Neuroscience, 12(1), 92–98. doi:10.1038/nn.2242.
Bollen, K. A., & Jackman, R. W. (1990). Regression diagnostics: An expository treatment of outliers and influential cases. In J. Fox & J. S. Long (Eds.), Modern methods of data analysis (pp. 257–291). Newbury Park, CA: Sage.
Budisavljevic, S., Dell'Acqua, F., Zanatto, D., Begliomini, C., Miotto, D., Motta, R., et al. (2016). Asymmetry and structure of the fronto-parietal networks underlie Visuomotor processing in humans. Cerebral Cortex. doi:10.1093/cercor/bhv348.
Bzdok, D., Laird, A. R., Zilles, K., Fox, P. T., & Eickhoff, S. B. (2013). An investigation of the structural, connectional, and functional subspecialization in the human amygdala. Human Brain Mapping, 34(12), 3247–3266. doi:10.1002/hbm.22138.
Carlson, J. M., Cha, J., & Mujica-Parodi, L. R. (2013). Functional and structural amygdala - anterior cingulate connectivity correlates with attentional bias to masked fearful faces. Cortex, 49(9), 2595–2600. doi:10.1016/j.cortex.2013.07.008.
Carter, R. M., & Huettel, S. A. (2013). A nexus model of the temporal-parietal junction. Trends in Cognitive Sciences, 17(7), 328–336. doi:10.1016/j.tics.2013.05.007.
Clayden, J. D., Jentschke, S., Munoz, M., Cooper, J. M., Chadwick, M. J., Banks, T., et al. (2012). Normative development of white matter tracts: similarities and differences in relation to age, gender, and intelligence. Cerebral Cortex, 22(8), 1738–1747. doi:10.1093/cercor/bhr243.
Clewett, D., Bachman, S., & Mather, M. (2014). Age-related reduced prefrontal-amygdala structural connectivity is associated with lower trait anxiety. Neuropsychology, 28(4), 631–642. doi:10.1037/neu0000060.
Corbetta, M., & Shulman, G. L. (2002). Control of goal-directed and stimulus-driven attention in the brain. Nature Reviews Neuroscience, 3(3), 201–215. doi:10.1038/nrn755.
Cullen, K. R., Westlund, M. K., Klimes-Dougan, B., Mueller, B. A., Houri, A., Eberly, L. E., et al. (2014). Abnormal amygdala resting-state functional connectivity in adolescent depression. JAMA Psychiatry, 71(10), 1138–1147. doi:10.1001/jamapsychiatry.2014.1087.
Davis, M., & Whalen, P. J. (2001). The amygdala: vigilance and emotion. Molecular Psychiatry, 6(1), 13–34.
Diez, I., Bonifazi, P., Escudero, I., Mateos, B., Munoz, M. A., Stramaglia, S., et al. (2015). A novel brain partition highlights the modular skeleton shared by structure and function. Scientific Reports, 5, 10532. doi:10.1038/srep10532.
Dosenbach, N. U., Fair, D. A., Miezin, F. M., Cohen, A. L., Wenger, K. K., Dosenbach, R. A., et al. (2007). Distinct brain networks for adaptive and stable task control in humans. Proceedings of the National Academy of Sciences of the United States of America, 104(26), 11073–11078. doi:10.1073/pnas.0704320104.
Dosenbach, N. U., Fair, D. A., Cohen, A. L., Schlaggar, B. L., & Petersen, S. E. (2008). A dual-networks architecture of top-down control. Trends in Cognitive Sciences, 12(3), 99–105. doi:10.1016/j.tics.2008.01.001.
Drevets, W. C., Price, J. L., Simpson Jr., J. R., Todd, R. D., Reich, T., Vannier, M., et al. (1997). Subgenual prefrontal cortex abnormalities in mood disorders. Nature, 386(6627), 824–827. doi:10.1038/386824a0.
Eden, A. S., Schreiber, J., Anwander, A., Keuper, K., Laeger, I., Zwanzger, P., et al. (2015). Emotion regulation and trait anxiety are predicted by the microstructure of fibers between amygdala and prefrontal cortex. Journal of Neuroscience, 35(15), 6020–6027. doi:10.1523/JNEUROSCI.3659-14.2015.
Etkin, A., Klemenhagen, K. C., Dudman, J. T., Rogan, M. T., Hen, R., Kandel, E. R., et al. (2004). Individual differences in trait anxiety predict the response of the basolateral amygdala to unconsciously processed fearful faces. Neuron, 44(6), 1043–1055. doi:10.1016/j.neuron.2004.12.006.
Etkin, A., Prater, K. E., Schatzberg, A. F., Menon, V., & Greicius, M. D. (2009). Disrupted amygdalar subregion functional connectivity and evidence of a compensatory network in generalized anxiety disorder. Archives of General Psychiatry, 66(12), 1361–1372. doi:10.1001/archgenpsychiatry.2009.104.
Etkin, A., Prater, K. E., Hoeft, F., Menon, V., & Schatzberg, A. F. (2010). Failure of anterior cingulate activation and connectivity with the amygdala during implicit regulation of emotional processing in generalized anxiety disorder. American Journal of Psychiatry, 167(5), 545–554. doi:10.1176/appi.ajp.2009.09070931.
Fair, D. A., Cohen, A. L., Dosenbach, N. U., Church, J. A., Miezin, F. M., Barch, D. M., et al. (2008). The maturing architecture of the brain's default network. Proceedings of the National Academy of Sciences of the United States of America, 105(10), 4028–4032. doi:10.1073/pnas.0800376105.
Finner, H. (1990). Some new inequalities for the range distribution, with application to the determination of optimum significance levels of multiple range tests. Journal of the American Statistical Association, 85(409), 191–194. doi:10.2307/2289544.
Finner, H. (1993). On a monotonicity problem in step-down multiple test procedures. Journal of the American Statistical Association, 88(423), 920–923. doi:10.2307/2290782.
Fischl, B. (2012). FreeSurfer. NeuroImage, 62(2), 774–781. doi:10.1016/j.neuroimage.2012.01.021.
Fox, M. D., Corbetta, M., Snyder, A. Z., Vincent, J. L., & Raichle, M. E. (2006). Spontaneous neuronal activity distinguishes human dorsal and ventral attention systems. Proceedings of the National Academy of Sciences of the United States of America, 103(26), 10046–10051. doi:10.1073/pnas.0604187103.
Friedel, E., Schlagenhauf, F., Sterzer, P., Park, S. Q., Bermpohl, F., Strohle, A., et al. (2009). 5-HTT genotype effect on prefrontal-amygdala coupling differs between major depression and controls. Psychopharmacology, 205(2), 261–271. doi:10.1007/s00213-009-1536-1.
Goldin, P. R., Manber-Ball, T., Werner, K., Heimberg, R., & Gross, J. J. (2009). Neural mechanisms of cognitive reappraisal of negative self-beliefs in social anxiety disorder. Biological Psychiatry, 66(12), 1091–1099. doi:10.1016/j.biopsych.2009.07.014.
Greicius, M. D., Krasnow, B., Reiss, A. L., & Menon, V. (2003). Functional connectivity in the resting brain: a network analysis of the default mode hypothesis. Proceedings of the National Academy of Sciences of the United States of America, 100(1), 253–258. doi:10.1073/pnas.0135058100.
Hariri, A. R., & Whalen, P. J. (2011). The amygdala: inside and out. F1000 Biology Reports, 3, 2. doi:10.3410/B3-2.
Honey, C. J., Sporns, O., Cammoun, L., Gigandet, X., Thiran, J. P., Meuli, R., et al. (2009). Predicting human resting-state functional connectivity from structural connectivity. Proceedings of the National Academy of Sciences of the United States of America, 106(6), 2035–2040. doi:10.1073/pnas.0811168106.
Inano, S., Takao, H., Hayashi, N., Abe, O., & Ohtomo, K. (2011). Effects of age and gender on white matter integrity. American Journal of Neuroradiology, 32(11), 2103–2109. doi:10.3174/ajnr.A2785.
Jbabdi, S., Sotiropoulos, S. N., Savio, A. M., Grana, M., & Behrens, T. E. (2012). Model-based analysis of multishell diffusion MR data for tractography: how to get over fitting problems. Magnetic Resonance in Medicine, 68(6), 1846–1855. doi:10.1002/mrm.24204.
Jenkinson, M., Bannister, P., Brady, M., & Smith, S. (2002). Improved optimization for the robust and accurate linear registration and motion correction of brain images. NeuroImage, 17(2), 825–841. doi:10.1006/nimg.2002.1132.
Jenkinson, M., Beckmann, C. F., Behrens, T. E., Woolrich, M. W., & Smith, S. M. (2012). Fsl. Neuroimage, 62(2), 782–790. doi:10.1016/j.neuroimage.2011.09.015.
Jones, D. K., Knosche, T. R., & Turner, R. (2013). White matter integrity, fiber count, and other fallacies: the do's and don'ts of diffusion MRI. NeuroImage, 73, 239–254. doi:10.1016/j.neuroimage.2012.06.081.
Kanaan, R. A., Chaddock, C., Allin, M., Picchioni, M. M., Daly, E., Shergill, S. S., et al. (2014). Gender influence on white matter microstructure: a tract-based spatial statistics analysis. PloS One, 9(3), e91109. doi:10.1371/journal.pone.0091109.
Khalsa, S., Mayhew, S. D., Chechlacz, M., Bagary, M., & Bagshaw, A. P. (2013). The structural and functional connectivity of the posterior cingulate cortex: comparison between deterministic and probabilistic tractography for the investigation of structure-function relationships. NeuroImage, 102, 118–127. doi:10.1016/j.neuroimage.2013.12.022.
Kim, H. (2014). Involvement of the dorsal and ventral attention networks in oddball stimulus processing: a meta-analysis. Human Brain Mapping, 35(5), 2265–2284. doi:10.1002/hbm.22326.
Kim, M. J., & Whalen, P. J. (2009). The structural integrity of an amygdala-prefrontal pathway predicts trait anxiety. Journal of Neuroscience, 29(37), 11614–11618. doi:10.1523/jneurosci.2335-09.2009.
Kollndorfer, K., Krajnik, J., Woitek, R., Freiherr, J., Prayer, D., & Schopf, V. (2013). Altered likelihood of brain activation in attention and working memory networks in patients with multiple sclerosis: an ALE meta-analysis. Neuroscience and Biobehavioral Reviews, 37(10 Pt 2), 2699–2708. doi:10.1016/j.neubiorev.2013.09.005.
Korgaonkar, M. S., Fornito, A., Williams, L. M., & Grieve, S. M. (2014). Abnormal structural networks characterize major depressive disorder: a connectome analysis. Biological Psychiatry, 76(7), 567–574. doi:10.1016/j.biopsych.2014.02.018.
Laeger, I., Dobel, C., Radenz, B., Kugel, H., Keuper, K., Eden, A., et al. (2014). Of 'Disgrace' and 'Pain' - Corticolimbic interaction patterns for disorder- relevant and emotional words in social phobia. PloS One, 9(11), e109949. doi:10.1371/journal.pone.0109949.
Liao, W., Chen, H., Feng, Y., Mantini, D., Gentili, C., Pan, Z., et al. (2010a). Selective aberrant functional connectivity of resting state networks in social anxiety disorder. NeuroImage, 52(4), 1549–1558. doi:10.1016/j.neuroimage.2010.05.010.
Liao, W., Qiu, C., Gentili, C., Walter, M., Pan, Z., Ding, J., et al. (2010b). Altered effective connectivity network of the amygdala in social anxiety disorder: a resting-state FMRI study. PloS One, 5(12), e15238. doi:10.1371/journal.pone.0015238.
Liao, W., Xu, Q., Mantini, D., Ding, J., Machado-de-Sousa, J. P., Hallak, J. E., et al. (2011). Altered gray matter morphometry and resting-state functional and structural connectivity in social anxiety disorder. Brain Research, 1388, 167–177. doi:10.1016/j.brainres.2011.03.018.
Liu, X., Watanabe, K., Kakeda, S., Yoshimura, R., Abe, O., Hayashi, K., et al. (2016). Relationship between white matter integrity and serum cortisol levels in drug-naive patients with major depressive disorder: diffusion tensor imaging study using tract-based spatial statistics. The British Journal of Psychiatry. doi:10.1192/bjp.bp.114.155689.
Lu, Q., Li, H. R., Luo, G. P., Wang, Y., Tang, H., Han, L., et al. (2012). Impaired prefrontal-amygdala effective connectivity is responsible for the dysfunction of emotion process in major depressive disorder: a dynamic causal modeling study on MEG. Neuroscience Letters, 523(2), 125–130. doi:10.1016/j.neulet.2012.06.058.
Modi, S., Trivedi, R., Singh, K., Kumar, P., Rathore, R. K., Tripathi, R. P., et al. (2013). Individual differences in trait anxiety are associated with white matter tract integrity in fornix and uncinate fasciculus: preliminary evidence from a DTI based tractography study. Behavioural Brain Research, 238, 188–192. doi:10.1016/j.bbr.2012.10.007.
Monk, C. S., Telzer, E. H., Mogg, K., Bradley, B. P., Mai, X. Q., Louro, H. M. C., et al. (2008). Amygdala and ventrolateral prefrontal cortex activation to masked angry faces in children and adolescents with generalized anxiety disorder. Archives of General Psychiatry, 65(5), 568–576. doi:10.1001/archpsyc.65.5.568.
Nakamae, T., Sakai, Y., Abe, Y., Nishida, S., Fukui, K., Yamada, K., et al. (2014). Altered fronto-striatal fiber topography and connectivity in obsessive-compulsive disorder. PloS One, 9(11), e112075. doi:10.1371/journal.pone.0112075.
Ochsner, K. N., & Gross, J. J. (2005). The cognitive control of emotion. Trends in Cognitive Sciences, 9(5), 242–249. doi:10.1016/j.tics.2005.03.010.
Patenaude, B., Smith, S. M., Kennedy, D. N., & Jenkinson, M. (2011). A Bayesian model of shape and appearance for subcortical brain segmentation. NeuroImage, 56(3), 907–922. doi:10.1016/j.neuroimage.2011.02.046.
Peeva, M. G., Tourville, J. A., Agam, Y., Holland, B., Manoach, D. S., & Guenther, F. H. (2013). White matter impairment in the speech network of individuals with autism spectrum disorder. Neuroimage Clinical, 3, 234–241. doi:10.1016/j.nicl.2013.08.011.
Pessoa, L. (2008). On the relationship between emotion and cognition. Nature Reviews Neuroscience, 9(2), 148–158. doi:10.1038/Nrn2317.
Power, J. D., Cohen, A. L., Nelson, S. M., Wig, G. S., Barnes, K. A., Church, J. A., et al. (2011). Functional network organization of the human brain. Neuron, 72(4), 665–678. doi:10.1016/j.neuron.2011.09.006.
Prater, K. E., Hosanagar, A., Klumpp, H., Angstadt, M., & Phan, K. L. (2013). Aberrant amygdala-frontal cortex connectivity during perception of fearful faces and at rest in generalized social anxiety disorder. Depression and Anxiety, 30(3), 234–241. doi:10.1002/da.22014.
Raichle, M. E. (2011). The restless brain. Brain Connectivity, 1(1), 3–12. doi:10.1089/brain.2011.0019.
Raichle, M. E., MacLeod, A. M., Snyder, A. Z., Powers, W. J., Gusnard, D. A., & Shulman, G. L. (2001). A default mode of brain function. Proceedings of the National Academy of Sciences of the United States of America, 98(2), 676–682. doi:10.1073/pnas.98.2.676.
Rauch, S. L., Shin, L. M., & Wright, C. I. (2003). Neuroimaging studies of amygdala function in anxiety disorders. Annals of the New York Academy of Sciences, 985, 389–410.
Raven, J., Raven, J. C., & Court, J. H. (2003). Manual for Raven's progressive matrices and vocabulary scales. Section 1: General overview. San Antonio, TX: Harcourt Assessment.
Ruigrok, A. N., Salimi-Khorshidi, G., Lai, M. C., Baron-Cohen, S., Lombardo, M. V., Tait, R. J., et al. (2014). A meta-analysis of sex differences in human brain structure. Neuroscience and Biobehavioral Reviews, 39, 34–50. doi:10.1016/j.neubiorev.2013.12.004.
Sotiropoulos, S. N., Moeller, S., Jbabdi, S., Xu, J., Andersson, J. L., Auerbach, E. J., et al. (2013). Effects of image reconstruction on fiber orientation mapping from multichannel diffusion MRI: reducing the noise floor using SENSE. Magnetic Resonance in Medicine, 70(6), 1682–1689. doi:10.1002/mrm.24623.
Sylvester, C. M., Corbetta, M., Raichle, M. E., Rodebaugh, T. L., Schlaggar, B. L., Sheline, Y. I., et al. (2012). Functional network dysfunction in anxiety and anxiety disorders. Trends in Neurosciences, 35(9), 527–535. doi:10.1016/j.tins.2012.04.012.
Taylor, W. D., MacFall, J. R., Gerig, G., & Krishnan, R. R. (2007). Structural integrity of the uncinate fasciculus in geriatric depression: relationship with age of onset. Neuropsychiatric Disease and Treatment, 3(5), 669–674.
Teipel, S. J., Bokde, A. L., Meindl, T., Amaro Jr., E., Soldner, J., Reiser, M. F., et al. (2010). White matter microstructure underlying default mode network connectivity in the human brain. NeuroImage, 49(3), 2021–2032. doi:10.1016/j.neuroimage.2009.10.067.
Thayer, J. F., & Brosschot, J. F. (2005). Psychosomatics and psychopathology: looking up and down from the brain. Psychoneuroendocrinology, 30(10), 1050–1058. doi:10.1016/j.psyneuen.2005.04.014.
Tromp, D. P., Grupe, D. W., Oathes, D. J., McFarlin, D. R., Hernandez, P. J., Kral, T. R., et al. (2012). Reduced structural connectivity of a major frontolimbic pathway in generalized anxiety disorder. Archives of General Psychiatry, 69(9), 925–934. doi:10.1001/archgenpsychiatry.2011.2178.
Walker, L., Chang, L. C., Koay, C. G., Sharma, N., Cohen, L., Verma, R., et al. (2011). Effects of physiological noise in population analysis of diffusion tensor MRI data. NeuroImage, 54(2), 1168–1177. doi:10.1016/j.neuroimage.2010.08.048.
Yendiki, A., Koldewyn, K., Kakunoori, S., Kanwisher, N., & Fischl, B. (2013). Spurious group differences due to head motion in a diffusion MRI study. NeuroImage, 88, 79–90. doi:10.1016/j.neuroimage.2013.11.027.
Acknowledgments
Data were provided by the Human Connectome Project, WU-Minn Consortium (Principal Investigators: David Van Essen and Kamil Ugurbil; 1U54MH091657) and funded by the 16 NIH Institutes and Centers that support the NIH Blueprint for Neuroscience Research and by the McDonnell Center for Systems Neuroscience at Washington University.
The computational resources (Stevin Supercomputer Infrastructure) and services used in this work were provided by the VSC (Flemish Supercomputer Center), and funded by Ghent University, the Hercules Foundation and the Flemish Government – Department EWI.
SCM and NDW are supported by Ghent University (Multidisciplinary Research Partnership “The integrative neuroscience of behavioral control”).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
De Witte, N.A.J., Mueller, S.C. White matter integrity in brain networks relevant to anxiety and depression: evidence from the human connectome project dataset. Brain Imaging and Behavior 11, 1604–1615 (2017). https://doi.org/10.1007/s11682-016-9642-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11682-016-9642-2